
Special Article-Cancer Immunology
J Immun Res. 2015;2(2): 1018.
Evaluation of Myeloid-derived Suppressor Cells and Components of Renin Angiotensin System in Urethaneinduced Lung Cancer
Jair Ribeiro Chagas1,2, Bruna Visniauskas1, Guiomar Nascimento Gomes3 and Valquiria Bueno4*
1Department of Psychobiology, UNIFESP Federal University of So Paulo, Brazil
2Department of Biosciences, UNIFESP Federal University of So Paulo, Brazil
3Department of Physiology, UNIFESP Federal University of So Paulo, Brazil
4Department of Microbiology, Immunology and Parasitology, UNIFESP Federal University of So Paulo, Brazil
*Corresponding author: Valquiria Bueno, Department of Microbiology Immunology and Parasitology, UNIFESP Federal University of So Paulo, Brazil
Received: February 19, 2015; Accepted: March 06, 2015; Published: March 09, 2015
Abstract
Urethane-induced lung cancer has been associated with an imbalance in the effector/suppression immune response since interferon-gamma production is increased along with elevated percentages of myeloid-derived suppressor cells. The goal in cancer therapy is to prevent the immunosuppressive effects caused by tumor and thus lead to the development of effective immune response. The depletion of myeloid-derived suppressor cells in experimental models causes less tumor burden and improves the immune response but doesn´t provide cure. As cancer is a complex and multifactorial disease the treatment also should be based on multiple targets.
A recently described target is the renin angiotensin system and its components (i.e. bradikynin receptors, angiotensin-converting enzyme) as they may play a role in cancer development. Therefore, our aim was to investigate the percentage of myeloid-derived suppressor cells at the tumor site and the possible correlation with bradikynin receptors and angiotensin-converting enzyme expressions in mice submitted to Urethane-induced lung cancer.
BALB/c mice were either injected with myeloid-derived suppressor cells, Urethane or only followed for 120 days (control). Lung, spleen, and blood were phenotyped for myeloid-derived suppressor cells and lung tissue was evaluated for the expression of renin angiotensin system components.
In tumor-bearing mice it was observed a significant increase at the percentage of myeloid-derived suppressor cells in the spleen along with angiotensinconverting enzyme increased gene and decreased protein expressions in lung.
Urethane administration was crucial to lung tumor development, to the increase of peripheral myeloid-derived suppressor cells and to the changes observed in angiotensin-converting enzyme (ACE).
In conclusion, tumor microenvironment seems to modulate myeloid-derived suppressor cells expansion and ACE gene and protein expressions. Myeloidderived suppressor cells and renin angiotensin system components could be potential targets for cancer diagnostics and therapy.
Keywords: Lung cancer; Urethane; Myeloid-derived suppressor cells; Angiotensin-converting enzyme; Bradykinin
Introduction
Our group has shown previously that Urethane-induced lung cancer leads to an increase in the percentage of myeloid-derived suppressor cells (MDSC) in spleen and lung along with transforming growth factor-beta (TGF-β) expression by tumor cells. In addition, Foxp3+ cells were elevated in lung tissue of Urethane-injected mice [1, 2]. These findings suggest a suppressive status after lung cancer induced by Urethane. On the other hand, there was an increase of toll like receptor 4 (TLR4) expressions in lung tissue and interferongamma (IFN-γ) production ex-vivo by splenocytes suggesting immune response against tumor [1, 2]. We hypothesized that there is an imbalance of effector immune response versus suppression/ tolerance culminating with cancer development.
It has been shown that one of the mechanisms responsible for tumor growth is the increased generation of MDSC in bone marrow followed by their migration to blood and tumor site [3]. These cells act suppressing the immune system through the secretion of factors such as arginase-1 and TGF-β [reviewed in [4, 5]. Another possible role played by tumor cells and/or MDSC is the interference with hematopoiesis [3]. The increase of myelopoiesis can lead to the inhibition in the complete maturation of myeloid cells and thus to the decrease in the number of inflammatory macrophages and elevation in the number of suppressive immature myeloid cells [6]. In agreement, we found an increased percentage of MDSC in bone marrow of mice induced to lung cancer by Urethane administration [2].
