
Review Article
J Immun Res. 2014;1(1): 4.
Immunosuppression Induced by Avian Leukosis and Sarcoma Virus
Ning Cui1,2, Ziqiang Cheng1 and Xiaomin Zhao1,2*
1College of Veterinary Medicine, Shandong Agricultural University, China
2Shandong Provincial Key Laboratory of Animal Biotechnology and Disease Control and Prevention, Shandong Agricultural University, China
*Corresponding author: Xiaomin Zhao, College of Veterinary Medicine, Shandong Agricultural University, Tai’an, China
Received: Aug 05, 2014; Accepted: Aug 25, 2014; Published: Aug 26, 2014
Abstract
Avian Leukosis/Sarcoma Viruses (ALSVs) belong to the genus Alpha retrovirus of family Retroviridae. ALSVs that occur in chickens have been divided into 6 envelope subgroups (A–E and J) based on the differences in their viral envelope glycoproteins. ALSV infections usually induce several kinds of neoplasm of infected hosts which lead to severe morbidity and mortality. Most importantly, hosts infected with ALSVs often develop immunosuppression, similar as Acquired Immunodeficiency Syndrome (AIDS) caused by Human Immunodeficiency Virus (HIV) in human. Immunosuppression can be induced by different subgroups of ALSVs in different mechanisms. Some possiblemechanisms were reviewed in this short paper.
Keywords: Avian leukosis/Sarcoma virus; Retrovirus; Immunosuppression; Mechanism
Introduction
Immunosuppression means impairment of the immune system that causes a weaker or even no response to antigens in hosts. Studies have demonstrated that many factors cause immunosuppression, including nutritions, diseases, stresses, microbes and so on, in which virus-induced-immunosuppression is most common and especially severe. Immunosuppression associated with a virus infection was first described in patients who lost their tuberculin sensitivity during and after measles about 100 years ago. It was not until the occurrence of Acquired Immunodeficiency Syndrome (AIDS) that virus-induced immunosuppression has attracted a renewed attention and detailed investigation [1-4]. Immunosuppression can be induced through diverse mechanisms, including direct toxicity to target cells, fetal infection leading to tolerance, viral proteins acting on infected cells or uninfected bystander cells leading to cell death and aberrant production of cytokines, and suppressor T lymphocytes.
Avian Leukosis/Sarcoma Viruses (ALSVs) are a group of retroviruses frequently causing immunosuppression [5,6]. Members of this group of viruses have similar physical and molecular characteristics and share a common group-specific antigen envelope glycoprotein. Infection of cells is dependent on the presence, in the cell membrane, of host gene-encoded receptors specific for particular virus envelope subgroups and on fusion of viral and cell membranes [7]. The virion envelope contains 2 glycoproteins encoded by the env gene: SU (surface, gp85), the viral surface knob-like structure, that contains the receptor binding site and determines viral envelope subgroup specificity of the ALSV; and TM (Transmembrane, gp37), harboring functional elements required for fusion with host cells, a fusion peptide, two Heptads Repeats (HR) and a Trans Membrane region (TM) [8,9]. Using interference patterns of different strains of leukosis virus against different strains of Rous Sarcoma Virus (RSV), ALSVs that occur in chickens have been divided into 6 envelope subgroups, designated A, B, C, D, E and J [6,10-12]. A few studies have revealed some possible mechanisms of ALSVs-induced immunosuppression,but detailed mechanisms for immunosuppression induced by these viruses have not been fully understood.
ALSVs-induced immunosuppression
In addition to inducing tumor growth and subsequent mortality, ALSVs impart immunosuppressive effects that lead to decreases in the immunologic function and productivity. Chickens that are infected congenitally with ALV become immunologically tolerant to the virus, that they do not develop immune responses to the virus, but develop a persistent viremia in the absence of neutralizing antibodies [13,14]. The younger the chicken at infections, the longer the duration of a viremia, and the longer for the antibody to develop. Chickens with a tolerant viremic infection are more likely to develop neoplasms because of more virus loads. Infection with ALV can lead to a depression in primary and secondary antibody responses and cell-mediated immunity [15] of hosts to unrelated antigens, making it easy for secondary infections or opportunistic pathogens to develop clinical features. This will result in a severe co-infection phenomenon which increases morbidities and mortalities [16,17]. Decreases in productivity performed as decline in weight gain, egg production, fertility, and hatchability directly cause tremendous economic losses.
