
Perspective
J Immun Res. 2015;2(1): 1011.
Obesity: An Immunological Perspective
Vasso Apostolopoulos*, Maximilian de Courten, Lily Stojanovska
Centre for Chronic Disease, College of Health and Biomedicine, Victoria University, Australia
*Corresponding author: Vasso Apostolopoulos, Centre for Chronic Disease, College of Health and Biomedicine, Victoria University, Australia,
Received: November 17, 2014; Accepted: November 28, 2014; Published: December 01, 2014
Abstract
Current approaches in the treatment of obesity include medication, surgery, physical activity, diet modifications and overall lifestyle changes. However the obesity epidemic still prevails. An understanding of the immunological processes that regulate obesity may contribute to better treatment modalities for obesity and obesity-related disorders.
Keywords: Obesity; Immunological processes; Regulatory T Cells; Natural killer cells
Abbreviations
IFN: InterFeroN; iNKT: inducible Natural Killer T; IL: InterLeukin; MCP-1: Monocyte Chemoattractant Protein; NKT: Natural Killer T; ObR: Leptin Cell Surface Receptor; TGF: Tumor Growth Factor; TNF: Tumor Necrosis Factor; Treg: Regulatory T Cells
Introduction
Obesity is a medical condition whereby excess body fat may have a negative effect on health. Indeed, the ancient Greeks recognized obesity as a medical disorder and Sushruta, the ancient Indian surgeon described a link between obesity to heart disease and diabetes [1]. During the Middle Ages however, obesity was a sign of prosperity and wealth. It wasn´t until the mid-1900s that obesity was scientifically associated with various health conditions. Today, obesity is the 6th most important risk factor contributing to overall burden of disease and is the leading preventable cause of death. Worldwide, 1.2 billion adults (1/7) and 10% of children are overweight or obese and in the USA alone it accounts to over $190 billion of all medical expenditure. Obesity is defined by body mass index and fat distribution as measured by waist-hip ratio [2]. Obesity increases the likelihood of metabolic syndrome (heart disease, type 2 diabetes, insulin resistance, hyperlipidemia), non-alcoholic fatty liver, osteoarthritis, cancer, gallbladder disease, autoimmunity, infertility, and obstructive sleep apnoea, thus, contributing to decreased life expectancy [3]. In fact, obesity contributes to approximately 70% of cases of diabetes [4]. Obesity involves a complex pathological process which is reflected by environmental and genetic interactions, although a few cases are caused primarily by medical disorders (ie, Prader-Willi syndrome, Cushings syndrome), medications or psychiatric illness. In addition, lifestyle factors such as, inactivity, poor eating habits in particular, overconsumption of carbohydrates and lack of sleep are contributing factors and appear to be the major factors leading to the current obesity epidemic.
The Immunological Network in Obesity
Chronic inflammation and obesity
Obesity creates an inflammatory state and contributes to the development of chronic conditions, including cancer, autoimmunity and atherosclerosis. High levels of tumor necrosis factor (TNF) alpha, monocyte chemo attractant protein (MCP-1), interleukin (IL)- 1 and IL-6 present in adipose tissues or in the circulation correlates to insulin resistance and metabolic syndrome [5]. In addition, high levels of macrophages present in adipose tissues secrete significant amounts of pro-inflammatory cytokines, TNF alpha and IL-6 which contribute towards insulin resistance. Macrophages also secrete iNOS [6]. iNOS, inducible nitric oxide synthase, is involved in immune responses responsible for cardiovascular disease. Furthermore, T cells, chemokines (such as RANTES) and interferon (IFN)-gamma are abundantly found in adipose cells, suggesting a role of T cells in obesity (Figure 1). The role of macrophages and T cells in adipose tissues remains to be determined, however, depletion of such cells may give us greater insights in the understanding and management of obesity-associated co-morbidities. Indeed, the long term depletion of T cells in mice has been shown to reverse insulin resistance in obesity [7], however, whether adipose T cell depletion has similar effects in humans is not clear. Further, T helper (Th)-17 cells are associated with autoimmune diseases as demonstrated in models for multiple sclerosis and colitis. In diet induced obese mice, high levels of IL- 17 are secreted by Th17 cells [8], suggesting an association between obesity with inflammatory cells and autoimmune diseases.