Although MDSCs depletion causes less tumor burden and improved immune response in experimental models, this procedure has not been associated with cure [7, 8]. Considering that cancer is a complex and multifactorial disease the treatment also should be based on multiple targets.
A recently described target is the renin angiotesing system (RAS) as some components are proposed to play a role in cancer development [9]. RAS is a network of enzymes and peptides and its key peptidase angiotensin-converting-enzyme (ACE, EC.3.4.15.1) cleaves several substrates including bradykinin (B). The intrarenal hormone bradikynin modulates renal functions [10,11] and renal cells growth and proliferation [12, 13] exerting its multiple functions via B1 and B2 receptors [14, 15]. In addition to the classic functions of ACE and B1/B2 it has been suggested the participation of these components in cancer pathophysiology including the expression of B1 and B2 in clinical specimens of several cancers such as small cell and non-small cell carcinomas of the lung [16, 17, 18].
Shen et al. using experimental models of tumor and chronic inflammation showed that mice over expressing ACE in myeloid cells present reduced number of MDSCs in blood and spleen [19] which confirms previous findings that ACE is associated with some aspects of myelopoiesis regulation [20, 21]. All these findings strongly suggest the participation of RAS members in cancer development which could occur via modulation of MDSC and increased immunosuppression favoring tumor growth.
In humans there are some conflicting data relating ACE and cancer development. Whereas meta-analysis showed no association of ACE gene insertion/deletion polymorphism and lung cancer development (Wang et al. [22]), the use of angiotensin-converting enzyme inhibitors was associated with an increased rate of lung cancer in a nested case-control analysis (Azoulay et al. [23]). Experimental models have shown that ACE inhibitors can decrease tumor growth as observed by Attoub et al. [24] in athymic mice injected with highly tumorigenic LNM35 human lung cells (xenografts) and treated with captopril. Moreover, Araujo et al. [25] showed in renal cell carcinoma model that ACE blockade was associated with smaller tumors and fewer lung metastases compared with the controls.
Thus, RAS members (ACE, B1 and B2 receptors) could be used in addition to MDSCs as targets for cancer diagnostic, prediction of outcome, and therapy. In this line, Nowak et al. [26] showed that ACE activity was significantly lower in lung tissue from patients undergoing tumor resection than in individuals with normal lung tissue.
In addition to ACE expression during cancer development the multiple cross talks between bradykinin receptors (B1 and B2) and renin-angiotensin system affecting cancer development have been studied [27] but no evaluation has been performed on bradykinin receptors and MDSC in cancer.
Therefore, our aim was to investigate at the tumor site whether there is an increase in myeloid-derived suppressor cells and the possible correlation with bradikynin receptors and angiotensinconverting enzyme expressions in mice submitted to Urethaneinduced lung cancer.
Material and Methods
Animals and experimental design
Eight to 10-week-old male BALB/c mice (bred in CEDEME/ UNIFESP) were placed in cages and cared for in accordance with the Principles of Laboratory Animal Care (NIH publication 86-23, revised 1985) and the regulations of the Brazilian Committee on Animal Experimentation. The project was submitted and approved by the Animal Ethics Committee of UNIFESP (protocol 0117/09).
Control: Mice were just followed during 120 days (n=5)
MDSC: Mice were adoptively transferred with myeloid-derived suppressor cells and followed during 120 days in order to evaluate whether the increased percentage of MDSC could lead to tumor development (n=5)
UR: (urethane induced-lung cancer) Mice were injected with Urethane and followed during 120 days (n=5).
In order to induce lung cancer Urethane (Sigma Chemical Company, St Louis, MO) dissolved in 0.9% NaCl was injected in BALB/c mice (two doses i.p. with the interval of 48 h; 1.5 g/kg each dose).
Animals were followed for 120 days when they were anesthetized with Xylazine (Agribrands, Brazil) and Ketamine (Vetbrands, Brazil) diluted in 10 ml of sterile PBS (phosphate buffered solution-OXOID LTD Hampshire England) for the harvesting of spleen, blood and lung.