Target cells of subgroup J ALV are myeloid cells and immunosuppression induced by ALV-J appears to be associated with both T and B cells [11,18]. Viruses of other subgroups, such as A, B, C, and D, mainly infect B lymphocytes [12]. They are well known potent inducers of wasting disease and anemia, the severity of which, however, appears to be strain dependent. The genetic sequences of the eve loci are related to subgroup E of ALVs and are present as either complete or defective genomes in almost all normal chickens [19-23]. Viruses of subgroup E are of equally important in causing immunosuppression alone and affecting the immunosuppression by exogenous viruses [24-26].
Immunosuppression mechanisms caused by different ALSV subgroups
Acute transforming ALSVs contain viral oncogene in their genomes, and they induce neoplastic transformation, in vivo or in vitro, within a few days [27,28]. While slow transforming ALVs do not carry viral oncogenes and they induce tumors by a “promoter insertion” or a related mechanism that activates a cellular oncogene to bring about neoplastic transformation and development of tumors over many weeks or months [8, 29-31]. There are two distinct ways to cause immunosuppression in acute transforming process of tumor genesis different from slow transforming ALVs. One is immunosuppression caused directly by tumor related antigens [32], and another is immunosuppression caused by the induction and activation of suppressor T lymphocytes and macrophages. Suppressor cells play their inhibitory effect via an interaction between suppressor cells and effect cells or through inhibiting factors [32].
Subgroup B and D ALVs are capable of inducing Cytopathic Effects (CPEs) upon infection of cultured avian cells [33,34]. The CPE is explained by use of a death receptor for subgroups B and D, designated TVBs3, as a Tumor Necrosis Factor (TNF) receptor-related death receptor with a cytoplasmic death domain [33, 35-37]. This is most possibly similar to immunosuppression caused directly by tumor related antigens. Symptoms of the disease induced by viruses of ALV subgroup C are most obvious, and a key feature was a depletion of B lymphocytes in the thymus, bursa and spleen within 2 to 3 weeks after hatching. The receptor for the subgroup C ALV, TVC, is related to mammalian butyrophilins, members of the immunoglobulin super family [38]. Although a documented cythopathogenicity of subgroup C to DF-1 cells indicates that some death-promoting activity of the TVC receptor might be stimulated upon binding of the retrovirus, the signaling pathway might be different from those activated by subgroups B and D [39]. However, the direct toxic effect of ALSV to infected cells may not be a principal cause of lymphoid tissue depletion; more probably, uninfected bystander cells are attacked in an indirect way as has been shown in an anther retroviral infection. Studies from HIV indicated that the virus infects CD4+ T helper lymphocyte (Th) and kills infected cells via Cytotoxic T Lymphocyte (CTL)-mediated mechanisms [1,40]. More seriously, viral Env glycoproteins trigger autophagy in uninfected bystander CD4 T cells, leading to apoptosis and thus contributing to a large loss of Th lymphocytes [41-43]. In fact, HIV or SIV infection causes a universal activation of all lymphocyte population (CD4+, CD8+, NK, and B cells) and a high proportion of activated cells undergo rapid apoptosis. Due to the vital role of these cells in regulating and amplifying the immune response, any decline in their number results in deficiencies in both humeral and cell-mediated immunity. Whether ALVs kill uninfected by stander cells via the same manner as HIV still needs further proofs. Binding of HIV-1 Env to both a primary receptor (CD40) and a co-receptor (mainly CCR5 and CXCR4) on the surface of susceptible cells trigger autophagy in uninfected bystander CD4 T cells, which most likely contributes to an immunodeficiency [41]. CD4 is also a member of the immunoglobulin super family. Thus viruses of ALV subgroup C most possibly share the similar mechanism as HIV-1 [41].
Previous studies showed that subgroup A ALV might induce the least immunosuppression in vivo. The receptor for subgroup A ALV, designated TVA, is related to the human low-density lipoprotein receptor [44,45]. Congenital infection with Rous-Associated Viruse-1 (RAV-1) caused no detectable immunodepression during the early and late stages of infections [46], but affected a T cell population during the advanced stage of the disease [47]. Besides, only RAV- 1 infection can cause immunodepressions in chickens that lack endogenous virus gene expressions [48,49].
