
Research Article
J Immun Res. 2015;2(2): 1020.
Immunostimulatory- Fusogenic and Conventional Liposome Adjuvants Induce Qualitatively and Quantitatively Distinct Innate and Adaptive Immune Responses
Faisal SM1*, Scaria J2,3 and Chang YF2
1Laboratory of Zoonotic and Infectious Diseases, National Institute of Animal Biotechnology, Hyderabad, India
2Department of Population Medicine and Diagnostic Sciences, College of Veterinary Medicine, Cornell University, Ithaca, New York, USA
3Department of Veterinary and Biomedical Sciences, South Dakota State University, Brookings, SD 57007, USA
*Corresponding author: Faisal SM, National Institute of Animal Biotechnology, 4th Floor, Axis Clinical Building, Miyapur, Hyderabad, India
Received: May 23, 2015; Accepted: June 30, 2015; Published: July 02, 2015
Abstract
An ideal adjuvant should possess both immunostimulatory and antigen delivery properties for simultaneous induction of innate and adaptive immune response to clear pathogens from host. The majority of pharmaceutical based adjuvants currently being used are particle based delivery systems, such as liposome formulations. However, the mechanism of their action is largely unknown. To identify the regulatory gene cascade that triggers the innate immune response following liposome adjuvant injection, we applied microarray based transcriptional profiling of tissue sites (muscle and peritoneum) of mouse injected with conventional (CL) and immunostimulatory fusogenic liposomes (IFL). While CL and IFL induced large number of shared innate immune genes, IFL activated quantitatively and qualitatively stronger immune response than CL. IFL induced upregulation of pro-inflammatory response genes and triggered a rapid influx of antigen presenting cells (APCs) as compared to CL. Comparisonof gene interaction network revealed several fold increase of gene interactions at the injection site with IFL as compared to CL. The induction of innate response by IFL was correlated to strong adaptive response against encapsulated antigen ovalbumin (OVA) indicated by strong T cell proliferation and cytokine production.Notably, CL induced a biased Th2 response whereas IFL favored a predominant Th1 or mixed of Th1/ Th2 response. Our data indicate that IFL induced quantitatively and qualitatively distinct and strong innate immune signals which correlated to strong adaptive immune response. These results provide novel insights into understanding the mechanism of action of liposomes and may be utilized for development of improved liposome based vaccines.
Keywords: Liposome; Adjuvants; Immunostimulatory; Transcriptional gene profiling; Mouse model
Abbreviations
CL: Conventional Liposomes; IFL: Immunostiomulatory- Fusogenic Liposomes; OVA: Ovalbumin; APC: Antigen Presenting Cell; CTL: Cytotoxix T cell; MPLA: Monophosphoryl Lipid A
Introduction
It is well established that both the magnitude and the quality of the adaptive immune response largely depends on the efficient induction of the innate immune system [1-3]. The efficacy of traditional vaccines based on attenuated and live organisms is mainly attributed to two properties; their invasiveness which provides efficient delivery to antigen presenting cells (APCs) and presence of naturally occurring components of pathogen which stimulates innate immunity.