Spleen and blood were evaluated for the percentages of CD11b+ Gr-1+ cells by flow cytometry. Lung lobes were segmented and used for: flow cytometry to evaluate the percentages of CD11b+ Gr-1+ cells, histology (nodules identification and measurement), PCR (mRNA), and immunohistochemistry.
Myeloid-derived suppressor cells enrichment for adoptive transfer
Our goal was to increase the percentage of myeloid-derived suppressor cells (by adoptive transfer) in naïve BALB/c mice to evaluate whether these cells in higher percentage could induce lung cancer development even in the absence of the carcinogen Urethane.
In order to obtain an enrichment of MDSCs for adoptive transfer we injected BALB/c mice (i.p.) twice with 1.5g/kg of Urethane (Sigma Chemical Company, St Louis, MO) dissolved in 0.9% NaCl and with an interval of 48 hours between administrations. Twenty days later mice were anesthetized and lungs were removed for cell harvesting, and a single cell suspension was obtained. Lung cells were stained with CD11b APC (BD Biosciences Pharmingen) and Gr-1 Pe (Immuno- Tools, Germany). Cells were sorted by flow citometry using FACS Aria (BD Biosciences, San Jose, CA). It was obtained 95% of myeloid derived suppressor cells purity (shown below) (Figure 1).
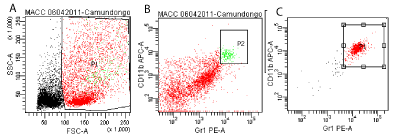
Figure 1: Representative dot plot from one animal showing the sorting of lung cells (myeloid derived suppressor cells, CD11b+Gr-1+). Total lung cells from BALB/c
mice after 20 days of Urethane injection (A). Pre sorting of total lung cells stained and gated for CD11b and Gr-1 (P2=1.8% from total lung cells) (B). Pos sorting
of CD11b+Gr-1+ lung cells (P1=95.8% of purity) (C).
Myeloid-derived suppressor cells adoptive transfer
0.8 to 1x106 sorted lung cells (CD11b+ Gr-1+, MDSCs) in sterile saline were injected in the penile vein of each anesthetized naïve BALB/c mice.
Harvesting of spleen, lung, and blood for single cell suspension
After 120 days of following Control, MDSC adoptive transfer (MDSC), and Urethane-induced (UR) groups, mice were anesthetized for the harvesting of spleen, blood and lung.
Spleen and lung single cell suspensions were prepared by pressing each organ through a 400μm sterile nylon mesh. Spleen and lung single cell suspension were placed in individual tubes and submitted to 1 min of distilled water to cause hemolysis.
Blood was diluted in an equal volume of PBS and added to a Falcon tube containing 1mL of Ficoll followed by centrifugation (30 min, 1800 rpm, room temperature). Peripheral blood mononuclear cells were collected and washed in PBS.
Flow cytometry
Single cell suspensions from spleen, lung and blood (1×106) were incubated for 20 min with the rat anti-mouse (BD Biosciences Pharmingen) CD11b APC and Gr-1 Pe (Immuno-Tools, Germany) for the evaluation of myeloid-derived suppressor cells (MDSC). Cells were washed in PBS and suspended in PBS-BSA for flow cytometry analysis. Cells were analyzed in a FACSCalibur (Cell Quest Pro Software) Cell Cytometer (BD Biosciences). At least 10,000 events were evaluated.
Histological analysis
Lungs were fixed in 4% buffered formalin followed by paraffin embedded, cut into 5 μm sections that were placed on glass slides, stained with hematoxylin and eosin (H&E), and these sections were reviewed by a pathologist (blinded to the procedures).
Nodules measurement
Lung tissue stained for H&E as described above was observed in an image system using 500 x magnifications. Each nodule was photographed, saved in the software Image Pro Plus 3.0, drawn round with an electronic pen and its area was immediately calculated by UHTSCSA Image Tool 3.0.