Apoptosis can be induced by binding of a soluble ALV-E Surface envelope protein (SU) to its receptor, at least in quail or turkey cells. Cellular receptors for the noncytopathic subgroup E of ALV (ALV-E), TVBT, a turkey subgroup E-specific ALV receptor, and TVBS1, a chicken receptor for subgroups B, D, and E ALV, are functional death receptors that can trigger cell death by apoptosis [33]. Except cell death induced by subgroup E ALV, the normal presence of ev loci may suppress the immune response to some exogenous ALVs by inducing partial immunological tolerance [25,26,50]. Thus, the presence of the ev loci makes an increased incidence of lymphoid leukosis in the field and experimentally [6]. For example, embryonic infection with Rous-Associated Viruse-0 (RAV-0) causes a more persistent viremia and more neoplasms following infection with exogenous ALV [24]. Similarly, expression of EV21 ALV by the ev21 locus has a tolerating effect on response to exogenous ALV. Besides, there was a strong additive effect between ev6 and ev9 in reducing an antibody response to exogenous ALSVs infection [51]. But a biological value of endogenous viruses is controversial, because in certain circumstances they can be of value as the presence of ev2 or ev3 has been reported to protect birds from a non-neoplastic syndrome caused by infection with subgroup A ALV [48,49]. Because these ev-genes express endogenous viral protein or complete endogenous virus [23], it has been proposed that the induction of immunological tolerance is attributable to common epitomes between endogenous and exogenous virus [48], which is dependent on the expression of viral proteins, and there is possibly synergistic effect between these proteins [51]. Likewise, embryonic infection with any of an exogenous ALSV subgroup may lead to a tolerance to other subgroups.
Subgroup J ALV mainly attacks myeloid cells, causes a malignant growth, interferes synthesis of functional IL-2, influences mature and differentiation of B and T lymphocytes, and thus induces immunosuppression [52,53]. The immunosuppression mechanism induced by subgroup J ALV might distinct from other subgroups because the host cell receptor used by the subgroup J ALV has been identified as the chicken Na(+)/H(+) Exchanger type 1 (chNHE1) protein [54]. Recent studies in immunosuppression mechanisms in cancers revealed that tumor exosomes play an important role in inducing myeloid-derived suppressor cells, which promote a tumor progression [55-57]. Some researchers have sought clues from exosomes secreted by ALV-J infected cells. Their studies suggested that exosomes secreted by ALV-J infected cells contain virus-encoding Env and Gag proteins and showed similar immunosuppression effects in immune cells as subgroup J ALV. Although the role of exosomes in subgroup J ALV induced immunosuppression is still unclear, chicken biliary exosomes were demonstrated to possess a capacity to influence immune responses of lymphocytes and inhibit subgroup J ALV [58].
Other factors playing roles in immunosuppression
Obviously, cell death, either caused by death receptor-mediated CPEs or Env glycoprotein-triggered autophagy, plays an essential role in inducing immunosuppression in slow transforming ALVs. Damage of immune cells will surely cause aberrant responses of cytokines produced by these cells. Since it is cascade reactions to produce cytokines and for cytokines to play their role, a globe dysregulation of cytokines in vivo will surely occur. There is also evidence that as AIDS and other retroviruses progress cytokines dysregulation occurs [59,60]. Dysregulation of cytokines in turn aggravate virus-induced immunosuppression. In addition, many DNA viruses produce a number of proteins that act as ‘viroceptors’, which resemble and compete with cellular receptors of the host, biding the cytokines and reducing its physiological activity. But there is no evidence of such possible manners for ALSVs to perturb cytokine homoeostasis except the damage of immune cells.
Conclusion
Although ALSVs-induced immunosuppression can occur through diverse mechanisms (Figure 1), the virus-encoding Env glycoprotein’s play a major role in almost all of the possible mechanisms. Env is not only a key protein for ALSVs to recognize corresponding receptors and enter target cells, but also mediates the death of uninfected bystander immune cells. Further studies on Env-related mechanisms of immunosuppression will be of great value in seeking potential new therapeutic targets direct against immunosuppression. Regulation of autophagy also provided a possible way, but it depends on clear awareness of the role of autophagy in ALSVs infection. Considering the complexity of immunosuppression phenomenon and the complexity of ALSVs group, researches on ALSVs-induced immunosuppression still need much effort. Since ALSV has been intensively used as a model to study retroviruses, there is no doubt that any breakthrough in ALSVs studies will bring new insights into retroviruses.
