
Review Article
J Immun Res. 2024; 10(1): 1049.
Nanotechnology-Derived Combination Therapies for The Treatment of Osteosarcoma
Bingkai Fan; Qun Yang; Jie Cai*
Department of Orthopedics, The First People’s Hospital of Xiaoshan District, Xiaoshan Affiliated Hospital of Wenzhou Medical University, China
*Corresponding author: Jie Cai Department of orthopaedics, The First People’s Hospital of Xiaoshan District, Xiaoshan Affiliated Hospital of Wenzhou Medical University, 311200, Zhejiang, China. Email: jiecai228@126.com
Received: Septermber 04, 2024 Accepted: September 24, 2024 Published: Octoner 01, 2024
Abstract
Chemotherapy for osteosarcoma is associated with significant adverse effects due to its systemic toxicity. Nanotechnology offers a promising strategy to mitigate these limitations. Nanotherapy, utilizing nanoparticle-based drug delivery systems, has emerged as a novel approach for the treatment of osteosarcoma. The surgical resection of osteosarcoma often results in severe bone defects that necessitate medical intervention. Recent studies have demonstrated that multifunctional nanoparticle delivery systems not only actively inhibit tumor growth but also promote new bone formation. The rapid advancements in nanoparticle-based tumor therapies have opened new avenues for enhancing the therapeutic outcomes in osteosarcoma-associated bone defects. This review examines the current state of immunotherapy using multifunctional nanoparticles for osteosarcoma treatment. With further optimization and screening, these multifunctional nanoparticles hold potential for successful clinical application in osteosarcoma therapy.
Keywords: Nanoparticles; Tumor immunity; Osteosarcoma; Multifunctional system
Introduction
Osteosarcoma (OS) is the most prevalent and aggressive type of primary bone malignancy, primarily affecting children under 19 years of age [1]. Originating from bone-forming cells, it is also known as osteogenic sarcoma and is predominantly observed in pediatric and adolescent populations [2]. In China alone, it is estimated that there are over 28,000 new cases of bone cancer annually, with approximately 20,700 associated deaths [3].The symptoms of osteosarcoma include joint pain, swelling, bone destruction, and fractures. Clinical diagnosis typically involves X-ray imaging to identify the tumor, supplemented by CT scans to assess the extent and location of tumor growth [4]. Standard treatment options for osteosarcoma include chemotherapy and radiotherapy. Current therapeutic approaches often involve preoperative neoadjuvant chemotherapy, complete surgical resection of the tumor, and high-dose chemotherapy regimens [5]. Reports indicate that the success rate of surgical tumor removal in cases of localized osteosarcoma is below 20%. However, when combined with chemotherapy, the success rate significantly increases to approximately 70% [6]. As a result, a combination of surgery and chemotherapy is commonly employed in osteosarcoma treatment.
The primary chemotherapeutic agents used include doxorubicin, cisplatin, methotrexate, ifosfamide, and epirubicin, among others [7]. Neoadjuvant chemotherapy is typically administered before surgery to reduce tumor size and facilitate surgical resection, while adjuvant chemotherapy is given postoperatively, often extending over a year [8]. For the past two decades, high-dose and multi-drug neoadjuvant chemotherapy has been the gold standard for treating osteosarcoma [9]. However, systemic chemotherapy is associated with significant cardiotoxicity and nephrotoxicity, particularly at high doses. Moreover, due to the heterogeneity and genetic complexity of osteosarcoma, chemotherapy regimens often yield suboptimal responses in the treatment of this malignancy. Unfortunately, the survival rate for osteosarcoma has remained stagnant over the past few decades [10]. This is particularly concerning given that the bone microenvironment serves as a conducive environment for the initiation, progression, and metastasis of osteosarcoma. The high multidrug resistance (MDR) and immunosuppressive nature of the osteosarcoma microenvironment have contributed to the stagnation in therapeutic advancements over the last 30 years [11]. Consequently, the conventional approach of combining chemotherapy with surgery has failed to significantly improve long-term survival outcomes for osteosarcoma patients, highlighting the urgent need for the development of novel, effective treatment strategies.
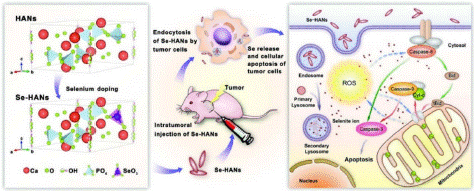
Figure 1: Preparation and working principle of anti-tumor nanoparticles: Se-ANN uses selenite instead of phosphate in ANN. Reprinted from with permission (copyright acquired from Wang et al. [17]).
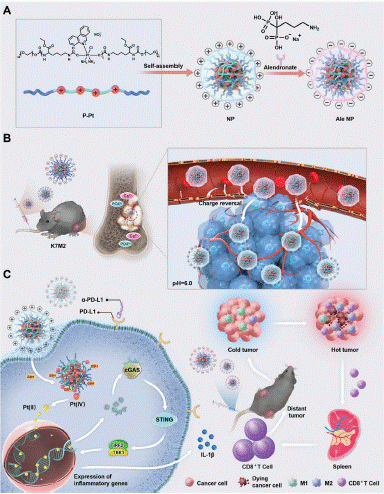
Figure 2: Meifang Shen, et al. Alendronate Triggered Dual-Cascade Targeting Prodrug Nanoparticles for Enhanced Tumor Penetration and STING Activation of Osteosarcoma (copyright acquired from Shen et al. [20]).
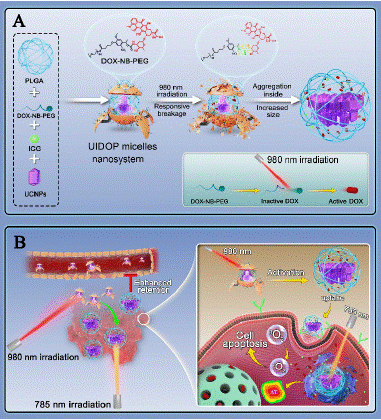
Figure 3: (A) Construction of light-controlled nanosystem and the process of light-controlled particle size change; (B) The process of light-controlled nanosystem for synergistic treatment of tumors (copyright acquired from Luo et al. [23]).
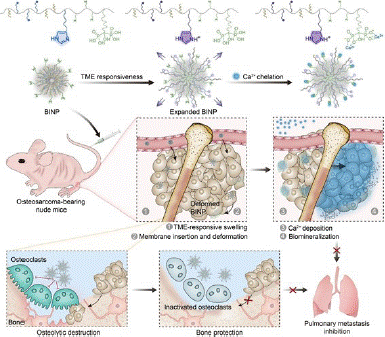
Figure 4: A Biomineralization-Inducing Nanoparticle (BINP) is used for closed treatment of osteosarcoma (copyright acquired from Chen et al. [29]).