ACE, B1and B2 receptor, and VEGFA mRNA expression in lung tissue
Total lung RNA was extracted using the TRIzol reagent (Life Technologies). Following DNase I treatment, 3μg of RNA was reverse transcribed into cDNA using Super Script II Reverse Transcriptase (Life Technologies). Quantitative SYBR Green real-time PCR was performed with the Step One Plus Instrument (Life Technologies). Each 25μl of SYBR Green reaction consisted of 25 ng of cDNA, 12.5μl of 2× SYBR Green Universal PCR Master Mix (Life Technologies), and 200 nM of each forward and reverse primer (Table 1). Realtime PCR were performed using the temperature protocol 50 °C for 2 min, 95 °C for 10 min, and 50 cycles of 95 °C for 15 s and 60 °C for 1 min, followed by a dissociation curve protocol for evaluation of the specificity of the amplicon produced in each reaction. A distinct peak indicated that a single DNA sequence was amplified during PCR. Standard curves were measured for each primer set and cDNA sample to verify the efficiency of the reaction. As the efficiency of all reactions was >95%, 2–ΔΔCt parameter was used to obtain quantitative values in which ΔCt represents the subtraction of the beta-actin and GAPDH Ct values from the target gene values.
Gene
Forward primer (5′-3′)
Reverse primer (5′-3′)
ACE
CGGTTTTCATGAGGCTATTGG
TCGTAGCCACTGCCCTCACT
VEGF-A
CTGTGCAGGCTGCTGGTAACGATGAAGC
CCGGTGAGAGGTCTGGTTCCCGAAACC
B1
CCAGGGTTCGTCATCACTATCTG
GCAAAAGGAAGAAGGACAAGACTAA
B2
CCCTTCCTCTGGGTCCTCTT
CA CAGAACACGCTGAGGACAAAGA
β-actin
AGGCCAACCGTGAAAAGATG
CCAGAGGCATACAGGGACAAC
GADPH
TGCCCCCATGTTTGTGATG
GCTGACAATCTTGAGGGAGTTGT
Table 1: Primer sequences of cDNAs coding for Angiotensin-converting enzyme (ACE), VEGF-A and bradykinin B (1 and 2) receptors, β-actin, and GADPH used for real-time PCR.
Immunohistochemistry
Lung sections (5 μm) were deparaffinized in xylene, rehydrated in graded ethanol, and then pretreated in a microwave (Eletrolux, SP, Brazil) with 10 mM citric acid buffer (pH 6) for 3 cycles of 5 min each at 850 W for antigen retrieval. The sections were pre incubated with 0.3% hydrogen peroxidase in PBS for 5 min to inactivate endogenous peroxidases and blocked with 5% normal goat serum in PBS for 10 min. The specimens were then incubated with anti-ACE monoclonal antibody (H-170, sc-20791, Santa Cruz Biotechnology, CA, USA) diluted 1:500. The incubation was performed overnight at 4°C and followed by two washes in PBS for 10 min. The sections were then incubated with anti-rabbit biotin conjugated secondary antibody (Vector Laboratories, CA, USA) diluted at 1:200 in PBS for 1 h. The sections were washed twice with PBS followed by incubation with the preformed avid in biotin complex conjugated to peroxidase (Vector Laboratories, CA, and USA) for 45 min. The bound complexes were visualized with 0.05% 3-39-diaminobenzidine (DAB) solution.
Statistical analysis
Data are expressed as mean and standard deviation and the statistical analysis were performed by ANOVA followed by Tukey’s pairwise comparisons. The level of statistical significance was defined as p-value <0.05.
Results
Mice from the groups Control and adoptively transferred with MDSC did not develop lung cancer whereas mice injected with Urethane developed 1 to 4 lung nodules (Figure 2A). Lung nodules in Urethane injected mice measured from 0.007 to 0.022mm2 (data not shown).
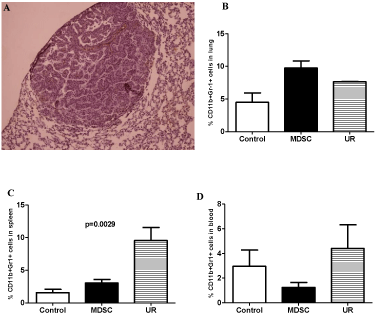
Figure 2: Lung histology showing a nodule in a mouse from Urethaneinduced
cancer group (A). Flow cytometry results showing myeloid-derived
suppressor cells (MDSC; CD11b+Gr-1+) percentage in lung (B), spleen (C),
and blood (D) of BALB/c mice followed by 120 days. Groups: Control, MDSC
(adoptive transfer of myeloid-derived suppressor cells), and UR (Urethane
injection).