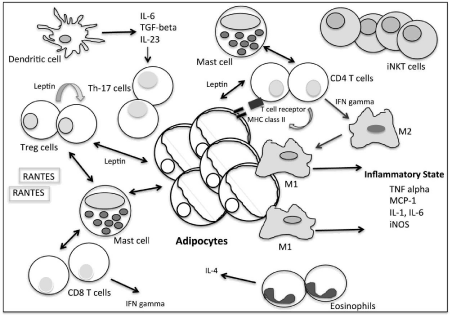
Figure 1: Adipose tissue in obesity. A complex immunological network.
Regulatory T cells and Leptin connection
Regulatory T cells (Treg) are a subpopulation of T cells which suppress the activation and proliferation of effector T cells, keeping tolerance to self-antigens, abrogating autoimmune cells and modulating immune responses. Leptin, a hormone secreted by fat cells reflects the amount of fat stored in the body. In addition, Treg cells also secrete leptin which acts as a negative signal for the proliferation of Treg cells [9]. In obese patients, high leptin, high C-reactive protein levels or high HbA1c levels in the circulation correlates with reduced circulatory Treg cells [10]. Reduced Treg cells have implications in the development of a number of chronic diseases, including cardiovascular diseases and metabolic complications.
Macrophages and dendritic cells in adipose tissues
Adipose tissues consist of Treg cells and eosinophils which balance local inflammation and influx of M1 macrophages, T cells and B cells. Adipose tissues contain resident macrophages and in obesity they are greatly enhanced where they secrete cytokines leading to inflammation and insulin resistance. It is believed that adipocyte death leads to the recruitment of pro-inflammatory M1 macrophages, however, M2 macrophages surround adipocytes and accumulate in the absence of adipocyte death (Figure 1). In recent studies, it was surprising to note that MHC class II was markedly increased in adipocytes of obese subjects [11]. Similarly, in mouse adipocytes, MHC class II was enhanced within 2 weeks of a high fat diet, and were able to stimulate CD4+ T cells in vitro [11]. Interestingly, MHC class II deficiency decreased adipose inflammation and insulin resistance in high fat diet mice compared to wild-type mice, despite having similar adiposity. Hence, the expression of MHC class II on adipocytes plays a role in adaptive immunity in obesity and its down-regulation may aid in therapeutic approaches against obesity.
Dendritic cells are present in adipose tissue in obese mice in an immature phenotype with lower levels of expression of CD40, CD80, CD86, MHC class I and II. However, high levels of IL-6, tumor growth factor (TGF)-beta and IL-23 cytokines are secreted and dendritic cells stimulate the generation of Th17 cells [12]. Hence dendritic cells contribute to the pro-inflammatory state in obesity.
What do eosinophils and mast cells do in adipose tissue?
Eosinophils are granulocytic leukocytes found in peripheral blood and tissues and are usually associated with inflammatory states, helminth immunity and allergies. They are also present in adipose tissues, where they primarily secrete IL-4 and in their absence macrophages are significantly enhanced (Figure 1). In the absence of eosinophils, high fat diet mice, increase body fat composition and body weight develop impaired glucose tolerance and insulin resistance [13]. Hence, eosinophils play a role in regulating macrophage activation in adipose tissue and aid in keeping the inflammatory balance.