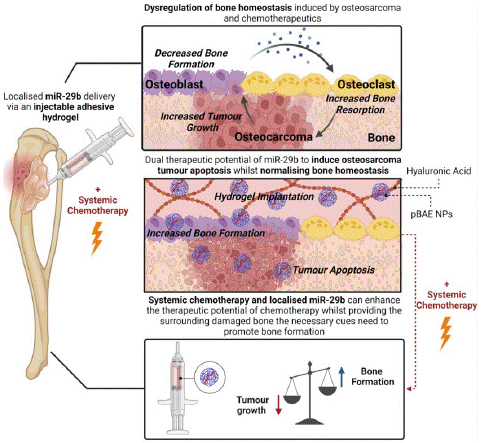
Figure 5: Local delivery of PBAE nanoparticles to the primary tumor site, as well as systemic delivery of chemotherapy, can inhibit tumor growth and provide surrounding damaged bone to normalize bone homeostasis (copyright acquired from Freeman et al. [36]).
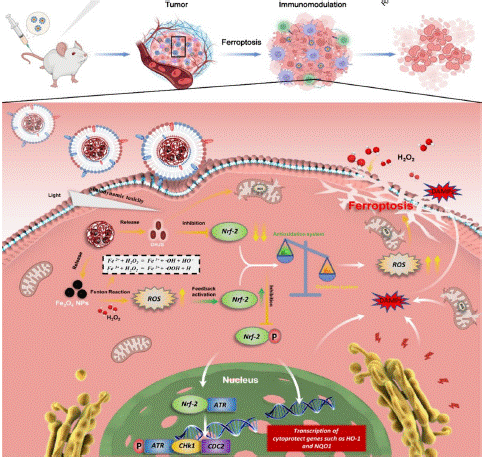
Figure 6: Schematic diagram of in vitro homologous targeting and mechanism of FDPM nanoparticles (copyright acquired from Yu et al. [40]).
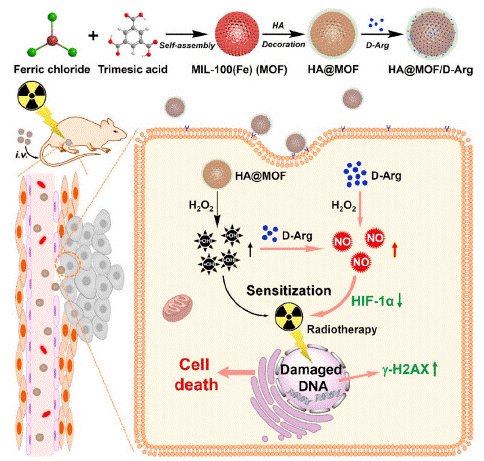
Figure 7: Schematic diagram of the synthesis pathway and function of HA@MOF/D-Arg for radiotherapy sensitization of osteosarcoma (copyright acquired from Owens et al. [44]).
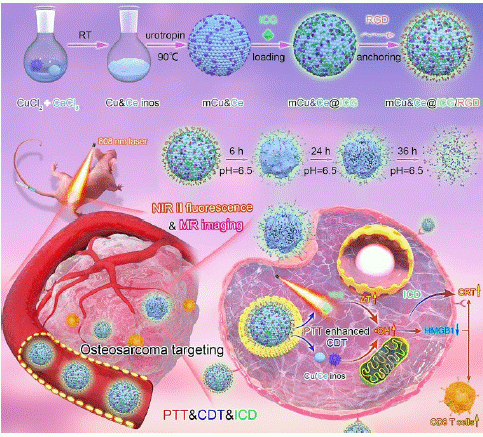
Figure 8: Schematic diagram of the construction process of osteosarcoma-targeted mCu&Ce@ICG/RGD for NIR-II fluorescence/MR bioimaging and PTT-CDT-ICD synergistic tumor inhibition (copyright acquired from Cheng et al. [47]).
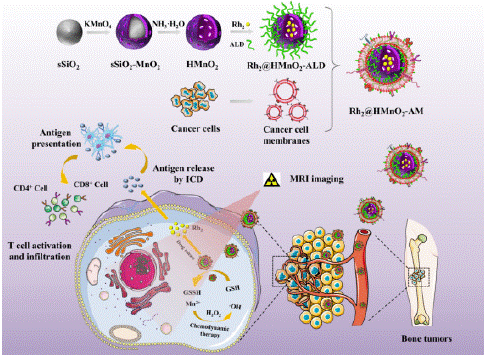
Figure 9: Synthetic procedure of Rh2@HMnO2-AM and mechanism of MRI-guided immuno-chemodynamic synergistic osteosarcoma therapy (copyright acquired from Fu et al. [50]).
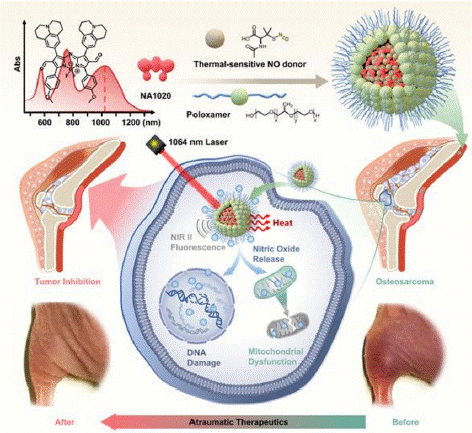
Figure 10: Synthetic procedure of Rh2@HMnO2-AM and mechanism of MRI-guided immuno-chemodynamic synergistic osteosarcoma therapy (copyright acquired from Fang et al. [55]).
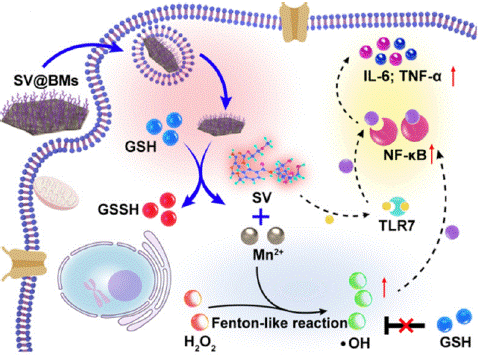
Figure 11: Synthetic procedure of Rh2@HMnO2-AM and mechanism of MRI-guided immuno-chemodynamic synergistic osteosarcoma therapy (copyright acquired from Lang et al. [60]).