The percentage of myeloid-derived suppressor cells (MDSC; CD11b+Gr-1+) in lung was higher in mice adoptively transferred with MDSC, whereas in spleen and blood a higher percentage of MDSC was observed in mice injected with Urethane (Figure 2).
Angiotensin-converting enzyme mean mRNA expression increased 10.5 times in lung of mice injected with Urethane and it was significantly higher (p=0.004 for beta-actin and p=0.001 for GAPDH) in mice injected with Urethane in comparison with Control and MDSC injected mice (Figure 3A and 3B). VEGF-A mean mRNA expression was 1.16 times higher in Urethane group compared to Control but did not reach statistical significance whereas this expression was decreased in MDSC group (Figure 3C and 3D). Bradykinin B1 receptor presented similar mRNA expression when Control, MDSC and Urethane groups were compared (Figure 3E and 3F). B2 receptor mean mRNA expression increased 2.5 times in the Urethane group but without reaching statistical significance in comparison with Control and MDSC groups (Figure 3G and 3H).
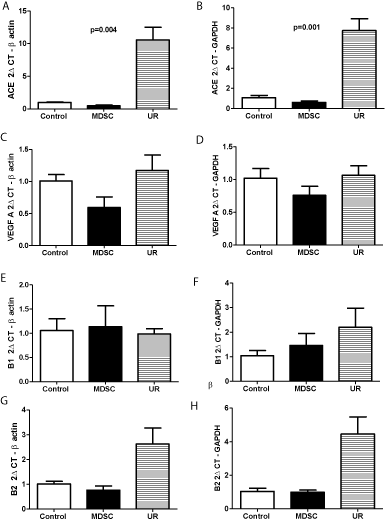
Figure 3: Angiotensin-converting enzyme (ACE), VEGF A, bradykinin B (1 and 2) receptors mRNA expression in BALB/c mice lungs. Groups: Control, MDSC
(adoptive transfer of myeloid-derived suppressor cells), and UR (Urethane injection). p=0.004 and p=0.001 Control and MDSC versus UR.
As mRNA for ACE was the only significantly higher expressed in Urethane-induced lung cancer we performed immunohistochemistry for ACE protein in the lung tissue. In the opposite of mRNA ACE expression by PCR, analysis by immunohistochemistry showed that Control and MDSC mice presented a more intense expression of ACE in lung epithelium than the Urethane group (Figure 4 A-C). In a detail of a lung nodule it is possible to observe that few cells in the tumor are positive for ACE (Figure 4D).
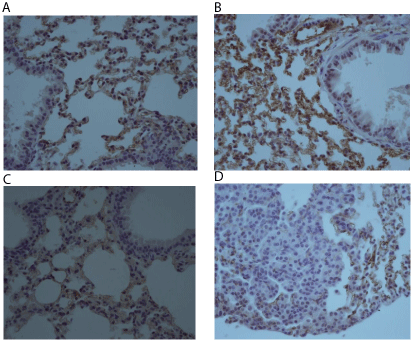
Figure 4: Angiotensin-converting enzyme (ACE) expression in lung tissue
(immunohistochemistry) from mice of Control group (A), MDSC group (B),
Urethane group (C), and detail of a lung nodule in Urethane group (D) (400X).
Discussion
In our model, the adoptive transfer of MDSC caused an accumulation of these cells in lung of naïve BALB/c mice. However, this increase of MDSC was not associated with the development of lung cancer suggesting that a carcinogen/mutation is required for tumor development. Moreover, tumor microenvironment seems to be needed for the increase of MDSC in extra-tumor sites as we did not observe any augmentation in the percentage of these cells in spleen and blood after adoptive transfer only. Lung tumor development after Urethane administration was associated with increased percentage of MDSC in lung, spleen, and blood compared to Control mice. In agreement with our findings, Younos et al. also observed that in naïve mice (i.v. injected CFSE-labeled MDSCs) myeloid-derived suppressor cells initially accumulated in lungs, while in tumor-bearing (TB) mice they were rapidly sequestered in the spleen. Moreover, they showed that in TB mice there is an extramedullary hematopoiesis (EMH) within lymphoid and parenchymal organs capable to generate myeloid progenitor cell (MPC) and thus contribute for the increase of MDSCs. They found that after MDSCs adoptive transfer the spleen is the primary site of myeloid cell proliferation and sequestration in TB mice and that cytokine secretion by tumor cells contribute to these processes [28].
