
Perspective
J Immun Res. 2015;2(1): 1015.
Friend or Foe? – The Role of TH17 Immunity in Host Protection
Hoe E1, Toh ZQ1, Marimla R1, Balloch A1 and Licciardi PV1,2*
1Pneumococcal Research Group, Murdoch Children’s Research Institute, Royal Children’s Hospital, Australia
1Department of Paediatrics, University of Melbourne, Australia
*Corresponding author: Dr. Paul V Licciardi, Pneumococcal Research Group, Murdoch Children’s Research Institute, Royal Children’s Hospital, Australia
Received: January 22, 2015; Accepted: February 04, 2015; Published: February 05, 2015
Introduction
The discovery and characterisation of T-helper 17 (Th17) lymphocytes was first described in 2005 [1,2]. This new lymphocyte subset challenged immunologists’ thinking of the day with respect to the immune system and the Th1/Th2 dogma described almost 30 years earlier by Mosmann and Coffman [3]. These Th17 cells were shown to have a potent pro-inflammatory effect important in protection of the host against bacterial or fungal infections [4]. A number of studies have since demonstrated that Th17 cells, in addition to Th1 cells, can also drive pathological responses in a number of inflammatory and autoimmune diseases such as multiple sclerosis, rheumatoid arthritis and psoriasis. However, the role of Th17 cells and its signature cytokine, IL-17A is also recognised to play a critical role in pathogen clearance. In humans, the mechanisms driving Th17 cell differentiation and its regulation in host protection are poorly understood. In this perspective article, we discuss the functional plasticity of Th17 cells in the context of autoimmunity and infection and how these responses may be targeted by newgeneration therapies.
TH17 Differentiation Pathways
In mice, differentiation of naïve T cells into Th17 cells occurs mainly in the presence of IL-6 and TGFβ, resulting in IL-17 (or IL- 17A) secretion and is characterised by the expression of the nuclear transcription factor RORγt [5] and STAT3 [6]. Although less well characterised in humans, this seems to require IL-1b and/or IL-23, the latter especially crucial for Th17 cell expansion, survival and stability [7]. In addition to IL-17A, Th17 cells also typically secrete IL-17F, IL-21and IL-22 under the transcriptional control of RORc (the human analog of RORγt) [8,9]. The function of Th17 cells is reciprocally regulated by another lymphocyte subset, the regulatory T cell (Treg) [10] which are either thymus-derived or induced in the periphery by TGFβ and express the transcription factor FoxP3 [11]. However, Th17 differentiation from naïve precursors is generally unstable compared to Treg and has been suggested to represent an intermediate phenotype that expresses both FoxP3 and IL-17 [12]. Moreover, conversion of Treg to Th17 cells in vitro is thought to be one mechanism that could explain their dual role in autoimmunity and pathogen clearance.
Two distinct Th17 cell populations are proposed to help explain the dual role of Th17 cells, those with a pathogenic role, termed Teff17, and those that are protective, termed Treg17 [12]. It is known that Teff17 cells require IL-23 as studies have shown that IL-23 and IL- 23R knockout mice are not susceptible to autoimmunity [13] and also lack GM-CSF, another pathogenic cytokine [14]. In contrast, Treg17 development requires TGFβ, which can suppress GM-CSF as well as inducing IL-10 secretion to protect against tissue inflammation. In humans, Th17 skewing from Treg is less well understood, although one study has shown that Treg cells can also secrete IL-17 and express RORγt [15]. This information may be important in our understanding of the link between Th17 and Treg responses in health and disease.