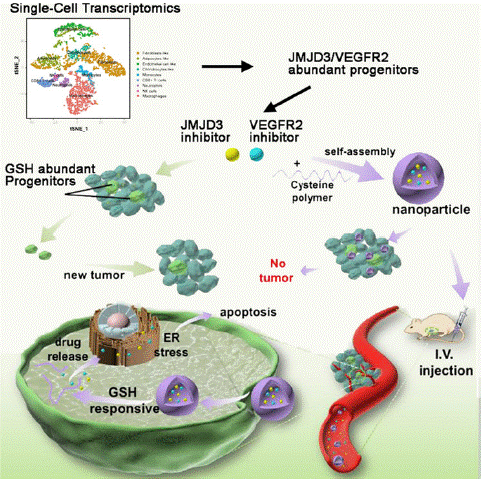
Figure 12: Nanoparticle enhanced combination therapy for stem-like progenitors defined by single-cell transcriptomics in chemotherapy-resistant osteosarcoma (copyright acquired from Wang et al. [64]).
Various nanoparticle-based platforms have been investigated for the diagnosis and treatment of osteosarcoma, offering the potential for improved therapeutic responses with minimal side effects [12]. These nanomaterials possess remarkable properties, including a high surface area-to-volume ratio, efficient drug encapsulation, controlled drug release, enhanced drug penetration, prolonged circulation time, and specific targeting of tumors, all while minimizing adverse effects [13]. Additionally, numerous studies have demonstrated that nanoparticles can enhance the efficacy of chemotherapeutic agents by acting as carriers for site-specific drug delivery [14]. As a result, nanoparticle-based tumor immunotherapy has gained recognition as a promising approach for the targeted delivery of drugs or genes in the treatment of osteosarcoma.
Characters and Properties of Multifunctional Nanoparticles for The Treatment of Osteosarcoma
Release of Therapeutic Inorganic Elements for Osteosarcoma
The use of biocompatible, tissue-engineered crystalline nanoparticles to release inorganic elements with anticancer properties represents a promising therapeutic strategy, offering new prospects for tissue repair and cancer treatment [15,16]. However, the precise mechanisms by which these therapeutic inorganic elements are released from the nanoparticle lattice to inhibit tumor growth remain unclear.
In this context, Professor Zhang's research group employed selenium-doped hydroxyapatite nanoparticles (Se-HANs), which not only have the potential to fill bone defects resulting from tumor resection but also to eradicate residual tumor cells [17]. Their study aimed to elucidate the mechanism by which selenium (Se) released from the Se-HAN lattice induces apoptosis in bone cancer cells in vitro and inhibits tumor growth in vivo. The findings revealed that Se-HANs promote tumor cell apoptosis by activating the intrinsic caspase-dependent apoptotic pathway in synergy with reactive oxygen species.
This mechanism was further confirmed through in vivo animal studies, where Se-HANs significantly induced tumor apoptosis, thereby inhibiting tumor growth while minimizing systemic toxicity. This work presents a viable paradigm for designing tissue-repairing inorganic nanoparticles that incorporate therapeutic ions within their lattices and release them in vivo to suppress tumor development.
2 Enhanced penetration of osteosarcoma drugs and STING activation
The complex physiological environment within bone tissue presents significant challenges to the effective delivery of chemotherapeutic drugs for osteosarcoma treatment, making the development of efficient drug delivery systems for osteosarcoma highly desirable [18,19].
Professor Yu has developed cationic platinum prodrug nanoparticles (Ale-NPs) based on Alendronate (Ale), designed to exhibit cascade reactivity within the Osteosarcoma tumor microenvironment [20]. Experimentally, leveraging the bone-targeting and charge-reversal effects triggered by Ale, Ale-NPs demonstrated a remarkable ability to deeply penetrate dense osteosarcoma tissues. Additionally, Ale-NPs facilitate the induction of Dendritic Cell (DC) maturation via the cyclic GMP-AMP synthase stimulator (cGAS-STING) pathway, thereby activating interferon genes. The highly efficient phenanthridine (Pt(II)) is released in response to the elevated glutathione (GSH) levels present in tumor cells, enabling dual-targeted cascade delivery of cationic platinum drugs in osteosarcoma. Ale-NPs not only effectively eradicated tumors in the deeper regions of osteosarcoma but also acted as potent STING agonists, effectively reversing the immunosuppressive microenvironment of osteosarcoma.
Overall, these Ale-triggered dual-cascade targeted prodrug nanoparticles significantly enhanced drug targeting and penetration in OS, thereby paving the way for improved clinical treatment of osteosarcoma.
Improve the Targeted Retention of Drugs in Osteosarcoma
Nanodrugs have demonstrated significant potential in the targeted treatment of tumors due to their strong targeting capabilities; however, the challenge of short retention times in osteosarcoma remains unresolved [21]. A critical issue that urgently needs to be addressed is how to functionalize nanocarriers in a way that allows for flexible control of nanoparticle size [22]. This would enable smaller particles to effectively target tumor sites, while also allowing for in situ size enlargement at the tumor site to prolong retention time. To tackle this challenge, Professor Cui's research team has developed a light-controlled, variable particle size nanosystem designed to enhance the targeted retention of drugs at osteosarcoma sites. This system involves the synthesis of UV-sensitive DOX-NB-PEG as a polymer carrier and chemotherapeutic agent, the use of Upconversion Nanoparticles (UCNPs) as photoinitiators, and Indocyanine Green (ICG) as both a phototherapeutic and imaging agent [23]. The UCNPs are surface-modified with PLGA, which is cross-linked with ICG and DOX to self-assemble into a nanosystem capable of controlling particle size in situ at the tumor site using near-infrared light. Upon activation by near-infrared light, the system can automatically remove the PEG from its surface.
Concurrently, its internal structure aggregates, forming larger nanoparticles that extend the retention time of the nanodrug at the tumor site, thereby achieving targeted and synergistic treatment of osteosarcoma.
Promoting Closed Treatment of Osteosarcoma
Biomineralization is a physiological process that encompasses nucleation, crystal growth, phase transition, and orientation evolution [24]. Notably, artificially induced biomineralization within osteosarcoma tumor tissue has emerged as an unconventional yet promising strategy for the treatment of malignant tumors [25]. However, the limited ion-chelating capacity of carboxyl-containing biomineralization initiators often results in insufficient mineralization, thereby reducing the anti-tumor efficacy in osteosarcoma [26,27].
To address this challenge, Professor Chen's research group has developed a biomineralization-inducing nanoparticle (BINP) for the localized treatment of osteosarcoma. BINP is constructed from dodecylamine-poly ((γ-dodecyl-L-glutamic acid)-co-(L-histidine))-block-poly(L-glutamic acid graft-alendronate) and integrates components for cell membrane insertion, Tumor Microenvironment (TME) responsiveness, and ion chelation [28]. Upon intravenous administration in osteosarcoma-bearing mice, BINPs respond to the acidic TME, exposing dodecyl groups on the extended nanoparticle surface, which promotes their insertion into cell membranes. Subsequently, the exposed bisphosphonate groups initiate continuous ion deposition, forming a mineralized barrier around the tumor that impedes material exchange between the tumor and the surrounding normal tissue. Compared to the control group, BINP-mediated blockade therapy demonstrated tumor inhibition rates of 59.3% and 52.1% in subcutaneous and orthotopic osteosarcoma models, respectively.