VEGF-A mRNA expression increased in Urethane group without reaching statistical significance in comparison with Control group. However, this angiogenesis-related factor was decreased in mice adoptively transferred with MDSC suggesting that the accumulation of MDSC at the lung site is not enough to promote tumor and angiogenesis. Instead, the tumor microenvironment including infiltrating MDSC is required for these processes. Srivastava et al. showed that mice injected with Lewis lung carcinoma and depleted of MDSC presented reduced tumor burden and decrease in mRNA expression of the pro-angiogenic factor VEGF-A, but without reaching tumor free status [8].
ACE expression (mRNA and immunohistochemistry) in lung from Control and MDSC adoptively transferred groups was similar. This finding suggests that the accumulation of MDSC after adoptive transfer did not interfere with ACE expression in lung. In opposition, tumor development after Urethane induction was associated with alterations in ACE expression (increased mRNA expression and decreased protein expression) at the lung suggesting that tumor and associated factors could regulate ACE expression. This divergence in mRNA and protein expression for ACE at the tumor site could be explained by post-transcriptional regulation. It has been shown that microRNAs (miR) play a role in regulating components of the renin angiotensin system (RAS). Martin et al. [29] observed that miR-155 can bind to the 3′-untranslated region (UTR) of human angiotensin II type 1 receptor (hAT1R) mRNAs and translationally repress the expression of this protein. Zheng et al. [30] showed that miR-155 overexpression in fibroblasts suppress AT(1)R 3’-UTR reporter construct activity by significantly decreasing target protein expression without reducing target mRNA levels. In addition, Li et al. [31] found that miR-155 and miR-21 were the most upregulated miRNAs during the process of MDSCs induction (bone marrow) by GM-CSF and IL- 6. In TB mice they observed higher levels of these miRNAs in MDSCs from bone marrow and spleen. These findings in association with ours strongly suggest that MDSCs and RAS components are regulated during tumor development. However, it has to be demonstrated whether ACE is subject to post-transcriptional regulation in lung cells during tumor development in the Urethane model.
Röcken et al. observed in colon carcinoma tissues (CRC) that ACE mRNA expression was higher than in non-lesion tissues. Using immunohistochemistry authors compared ACE protein in CRC and in colorectal adenomas (CRA) and found that ACE was highly expressed in CRC cells (apical membrane) whereas in CRA this marker was mostly weak and localized in the cytoplasm and less commonly at the apical membrane [32].
A possible link of ACE and MDSC in cancer development was demonstrated in ACE10/10 mice (over expression of ACE in myeloid cells) injected with B16-F10 melanoma cells. ACE10/10 mice presented significantly smaller tumors than wild type mice [19]. This result was associated with a decreased percentage of MDSC in spleen cells of ACE10/10 mice. Regarding to metastasis it was observed that the number of visible nodules in ACE10/10 lung was significantly lower than in wild type [19]. These data shows that a higher expression of ACE in myeloid cells was correlated with less primary tumor and metastasis which was associated with decreased percentage of MDSC. In this line, our results showed that decreased ACE protein expression at the lung was associated with tumor development and increase percentage of MDSC in spleen.
Histology analysis has shown the expression of bradykinin receptors in clinical specimens of several cancers including small cell and non-small cell carcinomas of the lung [17, 18]. In our model B2 receptor presented mRNA increased expression in Urethane-induced lung cancer mice but without reaching statistical significance. The increase of MDSC in lung after adoptive transfer only was not associated with a significant increase of B1 and B2 suggesting that instead the tumor microenvironment in association with infiltrating MDSC promote changes in bradykinin receptors.
We speculate based on our results and literature that modulation in the expression of MDSCs, VEGF-A, ACE, bradykinin and related factors occurs during tumor development and could tip the balance effector/suppressor immune response. However, further studies are needed to clarify the mechanisms associated with ECA, B1 and B2 receptors changes during tumor development.