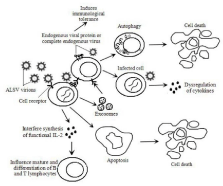
Figure 1: Hypotheses of mechanisms of immunosuppressant caused by ALSVs based on data from the literature. ALSV visions infect and enter target cells depend on Env-driven fusion of viral and cellular membranes. Immune cells or other cells are affected or killed by the following ways: a: Ev-genes express endogenous viral proteins or complete endogenous viruses that induce immunological tolerance; b: Infected cells express viral proteins or complete viruses trigger autophagy in uninfected bystander; c: Infected immune cells cause aberrant responses of cytokines leading to globe deregulations of cytokines in vivo; d: Exosomes secreted by infected cells show similar immunosuppressant effect in immune cells as visions; e: Cell apoptosis occurs in target cells upon infection by a death receptor; f: The visions attack myeloid cells and cause malignant growth, interfere synthesis of functional IL-2, influence mature and differentiation of B and T lymphocytes.
References
- Alimonti JB, Ball TB, Fowke KR. Mechanisms of CD4+ T lymphocyte cell death in human immunodeficiency virus infection and AIDS. J Gen Virol. 2003; 84: 1649-1661.
- Jekle A, Keppler OT, De Clercq E, Schols D, Weinstein M, Goldsmith MA. In vivo evolution of human immunodeficiency virus type 1 toward increased pathogenicity through CXCR4-mediated killing of uninfected CD4 T cells. J Virol. 2003; 77: 5846-5854.
- Prottengeier J, Koutsilieri E, Scheller C. The effects of opioids on HIV reactivation in latently-infected T-lymphoblasts. AIDS Res Ther. 2014; 11: 17.
- Taron-Brocard C, Le Chenadec J, Faye A, Dollfus C, Goetghebuer T, Gajdos, et al. Increased risk of serious bacterial infections due to maternal immunosuppression in HIV-exposed uninfected infants in a European country. Clin Infect Dis. 2014.
- Meyers P, Dougherty RM. Immunologic reactivity to viral antigens in chickens infected with avian leukosis viruses. J Natl Cancer Inst. 1971; 46: 701-711.
- Payne LN. Retrovirus-induced disease in poultry. Poult Sci. 1998; 77: 1204-1212.
- Dales S, Hanafusa H. Penetration and intracellular release of the genomes of avian RNA tumor viruses. Virology. 1972; 50: 440-458.
- Gallaher WR. Similar structural models of the transmembrane proteins of Ebola and avian sarcoma viruses. Cell. 1996; 85: 477-478.
- White JM, Delos SE, Brecher M, Schornberg K. Structures and mechanisms of viral membrane fusion proteins: multiple variations on a common theme. Crit Rev Biochem Mol Biol. 2008; 43: 189-219.
- Fadly AM and Nair V. Leukosis/sarcoma group. Saif YM, Fadly AM, Glisson JR, McDougald LR, Nolan LK, Swayne DE, editors. In: Diseases of Poultry 12th edn. Ames, IA: Blackwell Publishing. 2008; 514-568.
- Payne LN, Brown SR, Bumstead N, Howes K, Frazier JA, Thouless ME. A novel subgroup of exogenous avian leukosis virus in chickens. J Gen Virol. 1991; 72: 801-807.
- Payne LN, Nair V. The long view: 40 years of avian leukosis research. Avian Pathol. 2012; 41: 11-19.
- Meyers P. Antibody response to related leukosis viruses induced in chickens tolerant to an avian leukosis virus. J Natl Cancer Inst. 1976; 56: 381-386.
- Rubin H, Fanshier L, Cornelius A, Hughes WF. Tolerance and immunity in chickens after congenital and contact infection with an avian leukosis virus. Virology. 1962; 17: 143-156.
- Rup BJ, Hoelzer JD, Bose HR. Helper viruses associated with avian acute leukemia viruses inhibit the cellular immune response. Virology. 1982; 116: 61-71.
- Cui Z, Sun S, Zhang Z, Meng S. Simultaneous endemic infections with subgroup J avian leukosis virus and reticuloendotheliosis virus in commercial and local breeds of chickens. Avian Pathol. 2009; 38: 443-448.
- Guo F, Xue C, Wu C, Zhao X, Qu T, He X, et al. Effects of polysaccharide on chicks co-infected with Bordetella avium and Avian leukosis virus. Carbohydr Polym. 2014; 109: 71-76.
- Cui Z, Du Y, Zhang Z, Silva RF. Comparison of Chinese field strains of avian leukosis subgroup J viruses with prototype strain HPRS-103 and United States strains. Avian Dis. 2003; 47: 1321-1330.
- Borisenko L. Avian endogenous retroviruses. Folia Biol (Praha). 2003; 49: 177-182.