Additionally, the partial inhibition of osteoclasts by alendronate mitigated osteolysis and further suppressed lung metastasis. Consequently, BINP-induced selective biomineralization offers a promising therapeutic option for the clinical treatment of osteosarcoma.
Regulating Gene Expression in Osteosarcoma Cells
Surgical intervention for osteosarcoma typically involves either complete limb reconstruction or, in many cases, amputation. Both chemotherapy and osteosarcoma itself have been shown to disrupt bone homeostasis, leading to dysregulated osteolytic activity that significantly hinders the regenerative capacity of the surrounding bone following surgery [30,31]. MicroRNAs (miRNAs), which are small non-coding RNAs that regulate gene expression, offer therapeutic potential due to their ability to be delivered in vivo in a controlled manner [32]. They present numerous advantages, including the potential for targeted tumor suppression and bone remodeling [33]. However, miRNAs face challenges such as poor permeability in tumor tissues, rapid degradation when unmodified, and the activation of the innate immune system, which can result in unexpected toxicity and adverse side effects [34,35].
To address these issues, injectable Hyaluronic Acid (HA) hydrogels have been proposed as miRNA delivery systems [36]. These hydrogels can increase the bioavailability of miRNAs and reduce toxicity by limiting nonspecific uptake in healthy organs, focusing delivery at the primary tumor site. Additionally, Poly (β-amino Ester) (PBAE) nanoparticles have been developed as intracellular delivery vehicles for miRNA. The PBAE polymer chains interact electrostatically with RNA therapeutics during nanoparticle self-assembly, thereby enhancing encapsulation and loading efficiency.
Inducing Ferroptosis in Osteosarcoma Cells
Osteosarcoma is highly invasive, and ferroptosis—a novel form of programmed cell death characterized by iron accumulation and lipid peroxidation—has emerged as a promising cancer treatment strategy [37]. Inducing ferroptosis may help overcome drug resistance and immune escape mechanisms associated with tumor treatment [38,39]. However, achieving effective ferroptosis induction in osteosarcoma with current clinical doses of Fe₃O₄ nanoparticles presents challenges.
To solve this issues, Professor Li has provided new mechanistic insights and practical strategies for enhancing Fe₃O₄ nanoparticle-induced ferroptosis [40]. The study reveals that tumor cells can evade Fe₃O₄ nanoparticle-induced ferroptosis by upregulating Nrf-2 expression and activating the antioxidant defense system. To address this, a synergistic approach using DHJS in combination with Fe₃O₄ nanoparticles was employed to counteract Nrf-2 expression and induce ferroptosis in tumor cells.
The research involved constructing a biomimetic hybrid cell membrane composed of PLGA loaded with Fe₃O₄ and DHJS for osteosarcoma treatment. This formulation not only promoted ferroptosis in cancer cells but also induced macrophage M1 polarization and facilitated the infiltration of CD8+ T cells and dendritic cells into the tumors.
In summary, this study offers novel mechanistic insights and effective strategies for inducing ferroptosis with Fe₃O₄ nanoparticles. The developed biomimetic nanoparticles demonstrate synergistic ferroptosis and immunotherapeutic effects, presenting a promising approach for osteosarcoma treatment.
Improving Radiosensitivity of Osteosarcoma
Osteosarcoma is a prevalent bone cancer that frequently metastasizes to the lungs, making high-dose radiotherapy a valuable option for ablation of unresectable tumors [41,42]. However, this approach can result in severe side effects. To mitigate these issues, researchers have developed D-arginine-loaded Metal-Organic Framework (MOF) nanoparticles aimed at enhancing the radiosensitivity of osteosarcoma [43]. D-arginine, a metabolically inert enantiomer of L-arginine, functions as a Nitric Oxide (NO) donor and downregulates Hypoxia-inducible Factor-1α (HIF-1α) to alleviate tumor hypoxia [44]. Additionally, MOF nanoparticles generate free radicals that contribute to tumor cell destruction.
The study demonstrated that D-arginine-loaded nanoparticles significantly improved tumor ablation and inhibited lung metastasis in mouse models following radiotherapy [44]. The combination of nanoparticles and radiotherapy exhibited relatively low toxicity to both cells and mice when administered individually. Thus, MOF nanoparticles containing D-arginine prove to be a relatively safe and effective strategy for radio sensitizing osteosarcoma.
A multifunctional nanoplatform has been developed to further enhance radiosensitivity. In this system, D-arginine serves as a stable NO donor with good biocompatibility. The MOF component not only acts as a nanocarrier for targeted delivery of D-arginine but also increases the production of free radicals, which, in conjunction with NO, alleviates hypoxia and promotes tumor cell death. The nanoparticles reduced hypoxia by downregulating HIF-1α expression in both cellular and murine models. Consequently, HA@MOF/D-Arg combined with low-dose X-ray irradiation effectively inhibited tumor growth and prevented osteosarcoma recurrence and lung metastasis.
Comprehensive testing, including blood tests, hemolysis assays, DNA damage analysis, and H&E staining, revealed that HA@MOF/D-Arg or low-dose irradiation alone exhibited minimal toxicity to cells and mice. These findings suggest that D-arginine-loaded nanoparticles are a promising and safe tool for enhancing the effectiveness of radiotherapy in osteosarcoma treatment. Future research may explore the use of other Reactive Oxygen Species (ROS)-generating materials to improve D-arginine conversion to NO in vivo, and the potential application of NO generated by MOF/D-Arg for treating other conditions, such as cardiovascular diseases.
Enhanced Photothermal Therapy for Osteosarcoma
Osteosarcoma is characterized by its local destructiveness and high metastatic potential, creating an urgent need for nanoplatforms that offer both high therapeutic efficacy and precise diagnostic capabilities [45]. Multimodal optical imaging combined with programmed therapies, such as synergistic Photothermal-Chemodynamic Therapy (PTT-CDT) to induce Immunogenic cell Death (ICD), represents a promising strategy that ensures accurate bioimaging with high sensitivity [46]. This approach allows for significant treatment outcomes with minimal side effects.
Professor Yuan's research group has developed a straightforward one-step method to synthesize multifunctional Cu&Ce oxide nanospheres (mCu&Ce) with mesoporous structures [47]. The study reports that, following encapsulation of Indocyanine Green (ICG) and surface grafting with RGD peptide (mCu&Ce@ICG/RGD), this nanoplatform can precisely identify osteosarcoma and induce the robust release of ICG, Cu, and Ce ions within the tumor microenvironment (pH = 6.5).