Pathogenic Role of Th17 Responses in Autoimmunity
Th17 cells were first documented to induce severe tissue inflammation in the context of autoimmunity. Many studies in mice have confirmed the pathogenic role of Th17 cells in experimental models of human diseases such as experimental autoimmune encephalitis (multiple sclerosis, MS) [16], collagen-induced arthritis (rheumatoid arthritis, RA) [17] and colitis (inflammatory bowel disease) [18]. In humans, the role of Th17 responses in autoimmunity has mostly been based on studies examining biomarker correlations with clinical disease. For example, MS patients were found to have elevated IL-17 levels in the serum and cerebrospinal fluid [19-21]. Recently, it was shown that the higher IL-17A levels in MS patients was correlated with neuronal glutamate excitotoxicity and associated downstream blood-brain barrier disruption [22]. This supports in vitro evidence that Th17 cells have a greater capacity to penetrate the blood brain barrier (BBB) and infiltrate the parenchyma of the central nervous system than Th1 cells [23]. Similarly for RA, Th17 cells were of higher frequency in patients compared to healthy controls [24,25] and the expression of IL-17, TNF and IL-1 predicted later joint destruction [26]. Furthermore, it was demonstrated that the activity of these Th17 cells was inhibited by Tregs from RA patients that were up regulated by anti-TNFα treatment [27]. Emerging data on the use of anti-Th17 based therapies such as secukinumab (anti-IL-17A) for psoriasis are promising [28] and larger clinical trials with these newgeneration therapies will be of paramount importance.
Protective Role of Th17 Immunity
Some pathogens, particularly fungi and bacteria, are known to stimulate the production of IL-17, which is necessary to limit the spread of the organism. A number of studies have demonstrated that specific microbial ligands are able to induce cytokines such as IL-23 that drive Th17 development. Candida albicans, Klebsiella pneumoniae and Mycoplasma tuberculosis all require Th17 responses for their clearance, primarily through the upregulation of neutrophil function [29-31]. Recently, Th17 responses have been shown to protect mice against nasopharyngeal colonisation by Streptococcus pneumoniae, a major global pathogen responsible for the deaths of more than 1 million infants worldwide every year [32]. This is interesting as it suggests that antibody-independent mechanisms of protection are important in the mucosa in contrast to serotypespecific IgG, which is known to be the major correlate of protection against invasive pneumococcal disease.
The importance of IL-17-secreting Th17 cells in orchestrating the recruitment and activation of innate cells (neutrophils, monocytes and macrophages) in the upper respiratory tract and clearance of pneumococcal colonisation has been demonstrated [33,34]. This Th17 response was found to occur independently of antibodies and complement and was abrogated in the absence of the IL-17A receptor [35]. Importantly, high IL-17 expression was associated with low levels of pneumococcal nasopharyngeal carriage in both mice and young children [35-37] and stimulation of peripheral blood mononuclear cells ex vivo with pneumococcal pneumolysin generated substantial IL-17 [38,39]. In contrast, Tregs suppressed pneumococcal T cell responses in the mucosa, supporting the IL- 17-Treg counter-regulatory developmental pathway and providing a possible mechanism by which carriage is sustained [40]. More recently, higher lung Tregs were detected in mice resistant to pneumococcal pneumonia, highlighting the importance of TGFβ signalling in these animals [41]. However, further studies in humans are needed to confirm the protective effects of Tregs in the context of pneumococcal disease.
The discovery that Th17 immunity protects against pneumococcal colonisation underpinned the development of a Whole Cell Vaccine (WCV) that protects mice against colonisation, pneumonia and sepsis [42]. This vaccine comprises a non-encapsulated strain of pneumococcus that expresses multiple conserved proteins across many serotypes with the ability to stimulate CD4+ Th17-derived IL-17 responses [43]. This is a major advance in pneumococcal vaccinology due to its perceived ability to overcome many of the limitations of current pneumococcal conjugate vaccines such as serotype replacement and cost of the vaccine. The WCV has already completed a Phase 1 clinical study in healthy adults, demonstrating immunogenicity and an acceptable safety profile, leading to earlystage clinical evaluation in Kenya and later Indonesia to provide evidence that this vaccine provides broad protection for children at greatest risk of the disease.
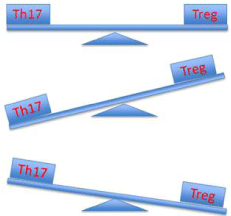
Figure 1: Schematic representation of the Th17-Treg axis in health and
disease. Top panel: Under healthy immune homeostasis, the Th17 and
Treg populations are counter-balanced; Middle panel: Protection against
autoimmune and auto-inflammatory diseases is associated with skewing
of the axis towards Treg cells ; Bottom panel: Th17 skewing is required for
protection against bacterial and fungal infections, especially Streptococcus
pneumoniae.