- Crittenden LB. Exogenous and endogenous leukosis virus genes--a review. Avian Pathol. 1981; 10: 101-112.
- Crittenden LB, Astrin SM. Genes, viruses and avian leukosis. Bioscience. 1981b; 31: 305-310.
- Robinson HL. Inheritance and expression of chicken genes that are related to avian leukosis sarcoma virus genes. Curr Top Microbiol Immunol. 1978; 83: 1-36.
- Smith EJ. Endogenous avian leukemia viruses. In G. F. De Boer (ed.), Martinus Nijhoff, Boston. Avian Leukosis. 1987; 4: 101-120.
- Crittenden LB, McMahon S, Halpern MS, Fadly AM. Embryonic infection with the endogenous avian leukosis virus Rous-associated virus-0 alters responses to exogenous avian leukosis virus infection. J Virol. 1987; 61: 722-725.
- Smith EJ, Fadly AM, Crittenden LB. Interactions between endogenous virus loci ev6 and ev21. 1. Immune response to exogenous avian leukosis virus infection. Poult Sci. 1990; 69: 1244-1250.
- Smith EJ, Fadly AM, Levin I, Crittenden LB. The influence of ev6 on the immune response to avian leukosis virus infection in rapid-feathering progeny of slow- and rapid-feathering dams. Poult Sci. 1991; 70: 1673-1678.
- Enrietto PJ, Wyke JA. The pathogenesis of oncogenic avian retroviruses. Adv Cancer Res. 1983; 39: 269-314.
- Moscovici C, L Gazzolo. Virus-cell interactions of avian sarcoma and defective leukemia viruses. Avian Leukosis. Martinus Nijhoff, Boston. 1987; 4: 153-170.
- Coffin JM, Hughes SH, Harold E Varmus. Retroviruses. Cold Spring Harbor Laboratory Press: Cold Spring Harbor, New York.1997.
- Fung YK, Lewis WG, Crittenden LB, Kung HJ. Activation of the cellular oncogene c-erbB by LTR insertion: molecular basis for induction of erythroblastosis by avian leukosis virus. Cell. 1983; 33: 357-368.
- Kung HJ, and JL Liu. Retroviral Oncogenesis. Nathanson N, editor. In: Viral Pathogenesis. Lippincott-Raven Publishers, Philadelphia. 1997; 235-266.
- Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007; 25: 267-296.
- Brojatsch J, Naughton J, Adkins HB, Young JA. TVB receptors for cytopathic and noncytopathic subgroups of avian leukosis viruses are functional death receptors. J Virol. 2000; 74: 11490-11494.
- Weller SK, Temin HM. Cell killing by avian leukosis viruses. J Virol. 1981; 39: 713-721.
- Adkins HB, Blacklow SC, Young JA. Two functionally distinct forms of a retroviral receptor explain the nonreciprocal receptor interference among subgroups B, D, and E avian leukosis viruses. J Virol. 2001; 75: 3520-3526.
- Adkins HB, Brojatsch J, Young JA. Identification and characterization of a shared TNFR-related receptor for subgroup B, D, and E avian leukosis viruses reveal cysteine residues required specifically for subgroup E viral entry. J Virol. 2000; 74: 3572-3578.
- Klucking S, Young JA. Amino acid residues Tyr-67, Asn-72, and Asp-73 of the TVB receptor are important for subgroup E avian sarcoma and leukosis virus interaction. Virology. 2004; 318: 371-380.
- Elleder D, Stepanets V, Melder DC, Senigl F, Geryk J, Pajer P, et al. The receptor for the subgroup C avian sarcoma and leukosis viruses, Tvc, is related to mammalian butyrophilins, members of the immunoglobulin superfamily. J Virol. 2005; 79: 10408-10419.
- Prŗková D, Vernerová Z, PilcÖk T, Stepanets V, Indrová M, Geryk J, et al. Differences in pathogenicity among strains of the same or different avian leukosis virus subgroups. Avian Pathol. 2007; 36: 15-27.
- Collins MH, Henderson AJ. Transcriptional regulation and T cell exhaustion. Curr Opin HIV AIDS. 2014; 9: 459-463.
- Espert L, Codogno P, Biard-Piechaczyk M. What is the role of autophagy in HIV-1 infection? Autophagy. 2008; 4: 273-275.
- Espert L, Biard-Piechaczyk M. Autophagy in HIV-induced T cell death. Curr Top Microbiol Immunol. 2009; 335: 307-321.