Upon entering osteosarcoma cells, mCu&Ce@ICG/RGD effectively generates high temperatures under near-infrared laser irradiation, which in turn promotes the production of hydroxyl radicals (•OH). This results in synergistic PTT/CDT tumor ablation both in vitro and in vivo. Concurrently, the heat and amplified Reactive Oxygen Species (ROS) stimulate ICD, leading to the activation of T cells and the development of systemic anti-osteosarcoma immune responses, thereby enhancing the efficacy of tumor immunotherapy.
Additionally, the Cu&Ce-based nanoplatform facilitates accurate early diagnosis of osteosarcoma through dual-mode bioimaging using Near-Infrared II (NIR-II) fluorescence and magnetic resonance imaging. In summary, this study presents a facile Cu&Ce nanoplatform with dual-modal bioimaging capabilities. It effectively targets osteosarcoma, inhibits cancer cells through PTT-enhanced CDT, and significantly enhances ICD for improved immunotherapy outcomes.
Promoting Chemodynamic Therapy for Osteosarcoma
Promoting Dhemodynamic Therapy (CDT) for osteosarcoma necessitates the optimization of various factors to enhance its efficacy and clinical applicability. By implementing diverse strategies, researchers and clinicians can improve the effectiveness of CDT and advance patient outcomes [48].
Osteosarcoma, a challenging malignant bone disease, demands ongoing advancements in effective treatment strategies. Although nanotechnology has shown promise in cancer treatment, its progress is significantly impeded by issues related to targeting efficiency and effectiveness [49]. To address these challenges, Professor He Chuanglong’s research team developed a novel approach using Alendronate (ALD) and K7M2 cell membrane (M)-modified Hollow Manganese Dioxide (HMnO2) nanoparticles as carriers, loaded with ginsenoside Rh2 (Rh2) [50]. The resulting Rh2@HMnO2-AM nanoparticles exhibit enhanced bone tumor targeting, tumor homing capabilities, and Glutathione (GSH)-sensitive drug release. Additionally, these nanoparticles demonstrate effective Magnetic Resonance Imaging (MRI) capabilities and can trigger Immunogenic Cell Death (ICD).
Consequently, Rh2@HMnO2-AM represents a promising, biocompatible nanoparticle platform for the treatment of osteosarcoma, particularly in combination with immunotherapy and chemodynamic therapy.
Combined Gas Therapy for Osteosarcoma
Enhancing tumor cell apoptosis to significantly inhibit in situ osteosarcoma has garnered considerable attention from researchers. Endogenous Nitric Oxide (NO) exhibits concentration-dependent physiological effects, with high levels inducing cytotoxicity, thereby establishing its potential as an anticancer agent within gas therapy frameworks [51,52]. NO offers multiple metabolic pathways, making it superior to synthetic chemotherapeutic drugs in terms of membrane permeability, therapeutic safety, and resistance to drug treatments in cancer therapy [53]. The combination of NO treatment with photothermal cancer therapy has demonstrated promising feasibility [54]. However, current methods still present side effects during photothermal therapy, potentially increasing the likelihood of cancer recurrence.
To address these challenges, the introduction of low-temperature photothermal therapy is proposed, aiming to enable non-invasive treatments while achieving the desired therapeutic outcomes. This study introduces a gas/light therapeutic nanocomposite (NA1020-NO@PLX) that exhibits superior photothermal conversion capabilities, making it suitable for treating in situ osteosarcoma in deep tissues [55]. Additionally, its excellent NIR-II imaging capability allows for precise tumor localization, thereby enhancing the visibility and effectiveness of the photothermal treatment process. Furthermore, the study explores the non-invasive treatment approach that enhances the apoptosis mechanism, demonstrating the feasibility of NO/low-temperature photothermal synergistic therapy for osteosarcoma.
This gas/light therapy strategy optimizes existing photothermal therapy modalities, offering a reproducible and non-invasive treatment process for deep tissue tumors, thus validating its potential for clinical application.
Stimulates Macrophage Activation in The Tumor Microenvironment
Osteosarcoma is a highly malignant and metastatic bone cancer with a poor prognosis, and its comprehensive treatment methods have seen little advancement over the past 30 years. Traditional treatment approaches for osteosarcoma primarily involve surgery and chemotherapy [56]. While early-stage osteosarcoma patients may achieve long-term survival rates exceeding 60% with standard treatments, the prognosis for those with recurrent or metastatic osteosarcoma remains dismal, with a 5-year survival rate of only 20% [57].
Recent research has focused on the immune microenvironment, aiming to treat osteosarcoma by activating immune cells [58]. Notably, the metallic element manganese has demonstrated potent immune-activating effects on Natural Killer (NK) cells and dendritic cells. Since macrophages are the most abundant immune cells within the osteosarcoma tumor microenvironment and play a crucial role in tumor growth and metastasis, understanding manganese's impact on these cells is of particular interest [59].
To simultaneously activate multiple immune cell types, researchers have developed manganese dioxide nanoparticles loaded with the small molecule agonist 1V209, which targets the TLR7 receptor to activate macrophages [60]. In vitro experiments showed that macrophages treated with these nanoparticles exhibited strong pro-apoptotic effects on osteosarcoma cell lines and significantly inhibited tumor growth and metastasis in mouse models. Further investigations revealed that manganese-containing nanoparticles increased the proportion of pro-inflammatory macrophages within tumors and promoted T cell infiltration.
The proposed mechanism involves manganese nanoparticles reacting with intracellular glutathione to release manganese ions, which subsequently generate reactive oxygen species through a Fenton-like reaction. This process activates the NF-κB signaling pathway, enhancing the inflammatory response in macrophages. This study expands the potential of manganese-based immunotherapy and underscores the importance of targeting macrophages in osteosarcoma treatment, providing a foundation for the development of novel therapeutic strategies.
Reduce Osteosarcoma Stem Cell Resistance
Osteosarcoma cells frequently exhibit resistance to standard treatments, underscoring the urgent need to enhance therapeutic efficacy and identify novel targets [61]. A small subset of osteosarcoma cells, possessing stem cell-like self-renewal properties, are particularly resistant to therapy and demonstrate high metastatic potential. Therefore, a comprehensive understanding of the characteristics and molecular mechanisms underlying these chemotherapy-resistant cells is crucial [62,63].
This study employed single-cell transcriptome sequencing to construct a cell type-specific gene expression profile from chemotherapy-resistant osteosarcoma patients. The analysis revealed that VEGFR2 and JMJD3 double-positive cells represent a quiescent, stem cell-like population [64]. Based on these findings, a hierarchy of stem cell-like/progenitor cells (marked by high JMJD3 expression) with inherent treatment resistance was established in osteosarcoma. The study further demonstrated that the synergistic inhibition of VEGFR2 and JMJD3 effectively suppressed osteosarcoma cell proliferation and tumor growth. Although this combined therapy induces apoptosis in osteosarcoma cells by activating the pro-apoptotic factor CHOP, leading to Endoplasmic Reticulum (ER) stress, stem cell-like/progenitor cells exhibit an adaptive response that allows them to survive.