Mast cells are known for their role in anaphylaxis and allergic responses. However, mast cells also play a significant role in tumor tissues, atherosclerotic lesions and are abundantly present in adipose tissues [14]. Mast cells, mature and differentiate in adipose tissues and interact with adipocytes to recruit inflammatory cells [15]. In obese mice and humans, the plasma mast cell protease levels and mast cells in visceral fat are significantly higher compared to lean counterparts [16]. In addition, mast cells interact with CD8+T cells in adipose tissues and secrete TNF-alpha, IL-8 and oncostatin M [17]. Furthermore, mast cells in adipose tissues interact with CD4+T cells, Treg cells and dendritic cells [18]. Detailed studies are required to understand the role of mast cells in adipose tissues in obesity, however, it appears that in obesity they are highly activated, and their de-activation could lead to improvements of weight in obese individuals. In fact, obese mice lose weight as a result of mast cell inactivation [18].
Are there immune cells that protect against obesity?
Natural killer T (NKT) cells share properties of natural killer cells and T cells. NKT cells bind to CD1d molecules, expressed on antigen presenting cells that presents lipids and glycolipids in complex with CD1d. Invariant NKT (iNKT) cells, a subset of NKT cells are innate T cells that influence inflammatory responses. iNKT cells were believed to be rare in humans, however, emerging evidence suggests they are present in adipose tissues and are up-regulated in obesity (Figure 1). Depletion of iNKT cells results in infiltration of pro-inflammatory macrophages and iNKT cells were restored following weight loss. Of relevance, mice lacking iNKT cells have enhanced weight gain, larger adipocytes and fatty livers and are insulin resistant. In adoptive transfer experiments, iNKT cells were associated with decreased body fat, triglycerides, leptin levels, fatty liver and improved insulin sensitivity [19]. The mechanism is believed to be via anti-inflammatory cytokine production by iNKT cells. Hence, iNKT cells could be used in the management of obesity.
Obesity and immune dysfunction: increased risks of disease outcomes
There is a link between obesity, metabolic diseases and immune dysfunction. Macrophages, lymphocytes and mast cells play a key role in initiating an inflammatory milieu in obese patients which leads to insulin resistance. Thus, it is not the mass of adipose tissue that leads to insulin resistance but the inflammatory state within the adipose tissue and systemically that leads to insulin resistance. Dysfunctional adipose tissue also leads to other metabolic disorders such as dyslipidemia and non-alcoholic steatohepatitis. Hence, adipose tissue is not merely a site for fat storage but a complex endocrine organ.
In obese humans, the total number of lymphocytes and NK cells are decreased and mitogen stimulation of lymphocytes is reduced [20]. Similar findings have been noted in mice [21]. As a result, obesity inhibits the ability of the immune system to appropriately respond to influenza virus infection in mice, suggesting increased mortality from viral infections [22]. In addition, obesity is associated with increased risks of urinary tract infections, pneumonia, periodontitis, pancreatitis, skin infections, cellulitis and influenza infections [reviewed in 23]. Furthermore, obese individuals respond weaker to vaccinations, such as hepatitis B vaccine compared to lean individuals. In a randomized trial of the triple antigen vaccine resulted in lower protection rates in obese vs lean subjects [23].
It is clear that obesity causes immune dysfunction with a chronic low grade inflammatory response. As a consequence, a number of diseases result, there is an inability to clear infections adequately and there are inadequate responses to vaccination. It is known that exercise and diet modifications have beneficial effects on the immune function. In fact, diet and moderate daily exercise has been shown to restore immune function in obesity [24].
Conclusion
With the obesity epidemic expanding at an alarming rate and the need to develop effective treatments, it is imperative to take a few steps back to understand the molecular basis and functional role of immune cells in obese individuals. Immune cells hold the key to developing improved treatments against obesity.
References
- Haslam D . Obesity: a medical history. Obes Rev. 2007; 8 Suppl 1: 31-36.
- Sweeting HN . Measurement and definitions of obesity in childhood and adolescence: a field guide for the uninitiated. Nutr J. 2007; 6: 32.