Conclusion
Our understanding of Th17 biology has advanced substantially over the last 10 years. In particular, the balance between suppressive Treg cells and inflammatory Th17 cells has long been considered an important aspect to preventing chronic inflammatory diseases, providing the impetus for development of novel therapeutic strategies aimed at augmenting Treg responses or blocking Th17 immunity. However, Th17 cells also have protective roles and further studies to understand their differentiation from Treg precursors will be critical in this approach. Harnessing protective Th17 immunity without the risk of inducing chronic tissue inflammation is of paramount importance. New-generation vaccines such as WCV offer significant promise in the prevention of pneumococcal disease while development of various IL-17 inhibitors has shown some benefit against autoimmune diseases.
References
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM et al: Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol.2005; 6: 1123-1132.
- Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005; 6: 1133-1141.
- Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989; 7: 145-173.
- Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006; 203: 2271-2279.
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006; 126: 1121-1133.
- Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009; 27: 485-517.
- Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003; 278: 1910-1914.
- Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008; 9: 641-649.
- Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007; 8: 950-957.
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006; 441: 235-238.
- Schmidt-Weber CB, Akdis M, Akdis CA. TH17 cells in the big picture of immunology. J Allergy Clin Immunol. 2007; 120: 247-254.
- Singh B, Schwartz JA, Sandrock C, Bellemore SM, Nikoopour E. Modulation of autoimmune diseases by interleukin (IL)-17 producing regulatory T helper (Th17) cells. Indian J Med Res. 2013; 138: 591-594.
- Croxford AL, Mair F, Becher B. IL-23: one cytokine in control of autoimmunity. Eur J Immunol. 2012; 42: 2263-2273.
- El-Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F, et al. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol. 2011; 12: 568-575.
- Ayyoub M, Deknuydt F, Raimbaud I, Dousset C, Leveque L, Bioley G, et al. Human memory FOXP3+ Tregs secrete IL-17 ex vivo and constitutively express the T(H)17 lineage-specific transcription factor RORgamma t. Proc Natl Acad Sci U S A. 2009; 106: 8635-8640.
- Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005; 201: 233-240.
- Lubberts E, Koenders MI, van den Berg WB. The role of T-cell interleukin-17 in conducting destructive arthritis: lessons from animal models. Arthritis Res Ther. 2005; 7: 29-37.
- Tesmer LA, Lundy SK, Sarkar S, Fox DA. Th17 cells in human disease. Immunol Rev. 2008; 223: 87-113.
- Tzartos JS, Friese MA, Craner MJ, Palace J, Newcombe J, Esiri MM, et al. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol. 2008; 172: 146-155.
- Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009; 361: 888-898.
- Montes M, Zhang X, Berthelot L, Laplaud DA, Brouard S, Jin J, et al. Oligoclonal myelin-reactive T-cell infiltrates derived from multiple sclerosis lesions are enriched in Th17 cells. Clin Immunol. 2009; 130: 133-144.
- Kostic M, Dzopalic T, Zivanovic S, Zivkovic N, Cvetanovic A, Stojanovic I, et al. IL-17 and glutamate excitotoxicity in the pathogenesis of multiple sclerosis. Scand J Immunol. 2014; 79: 181-186.
- Jadidi-Niaragh F, Mirshafiey A. Th17 cell, the new player of neuroinflammatory process in multiple sclerosis. Scand J Immunol. 2011; 74: 1-13.
- Shen H, Goodall JC, Hill Gaston JS. Frequency and phenotype of peripheral blood Th17 cells in ankylosing spondylitis and rheumatoid arthritis. Arthritis Rheum. 2009; 60: 1647-1656.
- Colin EM, Asmawidjaja PS, van Hamburg JP, Mus AM, van Driel M, Hazes JM, et al. 1,25-dihydroxyvitamin D3 modulates Th17 polarization and interleukin-22 expression by memory T cells from patients with early rheumatoid arthritis. Arthritis Rheum. 2010; 62: 132-142.