Single-cell transcriptome data also revealed that these stem-like/progenitor cells gain a survival advantage by upregulating Glutathione (GSH) synthesis, thereby resisting ER stress-mediated apoptosis. Importantly, the application of GSH-responsive nanoparticles, capable of effectively loading and releasing drugs, significantly enhanced the therapeutic efficacy of the synergistic treatment against these resistant cell populations.
In conclusion, this study confirmed the role of GSH in the resistance mechanisms of osteosarcoma stem cells. By encapsulating small molecule inhibitors targeting VEGFR2 and JMJD3 within GSH-responsive nanoparticles, the therapeutic impact of the combined treatment was substantially improved. This approach offers a promising framework for overcoming the resistance of stem-like/progenitor cells to conventional therapies, representing a significant advancement in osteosarcoma treatment.
Summary and Future Perspectives
The biological characteristics of osteosarcoma suggest that enhancing the human immune response may improve patient prognosis. However, the heterogeneity of osteosarcoma and the complexity of the immune system pose significant challenges to the success of immunotherapy for this malignancy. Osteosarcoma has historically resisted cure through single treatment regimens. In recent years, research into the tumor immune response has led to the development of various strategies to stimulate the immune system's response to osteosarcoma.
Nanotechnology offers promising new approaches for improving the diagnosis and treatment of osteosarcoma. Due to their ability to penetrate the tumor interstitial barrier and target tumor tissues through the conjugation with targeting agents such as antibodies, peptides, and small molecules, nanoparticles hold substantial potential in the management of osteosarcoma. However, there are notable challenges associated with nanoparticle formulations. While nanoparticles can enhance drug accumulation in osteosarcoma tissues compared to free drugs, the actual amount of drug that accumulates in the tumor remains relatively low compared to the total administered dose. Additionally, a significant proportion of nanoparticles tend to accumulate in organs such as the liver and kidneys, which may result in long-term toxicity, thus limiting the development and clinical application of nanoparticle-based therapies. Therefore, the establishment of a comprehensive evaluation system for nano-preparations is crucial for their advancement. A systematic assessment of the benefits and drawbacks of these nano-formulations will provide essential insights for the research and development of new nanoparticle-based therapies.
Future research should prioritize the development of novel treatment strategies that integrate nanotechnology. These innovative strategies currently under investigation include targeting specific signaling pathways, modulating the tumor microenvironment, immunotherapy, stem cell therapy, microbiome therapy, and metabolic therapy. Such approaches have the potential to significantly improve the prognosis of osteosarcoma patients. Research should focus on developing technologies that enable more precise and effective integrated strategies for the diagnosis and treatment of osteosarcoma. Leveraging the unique properties of nanoparticles, future studies should aim to combine new diagnostic modalities and treatment strategies within nano-platforms to achieve more potent anti-tumor effects. Furthermore, extensive preclinical studies are necessary to build a robust foundation for the clinical application of these novel multifunctional nanoparticles in osteosarcoma treatment.
Author Statements
Authors Contributions
Bingkai Fan and Jie Cai conceived the idea and designed the review, and Qun Yang, Bingkai Fan, and Jie Cai wrote the original draft. Bingkai Fan, Jie Cai, and Qun Yang helped to analyze the data and provided valuable advice. Bingkai Fan and Jie Cai co-wrote the manuscript. All the authors read and approved the final manuscript.
Disclosure Statement
The authors confirm that there is no conflict of interest.
References
- Beird HC, Bielack SS, Flanagan AM, Gill J, Heymann D, Janeway KA, et al. Osteosarcoma. Nat Rev Dis Primers. 2022; 8: 77.
- Liu S, Iorgulescu JB, Li S, Borji M, Barrera-Lopez IA, Shanmugam V, et al. Spatial maps of T cell receptors and transcriptomes reveal distinct immune niches and interactions in the adaptive immune response. Immunity. 2022; 55: 1940-1952.e5.
- Azharuddin M, Zhu GH, Sengupta A, Hinkula J, Slater NKH, Patra HK. Nano toolbox in immune modulation and nanovaccines. Trends Biotechnol. 2022; 40: 1195-1212.
- Li X, Guo X, Huang J, Lin Q, Qin B, Jiang M, et al. Recruiting T cells and sensitizing tumors to NKG2D immune surveillance for robust antitumor immune response. J Control Release. 2023; 353: 943-955.
- Maiorino L, Daßler-Plenker J, Sun L, Egeblad M. Innate Immunity and Cancer Pathophysiology. Annu Rev Pathol. 2022; 17: 425-457.
- González Díaz EC, Lee AG, Sayles LC, Feria C, Sweet-Cordero EA, Yang F. A 3D Osteosarcoma Model with Bone-Mimicking Cues Reveals a Critical Role of Bone Mineral and Informs Drug Discovery. Adv Healthc Mater. 2022; 11: e2200768.
- Han YH, Liu CG, Chen BQ, Fu CP, Kankala RK, Wang SB, et al. Orchestrated tumor apoptosis (Cu2+) and bone tissue calcification (Ca2+) by hierarchical Copper/Calcium-ensembled bioactive silica for osteosarcoma therapy. Chemical Engineering Journal. 2022; 435: 134820.
- Archilla-Ortega A, Domuro C, Martin-Liberal J, Muñoz P. Blockade of novel immune checkpoints and new therapeutic combinations to boost antitumor immunity. J Exp Clin Cancer Res. 2022; 41: 62.
- Wang Y, Zhang L, Zhao G, Zhang Y, Zhan F, Chen Z, et al. Homologous targeting nanoparticles for enhanced PDT against osteosarcoma HOS cells and the related molecular mechanisms. J Nanobiotechnology. 2022; 20: 83.
- Wang Y, Zhang L, Zhao G, Zhang Y, Zhan F, Chen Z, et al. Correction to: Homologous targeting nanoparticles for enhanced PDT against osteosarcoma HOS cells and the related molecular mechanisms. J Nanobiotechnology. 2022; 20: 286.
- Zhang D, Cheng S, Tan J, Xie J, Zhang Y, Chen S, et al. Black Mn-containing layered double hydroxide coated magnesium alloy for osteosarcoma therapy, bacteria killing, and bone regeneration. Bioact Mater. 2022; 17: 394-405.
- He G, Nie JJ, Liu X, Ding Z, Luo P, Liu Y, et al. Zinc oxide nanoparticles inhibit osteosarcoma metastasis by downregulating β-catenin via HIF-1α/BNIP3/LC3B-mediated mitophagy pathway. Bioact Mater. 2022; 19: 690-702.
- Yang C, Liu Y, Hu Y, Fang L, Huang Z, Cui H, et al. Myc inhibition tips the immune balance to promote antitumor immunity. Cell Mol Immunol. 2022; 19: 1030-1041.