In conclusion, Urethane administration was crucial to lung tumor development, and tumor microenvironment seems to interfere with the increase of MDSC and changes in angiotensin-converting enzyme (ACE) expression. Myeloid-derived suppressor cells and renin angiotensin system components could be potential targets for cancer diagnostics and therapy.
Acknowledgements
This work was supported by grants from Fundaço de amparo à Pesquisa - FAPESP, Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq and Associaço Fundo de Incentivo à Psicofarmacologia (AFIP).
References
- Rosin FC, Pedregosa JF, de Almeida JS, Bueno V. Identification of myeloid-derived suppressor cells and T regulatory cells in lung microenvironment after Urethane-induced lung tumor. Int Immunopharmacol. 2011; 11: 873-878.
- Teixeira D, Almeida JS, Visniauskas B, Gomes GN, Hirata AE, Bueno V. Myeloid-derived suppressor cells and associated events in urethane-induced lung cancer. Clinics (Sao Paulo). 2013; 68: 858-864.
- Sawanobori Y, Ueha S, Kurachi M, Shimaoka T, Talmadge JE, Abe J, et al. Chemokine-mediated rapid turnover of myeloid-derived suppressor cells in tumor-bearing mice. Blood. 2008; 111: 5457-5466.
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009; 9: 162-174.
- Bueno V, Sant'Anna OA, Lord JM. Ageing and myeloid-derived suppressor cells: possible involvement in immunosenescence and age-related disease. Age (Dordr). 2014; 36: 9729.
- Hammani I, Chen J, Murschel F, Bronte V, De Crescenzo G, Jolicoeur M. Immunosuppressive activity enhances central carbon metabolism and bioenergetics in myeloid-derived suppressor cells in vitro models. BMC Cell Biol. 2012; 13: 18.
- Morales JK, Kmieciak M, Graham L, Feldmesser M, Bear HD, Manjili MH. Adoptive transfer of HER2/neu-specific T cells expanded with alternating gamma chain cytokines mediate tumor regression when combined with the depletion of myeloid-derived suppressor cells. Cancer Immunol Immunother. 2009; 58: 941-953.
- Srivastava MK, Zhu L, Harris-White M, Kar UK, Huang M, Johnson MF, et al. Myeloid suppressor cell depletion augments antitumor activity in lung cancer. PLoS One. 2012; 7: e40677.
- Wegman-Ostrosky T, Soto-Reyes E, Vidal-Millán S, Sánchez-Corona J. The renin-angiotensin system meets the hallmarks of cancer. J Renin Angiotensin Aldosterone Syst. 2013.
- Mukai H, Fitzgibbon WR, Bozeman G, Margolius HS, Ploth DW. Bradykinin B2 receptor antagonist increases chloride and water absorption in rat medullary collecting duct. Am J Physiol. 1996; 271: R352-360.
- Bagaté K, Grima M, Imbs JL, Jong WD, Helwig JJ, Barthelmebs M. Signal transduction pathways involved in kinin B(2) receptor-mediated vasodilation in the rat isolated perfused kidney. Br J Pharmacol. 2001; 132: 1735-1742.
- El-Dahr SS, Dipp S, Baricos WH. Bradykinin stimulates the ERK-->Elk-1-->Fos/AP-1 pathway in mesangial cells. Am J Physiol. 1998; 275: F343-352.
- Jaffa AA, Miller BS, Rosenzweig SA, Naidu PS, Velarde V, Mayfield RK. Bradykinin induces tubulin phosphorylation and nuclear translocation of MAP kinase in mesangial cells. Am J Physiol. 1997; 273: F916-924.
- Hess JF, Borkowski JA, Young GS, Strader CD, Ransom RW. Cloning and pharmacological characterization of a human bradykinin (BK-2) receptor. Biochem Biophys Res Commun. 1992; 184: 260-268.
- Menke JG, Borkowski JA, Bierilo KK, MacNeil T, Derrick AW, Schneck KA, et al. Expression cloning of a human B1 bradykinin receptor. J Biol Chem. 1994; 269: 21583-21586.