- Haslam DW, James WP . Obesity. Lancet. 2005; 366: 1197-1209.
- Bray GA . Medical consequences of obesity. J Clin Endocrinol Metab. 2004; 89: 2583-2589.
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al . Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003; 112: 1821-1830.
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr . Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003; 112: 1796-1808.
- Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009; 15: 921-929.
- Winer S, Paltser G, Chan Y, Tsui H, Engleman E, Winer D, et al . Obesity predisposes to Th17 bias. Eur J Immunol. 2009; 39: 2629-2635.
- De Rosa V, Procaccini C, Calî G, Pirozzi G, Fontana S, Zappacosta S, et al. A key role of leptin in the control of regulatory T cell proliferation. Immunity. 2007; 26: 241-255.
- Wagner NM, Brandhorst G, Czepluch F, Lankeit M, Eberle C, Herzberg S, et al. Circulating regulatory T cells are reduced in obesity and may identify subjects at increased metabolic and cardiovascular risk. Obesity (Silver Spring). 2013; 21: 461-468.
- Deng T, Lyon CJ, Minze LJ, Lin J, Zou J, Liu JZ, et al . Class II major histocompatibility complex plays an essential role in obesity-induced adipose inflammation. Cell Metab. 2013; 17: 411-422.
- Chen Y, Tian J, Tian X, Tang X, Rui K, Tong J, et al . Adipose tissue dendritic cells enhances inflammation by prompting the generation of Th17 cells. PLoS One. 2014; 9: e92450.
- Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011; 332: 243-247.
- Liu J, Divoux A, Sun J, Zhang J, Clément K, Glickman JN, et al . Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med. 2009; 15: 940-945.
- Poglio S, De Toni-Costes F, Arnaud E, Laharrague P, Espinosa E, Casteilla L, et al. Adipose tissue as a dedicated reservoir of functional mast cell progenitors. Stem Cells. 2010; 28: 2065-2072.
- Altintas MM, Azad A, Nayer B, Contreras G, Zaias J, Faul C, et al. Mast cells, macrophages, and crown-like structures distinguish subcutaneous from visceral fat in mice. J Lipid Res. 2011; 52: 480-488.
- Salamon P, Shoham NG, Puxeddu I, Paitan Y, Levi-Schaffer F, Mekori YA . Human mast cells release oncostatin M on contact with activated T cells: possible biologic relevance. J Allergy Clin Immunol. 2008; 121: 448-455.
- Shi MA, Shi GP . Different roles of mast cells in obesity and diabetes: lessons from experimental animals and humans. Front Immunol. 2012; 3: 7.
- Lynch L, Nowak M, Varghese B, Clark J, Hogan AE, Toxavidis V, et al. Adipose tissue invariant NKT cells protect against diet-induced obesity and metabolic disorder through regulatory cytokine production. Immunity. 2012; 37: 574-587.
- Palmblad J, Hallberg D, Engstedt L . Polymorphonuclear (PMN) function after small intestinal shunt operation for morbid obesity. Br J Haematol. 1980; 44: 101-108.
- Moriguchi S, Kato M, Sakai K, Yamamoto S, Shimizu E . Exercise training restores decreased cellular immune functions in obese Zucker rats. J Appl Physiol (1985). 1998; 84: 311-317.
- Smith AG, Sheridan PA, Harp JB, Beck MA . Diet-induced obese mice have increased mortality and altered immune responses when infected with influenza virus. J Nutr. 2007; 137: 1236-1243.
- Bandaru P, Rajkumar H, Nappenveetil G. The impact of obesity on immune response to infection and vaccine: an insight into plausible mechanisms. Endocrinology and Metabolic Syndrome. 2013; 2: 2-9.
- Zhou Q, Leeman SE, Amar S . Signaling mechanisms in the restoration of impaired immune function due to diet-induced obesity. Proc Natl Acad Sci U S A. 2011; 108: 2867-2872.