- Wang Y, Wang N, Yang Y, Chen Y, Zhang Z. Cellular nanomechanics derived from pattern-dependent focal adhesion and cytoskeleton to balance gene transfection of malignant osteosarcoma. J Nanobiotechnology. 2022; 20: 499.
- Wang D, Wang W, Lu H, You C, Liang L, Liu C, et al. Charge transfer of ZnTPP/C60 cocrystal-hybridized bioimplants satisfies osteosarcoma eradication with antitumoral, antibacterial and osteogenic performances. Nano Today. 2022; 46: 101562.
- Fu Y, He G, Liu Z, Wang J, Li M, Zhang Z, et al. DNA Base Pairing-Inspired Supramolecular Nanodrug Camouflaged by Cancer-Cell Membrane for Osteosarcoma Treatment. Small. 2022; 18: e2202337.
- Chen K, Zhou R, Liang H, Liao Y, Zhu S, Dong X, et al. Reversing the pathological microenvironment by radiocatalytic sensitizer for local orthotopic osteosarcoma radiotherapy enhancement. Nano Today. 2023; 48: 101739.
- Wang Y, Wang J, Hao H, Cai M, Wang S, Ma J, et al. In Vitro and in Vivo Mechanism of Bone Tumor Inhibition by Selenium-Doped Bone Mineral Nanoparticles. ACS Nano. 2016; 10: 9927-9937.
- Fu L, Zhang W, Zhou X, Fu J, He C. Tumor cell membrane-camouflaged responsive nanoparticles enable MRI-guided immuno-chemodynamic therapy of orthotopic osteosarcoma. Bioact Mater. 2022; 17: 221-233.
- Li C, Zhang W, Wang R, Du XF, Jiang D, Liu B, et al. Nanocomposite multifunctional hydrogel for suppressing osteosarcoma recurrence and enhancing bone regeneration. Chemical Engineering Journal, 2022, 435: 134896.
- Shen M, Wang Y, Bing T, Tang Y, Liu X, Yu Y. Alendronate triggered dual‐cascade targeting prodrug nanoparticles for enhanced tumor penetration and sting activation of osteosarcoma. Adv Funct Mater. 2023; 33: 2307013.
- Zhou M, Zuo Q, Huang Y, Li L. Immunogenic hydrogel toolkit disturbing residual tumor “seeds” and pre-metastatic “soil” for inhibition of postoperative tumor recurrence and metastasis. Acta Pharm Sin B. 2022; 12: 3383-3397.
- Kong Y, Zhou L, Liao S, Wang C, Chen J, Cai X, et al. Dual peptide-engineered and gadolinium-doped polydopamine particles as targeted nanotheranostics for the treatment of osteosarcoma and related osteolysis. Chemical Engineering Journal. 2022, 444: 136516.
- Luo H, Kong L, Zhang F, Huang C, Chen J, Zhang H, et al. Light‐ controlled nanosystem with size‐flexibility improves targeted retention for tumor suppression. Advanced Functional Materials. 2021; 31: 2101262.
- Huang X, Wu W, Jing D, Yang L, Guo H, Wang L, et al. Engineered exosome as targeted lncRNA MEG3 delivery vehicles for osteosarcoma therapy. J Control Release. 2022; 343: 107-117.
- Fan Q, Zuo J, Tian H, Huang C, Nice EC, Shi Z, et al. Nanoengineering a metal-organic framework for osteosarcoma chemo-immunotherapy by modulating indoleamine-2,3-dioxygenase and myeloid-derived suppressor cells. J Exp Clin Cancer Res. 2022; 41: 162.
- Phuengkham H, Song C, Um SH, Lim YT. Implantable Synthetic Immune Niche for Spatiotemporal Modulation of Tumor-Derived Immunosuppression and Systemic Antitumor Immunity: Postoperative Immunotherapy. Adv Mater. 2018; 30: e1706719.
- Zhang S, Feng Y, Meng M, Li Z, Li H, Lin L, et al. A generally minimalist strategy of constructing biomineralized high-efficiency personalized nanovaccine combined with immune checkpoint blockade for cancer immunotherapy. Biomaterials. 2022; 289: 121794.
- Ge YX, Zhang TW, Zhou L, Ding W, Liang HF, Hu ZC, et al. Enhancement of anti-PD-1/PD-L1 immunotherapy for osteosarcoma using an intelligent autophagy-controlling metal organic framework. Biomaterials. 2022; 282: 121407.
- Chen, Tiantian, et al. “Biomimetic mineralization: Construction and biomedical applications of biohybrid materials.” Materials Chemistry Frontiers. 2024.
- Jin J, Yuan P, Yu W, Lin J, Xu A, Xu X, et al. Mitochondria-Targeting Polymer Micelle of Dichloroacetate Induced Pyroptosis to Enhance Osteosarcoma Immunotherapy. ACS Nano. 2022; 16: 10327-10340.
- Li M, Lin ZI, Yang J, Huang H, Liu GL, Liu Q, et al. Biodegradable Carbon Dioxide-Derived Non-Viral Gene Vectors for Osteosarcoma Gene Therapy. Adv Healthc Mater. 2023; 12: e2201306.
- Chen G, Xiong W, Gu Z, Gao Y, Hou J, Long L, et al. Mannosylated engineered trichosanthin-legumain protein vaccine hydrogel for breast cancer immunotherapy. Int J Biol Macromol. 2022; 223: 1485-1494.
- Wang T, Wang D, Yu H, Feng B, Zhou F, Zhang H, et al. A cancer vaccine-mediated postoperative immunotherapy for recurrent and metastatic tumors. Nat Commun. 2018; 9: 1532.
- Blass E, Ott PA. Advances in the development of personalized neoantigen-based therapeutic cancer vaccines. Nat Rev Clin Oncol. 2021; 18: 215-229.
- Jiang J, Wang R, Yang L, Sha Y, Zhao S, Guo J, et al. IL-11Rα-targeted nanostrategy empowers chemotherapy of relapsed and patient-derived osteosarcoma. J Control Release. 2022; 350: 460-470.
- Freeman FE, Dosta P, Shanley LC, Ramirez Tamez N, Riojas Javelly CJ, Mahon OR, et al. Localized Nanoparticle-Mediated Delivery of miR-29b Normalizes the Dysregulation of Bone Homeostasis Caused by Osteosarcoma whilst Simultaneously Inhibiting Tumor Growth. Adv Mater. 2023; 35: e2207877.
- Liu S, Zhang Q, He H, Yi M, Tan W, Guo J, et al. Intranuclear Nanoribbons for Selective Killing of Osteosarcoma Cells. Angew Chem Int Ed Engl. 2022; 61: e202210568.