- Okwam-Duodu D, Landry J, Shen XZ, Diaz R. Angiotensin-converting enzyme and the tumor microenvironment: mechanisms beyond angiogenesis. Am J Physiol Regul Integr Comp Physiol. 2013; 305: R205–R215.
- Wu J, Akaike T, Hayashida K, Miyamoto Y, Nakagawa T, Miyakawa K,et al. Identification of bradykinin receptors in clinical cancer specimens and murine tumor tissues. Int J Cancer. 2002; 98: 29-35.
- Chee J, Naran A, Misso NL, Thompson PJ, Bhoola KD. Expression of tissue and plasma kallikreins and kinin B1 and B2 receptors in lung cancer. Biol Chem. 2008; 389: 1225-1233.
- Shen XZ, Okwan-Duodu D, Blackwell WL, Ong FS, Janjulia T, Bernstein EA, et al. Myeloid expression of angiotensin-converting enzyme facilitates myeloid maturation and inhibits the development of myeloid-derived suppressor cells. Lab Invest. 2014; 94: 536-644.
- Lin C, Datta V, Okwan-Duodu D, Chen X, Fuchs S, Alsabeh R, et al. Angiotensin-converting enzyme is required for normal myelopoiesis. FASEB J. 2011; 25: 1145-1155.
- Shen XZ, Bernstein KE. The peptide network regulated by angiotensin converting enzyme (ACE) in hematopoiesis. Cell Cycle. 2011; 10: 1363-1369.
- Wang N, Yang D, Ji B1, Li J. Angiotensin-converting enzyme insertion/deletion gene polymorphism and lung cancer risk: A meta-analysis. J Renin Angiotensin Aldosterone Syst. 2014.
- Azoulay L, Assimes TL, Yin H, Bartels DB, Schiffrin EL, Suissa S. Long-term use of angiotensin receptor blockers and the risk of cancer. PLoS One. 2012; 7: e50893.
- Attoub S, Gaben AM, Al-Salam S, Al Sultan MA, John A, Nicholls MG, et al. Captopril as a potential inhibitor of lung tumor growth and metastasis. Ann N Y Acad Sci. 2008; 1138: 65-72.
- Araujo WR, Naves MA, Ravanin JN, Schor N, Teixeira VP. Renin-angiotensin system (RAS) blockade attenuates growth and metastatic potential of renal cell carcinoma in mice. Urol Oncol. 2015.
- Nowak K, Kölbel HC, Metzger RP, Hanusch C, Frohnmeyer M, Hohenberger P, et al. Immunotargeting of the pulmonary endothelium via angiotensin-converting-enzyme in isolated ventilated and perfused human lung. Adv Exp Med Biol. 2013; 756: 203-212.
- da Costa PL, Sirois P, Tannock IF, Chammas R. The role of kinin receptors in cancer and therapeutic opportunities. Cancer Lett. 2014; 345: 27-38.
- Younos IH, Dafferner AJ, Gulen D, Britton HC, Talmadge JE. Tumor regulation of myeloid-derived suppressor cell proliferation and trafficking. Int Immunopharmacol. 2012; 13: 245-256.
- Martin MM, Lee EJ, Buckenberger JA, Schmittgen TD, Elton TS. MicroRNA-155 regulates human angiotensin II type 1 receptor expression in fibroblasts. J Biol Chem. 2006; 281: 18277-18284.
- Zheng L, Xu CC, Chen WD, Shen WL, Ruan CC, Zhu LM, et al. MicroRNA-155 regulates angiotensin II type 1 receptor expression and phenotypic differentiation in vascular adventitial fibroblasts. Biochem Biophys Res Commun. 2010; 400: 483-488.
- Li L, Zhang J, Diao W, Wang D, Wei Y, Zhang CY, et al. MicroRNA-155 and MicroRNA-21 promote the expansion of functional myeloid-derived suppressor cells. J Immunol. 2014; 192: 1034-1043.
- Röcken C, Neumann K, Carl-McGrath S, Lage H, Ebert MP, Dierkes J, et al. The gene polymorphism of the angiotensin I-converting enzyme correlates with tumor size and patient survival in colorectal cancer patients. Neoplasia. 2007; 9: 716-722.