- Varbanov M, Espert L, Biard-Piechaczyk M. Mechanisms of CD4 T-cell depletion triggered by HIV-1 viral proteins. AIDS Rev. 2006; 8: 221-236.
- Bates P, Young JA, Varmus HE. A receptor for subgroup A Rous sarcoma virus is related to the low density lipoprotein receptor. Cell. 1993; 74: 1043-1051.
- Young JA, Bates P, Varmus HE. Isolation of a chicken gene that confers susceptibility to infection by subgroup A avian leukosis and sarcoma viruses. J Virol. 1993; 67: 1811-1816.
- Fadly AM, Lee LF, Bacon LD. Immunocompetence of chickens during early and tumorigenic stages of Rous-associated virus-1 infection. Infect Immun. 1982; 37: 1156-1161.
- Granlund DJ, Loan RW. Effect of lymphoid leukosis virus infection on the cell-mediated immune capacity of the chicken. J Natl Cancer Inst. 1974; 52: 1373-1374.
- Crittenden LB, Fadly AM, Smith EJ. Effect of endogenous leukosis virus genes on response to infection with avian leukosis and reticuloendotheliosis viruses. Avian Dis. 1982; 26: 279-294.
- Crittenden LB, Smith EJ, Fadly AM. Influence of endogenous viral (ev) gene expression and strain of exogenous avian leukosis virus (ALV) on mortality and ALV infection and shedding in chickens. Avian Dis. 1984; 28: 1037-1056.
- Smith EJ, Fadly AM. Influence of congenital transmission of endogenous virus-21 on the immune response to avian leukosis virus infection and the incidence of tumors in chickens. Poult Sci. 1988; 67: 1674-1679.
- Kuhnlein U, Fairfull RW, Gowe R, Kulenkamp A, Mou L, Zadworny D. Synergism between the endogenous viral loci ev6 and ev9 in inducing immunological tolerance to avian leukosis virus. Br Poult Sci. 1993; 34: 93-104.
- Landman WJ, Post J, Boonstra-Blom AG, Buyse J, Elbers AR, Koch G. Effect of an in ovo infection with a Dutch avian leukosis virus subgroup J isolate on the growth and immunological performance of SPF broiler chickens. Avian Pathol. 2002; 31: 59-72.
- Stedman NL, Brown TP, Brooks RL, Bounous DI. Heterophil function and resistance to staphylococcal challenge in broiler chickens naturally infected with avian leukosis virus subgroup J. Vet Pathol. 2001; 38: 519-527.
- Chai N, Bates P. Na+/H+ exchanger type 1 is a receptor for pathogenic subgroup J avian leukosis virus. Proc Natl Acad Sci USA. 2006; 103: 5531-5536.
- Cohen PA, Ko JS, Storkus WJ, Spencer CD, Bradley JM, Gorman JE, et al. Myeloid-derived suppressor cells adhere to physiologic STAT3- vs STAT5-dependent hematopoietic programming, establishing diverse tumor-mediated mechanisms of immunologic escape. Immunol Invest. 2012; 41: 680-710.
- Luczy Å, ski W, Krawczuk-Rybak M, Stasiak-Barmuta A. [Myeloid-derived suppressor cells - the new mechanism of immunosuppression in cancer]. Postepy Hig Med Dosw (Online). 2008; 62: 18-22.
- Xiang X, Poliakov A, Liu C, Liu Y, Deng ZB, Wang J, et al. Induction of myeloid-derived suppressor cells by tumor exosomes. Int J Cancer. 2009; 124: 2621-2633.
- Wang Y, Wang G, Wang Z, Zhang H, Zhang L, Cheng Z. Chicken biliary exosomes enhance CD4(+)T proliferation and inhibit ALV-J replication in liver. Biochem Cell Biol. 2014; 92: 145-151.
- Kenway-Lynch CS, Das A, Lackner AA, Pahar B. Cytokine/Chemokine Responses in Activated CD4+ and CD8+ T Cells Isolated from Peripheral Blood, Bone Marrow, and Axillary Lymph Nodes during Acute Simian Immunodeficiency Virus Infection. J Virol. 2014; 88: 9442-9457.
- Nolen BM, Breen EC, Bream JH, Jenkins FJ, Kingsley LA, Rinaldo CR, et al. Circulating mediators of inflammation and immune activation in AIDS-related non-hodgkin lymphoma. PLoS One. 2014; 9: 99144.