- Zhang R, Ye Y, Wu J, Gao J, Huang W, Qin H, et al. Immunostimulant In Situ Fibrin Gel for Post-operative Glioblastoma Treatment by Macrophage Reprogramming and Photo-Chemo-Immunotherapy. ACS Appl Mater Interfaces. 2023; 15: 17627-17640.
- Gao M, Chen Y, Wu C. Size-dependent chemosensitization of doxorubicin-loaded polymeric nanoparticles for malignant glioma chemotherapy. Bioengineered. 2021; 12: 12263-12273.
- Yu K, Chen Y, Zhang L, Zheng Y, Chen J, Wang Z, et al. Cancer-Erythrocyte Membrane-Mimicking Fe3O4 Nanoparticles and DHJS for Ferroptosis/Immunotherapy Synergism in Tumors. ACS Appl Mater Interfaces. 2023; 15: 44689-44710.
- Liu Y, Jiang Z, Tong S, Sun Y, Zhang Y, Zhang J, et al. Acidity-Triggered Transformable Polypeptide Self-Assembly to Initiate Tumor-Specific Biomineralization. Adv Mater. 2023; 35: e2203291.
- Qin X, Yang C, Xu H, Zhang R, Zhang D, Tu J, et al. Cell-Derived Biogenetic Gold Nanoparticles for Sensitizing Radiotherapy and Boosting Immune Response against Cancer. Small. 2021; 17: e2103984.
- Tian H, Cao J, Li B, Nice EC, Mao H, Zhang Y, et al. Managing the immune microenvironment of osteosarcoma: the outlook for osteosarcoma treatment. Bone Res. 2023; 11: 11.
- Du C, Zhou M, Jia F, Ruan L, Lu H, Zhang J, et al. D-arginine-loaded metal-organic frameworks nanoparticles sensitize osteosarcoma to radiotherapy. Biomaterials. 2021; 269: 120642.
- McMahon K, Eaton V, Cichon G, et al. Use of non-curative oncological care in osteosarcoma. 2022; 40: 78-78.
- Van Leent MMT, Priem B, Schrijver DP, de Drue A, Hofstraat SRJ, Zwolsman R, et al. Regulating trained immunity with nanomedicine. Nat Rev Mater. 2022; 7: 465–481.
- Cheng M, Kong Q, Tian Q, Cai W, Wang C, Yuan M, et al. Osteosarcoma-targeted Cu and Ce based oxide nanoplatform for NIR II fluorescence/magnetic resonance dual-mode imaging and ros cascade amplification along with immunotherapy. J Nanobiotechnology. 2024; 22: 151.
- Marchais A, Marques da Costa ME, Job B, Abbas R, Drubay D, Piperno-Neumann S, et al. Immune Infiltrate and Tumor Microenvironment Transcriptional Programs Stratify Pediatric Osteosarcoma into Prognostic Groups at Diagnosis. Cancer Res. 2022; 82: 974-985.
- Wu C, He W, Chen Y, Cai J, Zeng F, Lu Z, et al. Personalized Bacteria Loaded with Autoantigens for the Enhancement of Tumor Immunotherapy. Adv Healthc Mater. 2023; 12: e2203026.
- Fu L, Zhang W, Zhou X, Fu J, He C. Tumor cell membrane-camouflaged responsive nanoparticles enable MRI-guided immuno-chemodynamic therapy of orthotopic osteosarcoma. Bioact Mater. 2022; 17: 221-233.
- Capistrano IR, Paul S, Boere I, Pantziarka P, Chopra S, Nout RA, et al. Drug repurposing as a potential source of innovative therapies in cervical cancer. Int J Gynecol Cancer. 2022; 32: 1377–86.
- Sun C, Li S, Ding J. Biomaterials-Boosted Immunotherapy for Osteosarcoma. Adv Healthc Mater. 2024: e2400864.
- Liu Y, Qiao Z, Gao J, Wu F, Sun B, Lian M, et al. Hydroxyapatite-Bovine Serum Albumin-Paclitaxel Nanoparticles for Locoregional Treatment of Osteosarcoma. Adv Healthc Mater. 2021; 10: e2000573.
- Ritter J, Bielack SS. Osteosarcoma. Ann Oncol. 2010; 21: vii320-5.
- Fang Z, Zhang J, Shi Z, Wang L, Liu Y, Wang J, et al. A Gas/phototheranostic Nanocomposite Integrates NIR-II-Peak Absorbing Aza-BODIPY with Thermal-Sensitive Nitric Oxide Donor for Atraumatic Osteosarcoma Therapy. Adv Mater. 2023; 35: e2301901.
- Huo W, Yang X, Wang B, Cao L, Fang Z, Li Z, et al. Biomineralized hydrogel DC vaccine for cancer immunotherapy: A boosting strategy via improving immunogenicity and reversing immune-inhibitory microenvironment. Biomaterials. 2022; 288: 121722.
- Feng C, Jiang Y, Wang T, Tian D, Shen C, Wang Y, et al. Recent advances on nanostructured biomaterials in osteosarcoma treatment. Coordination Chemistry Reviews. 2023; 493: 215315.
- Zhou Y, Li G, Li H, Lai F, Duan P, Cheng M. Epithelial to Mesenchymal Transition Relevant Subtypes with Distinct Prognosis and Responses to Chemo- or Immunotherapies in Osteosarcoma. J Immunol Res. 2022; 2022: 1377565.
- Meng X, Zhang H, Zhang M, Wang B, Liu Y, Wang Y, et al. Negative CT Contrast Agents for the Diagnosis of Malignant Osteosarcoma. Adv Sci (Weinh). 2019; 6: 1901214.
- Liang C, Xiong N, Liu M, Chen Y, Li W, Xu J, et al. Manganese immunotherapy for treating osteosarcoma: Glycosylating 1V209 anchored MnO2 nanosheets prompt pro-inflammatory macrophage polarization. Nano Today, 2023, 48: 101670.
- Huang X, Wang L, Guo H, Zhang W, Shao Z. Single-cell transcriptomics reveals the regulative roles of cancer associated fibroblasts in tumor immune microenvironment of recurrent osteosarcoma. Theranostics. 2022; 12: 5877-5887.
- Gill J, Gorlick R. Advancing therapy for osteosarcoma. Nat Rev Clin Oncol. 2021; 18:609-624.
- Boufenghour W, Klymchenko AS, Foppolo S, Nazon C, Weingertner N, Martin S, et al. Engineering Novel 3D Models to Recreate High-Grade Osteosarcoma and its Immune and Extracellular Matrix Microenvironment. Adv Healthc Mater. 2022; 11: e2200195.
- Wang L, Huang X, You X, Yi T, Lu B, Liu J, et al. Nanoparticle enhanced combination therapy for stem-like progenitors defined by single-cell transcriptomics in chemotherapy-resistant osteosarcoma. Signal Transduct Target Ther. 2020; 5: 196.