- Kirkham BW, Lassere MN, Edmonds JP, Juhasz KM, Bird PA, Lee CS, et al. Synovial membrane cytokine expression is predictive of joint damage progression in rheumatoid arthritis: a two-year prospective study (the DAMAGE study cohort). Arthritis Rheum. 2006; 54: 1122-1131.
- McGovern JL, Nguyen DX, Notley CA, Mauri C, Isenberg DA, Ehrenstein MR. Th17 cells are restrained by Treg cells via the inhibition of interleukin-6 in patients with rheumatoid arthritis responding to anti-tumor necrosis factor antibody therapy. Arthritis Rheum. 2012; 64: 3129-3138.
- McInnes IB, Sieper J, Braun J, Emery P, van der Heijde D, Isaacs JD, et al. Efficacy and safety of secukinumab, a fully human anti-interleukin-17A monoclonal antibody, in patients with moderate-to-severe psoriatic arthritis: a 24-week, randomised, double-blind, placebo-controlled, phase II proof-of-concept trial. Ann Rheum Dis. 2014; 73: 349-356.
- Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007; 8: 369-377.
- Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis. 2004; 190: 624-631.
- Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001; 194: 519-527.
- Malley R. Antibody and cell-mediated immunity to Streptococcus pneumoniae: implications for vaccine development. J Mol Med (Berl). 2010; 88: 135-142.
- Zhang Z, Clarke TB, Weiser JN. Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. J Clin Invest. 2009; 119: 1899-1909.
- Trzciński K, Thompson CM, Srivastava A, Basset A, Malley R, Lipsitch M. Protection against nasopharyngeal colonization by Streptococcus pneumoniae is mediated by antigen-specific CD4+ T cells. Infect Immun. 2008; 76: 2678-2684.
- Lu YJ, Gross J, Bogaert D, Finn A, Bagrade L, Zhang Q, et al. Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLoS Pathog. 2008; 4: e1000159.
- Zhang Q, Arnaoutakis K, Murdoch C, Lakshman R, Race G, Burkinshaw R, et al. Mucosal immune responses to capsular pneumococcal polysaccharides in immunized preschool children and controls with similar nasal pneumococcal colonization rates. Pediatr Infect Dis J. 2004; 23: 307-313.
- Malley R, Trzcinski K, Srivastava A, Thompson CM, Anderson PW, Lipsitch M. CD4+ T cells mediate antibody-independent acquired immunity to pneumococcal colonization. Proc Natl Acad Sci U S A. 2005; 102: 4848-4853.
- Mureithi MW, Finn A, Ota MO, Zhang Q, Davenport V, Mitchell TJ, et al. T cell memory response to pneumococcal protein antigens in an area of high pneumococcal carriage and disease. J Infect Dis. 2009; 200: 783-793.
- Schmid P, Selak S, Keller M, Luhan B, Magyarics Z, Seidel S, et al. Th17/Th1 biased immunity to the pneumococcal proteins PcsB, StkP and PsaA in adults of different age. Vaccine. 2011; 29: 3982-3989.
- Pido-Lopez J, Kwok WW, Mitchell TJ, Heyderman RS, Williams NA. Acquisition of pneumococci specific effector and regulatory Cd4+ T cells localising within human upper respiratory-tract mucosal lymphoid tissue. PLoS Pathog. 2011; 7: e1002396.
- Neill DR, Fernandes VE, Wisby L, Haynes AR, Ferreira DM, Laher A, et al. T regulatory cells control susceptibility to invasive pneumococcal pneumonia in mice. PLoS Pathog. 2012; 8: e1002660.
- Gonçalves VM, Dias WO, Campos IB, Liberman C, Sbrogio-Almeida ME, Silva EP, et al. Development of a whole cell pneumococcal vaccine: BPL inactivation, cGMP production, and stability. Vaccine. 2014; 32: 1113-1120.
- Moffitt KL, Malley R, Lu YJ. Identification of protective pneumococcal T(H)17 antigens from the soluble fraction of a killed whole cell vaccine. PLoS One. 2012; 7: e43445.