Thus the success of modern vaccines based on subunit antigens relies on inclusion of both immunopotentiators and delivery systems as adjuvants. Liposome seems to fulfill these criteria and have been widely studied as adjuvant/antigen delivery systems against various infections and have shown better performance than Freund’s adjuvant or alum [4-7]. The success of liposomes as vaccine adjuvants has been demonstrated against several diseases such as HIV [8-11] tuberculosis [6, 12, 13], malaria [3, 14, 15] and leshmaniasis [16, 17] indicating that liposomal systems have a real chance of becoming the standard for vaccine adjuvants of the future. Several liposome products have been licensed and others are in various phases of clinical trials [18-21]. Conventional liposomes find limited applications as they are inert or non-stimulatory (requires tagging with immunostimulants like LPS, toxins or cytokines) and fail to deliver antigen to the cytosol for MHC I presentation and subsequent induction of cytotoxic T cells (CTLs) response [22-24]. Several bacterial cell wall/membrane components or pathogen associated molecular patterns (PAMPs) such as Monophosphoryl Lipid A (MPL, detoxified LPS), Muramyl dipeptide (MDP), Trehalose Dimycolate (TDM) and Lipopeptides (P3CSS) have been widely exploited as immunepotentiators/ immunomodulators [25-28]. PAMPs induce robust innate response through TLR4 dependent mechanism that can promote Th1 response. TL4 based adjuvants like AS04 (Monophosphoryl Lipid A adsorbed on alum), GLA-SE (Glucopyranosyl Lipid A formulated with stable emulsion) have been used in various vaccines, inducing Th1 biased immune response [29-31]. Liposomes composed of total polar lipids (TPL) isolated from various non-pathogenic and/or attenuated bacteria like E. coli (escheriosomes), attenuated mycobacterial vaccine strain BCG (mycosomes), Archaebacteria (Archaeosomes), non-pathogenic Mycobacterium smegmatis (smegmosomes) and Leptospira biflexa (leptosomes) have shown to be very potent adjuvant/antigen delivery vehicles capable of inducing strong immune responses and significant levels of protection against various infections in animal models [3, 10, 32-39]. These liposomes being immunostimulatory and fusogenic were able to activate both innate and adaptive immune responses simultaneously. With increased understanding of immune response and considerable success of liposomal adjuvants their mechanism of action is largely unknown. While mechanism of most of the currently used adjuvants like alum, MF59, CpG are being explored by exploiting transcriptional gene profiling, only few studies have reported on adjuvant mechanisms of liposomes [31, 40-46]. Here, we have explored the adjuvant mechanism of liposomes by applying microarray based transcriptional profiling. Injection of conventional (CL) or immunostimulatory/fusogenic liposomes (IFL) at mouse muscle or peritoneum induced distinct differences in magnitude and quality of innate immune responses. A strong innate response correlated to antigen (OVA) specific adaptive response (Figure 1). While IFL induced high amounts of pro-inflammatory mediators and the influx of large numbers of various cell types leading to mixed Th1/ Th2 response, the CL induced a modest and biased Th2 response with the involvement of only few mediators and cell recruitment.
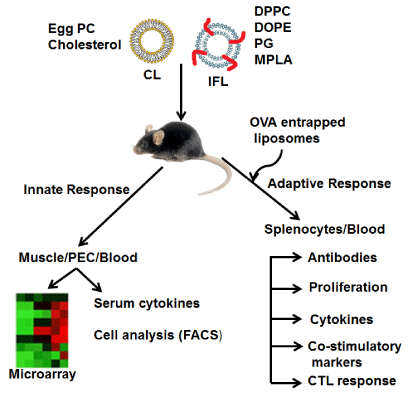
Figure 1: Schematic presentation of analyzing the immune response against
liposome adjuvant in mouse. Conventional and Immunostimulatory-fusogenic
liposomes were injected in mouse muscle or peritoneum. 24 hrs post injection
muscles from both quadriceps were excised or peritoneal exudate cells
(PECs) were harvested. Total RNA was isolated, converted to cDNA and
subjected to Microarray analysis. Serum collected at various time points
from mice injected with liposomes in muscle or peritoneum were analyzed
for cytokines using ELISA kits. PECs collected at various time points were
subjected to cell analysis by FACS. In separate experiment mice were
injected with OVA entrapped liposomes intraperitoneally (Day 0 and 14) and
antigen specific adaptive response was analyzed at day 14 and 21 in terms of
antibody response, T cell proliferation, cytokines, expression of costimulatory
molecules and CTL response.
Materials and Methods
Animals and reagents
Female C57BL/6 mice (Jackson laboratories) 6 to 8 weeks old were used in the study. The animals were maintained and treated under specific pathogen-free conditions. The experiments were conducted according to protocol approved by Institutional Animal Care and Use Committee (IACUC). The J774A.1 (macrophage) cell lines, EL-4 and EG.7 (EL-4 cell line stably transected with the OVA gene) were obtained from ATCC and maintained in their recommended medium. Ovalbumin (OVA), Mytomycin C, Phosphotidylcholine (PC), Cholesterol, Dipalmitoyl phosphotidylcholine (DPPC), Diolyl phosphotidylethanolamine (DOPE), Phosphatidylglycerol (PG), Monosphosphoryl Lipid A (MPLA), L-phophatidylethanolamine- N-(4-nitrobenzo-2-oxa-1,3-diazole) (NBD-PE) and N-(lissamine rhodamine B sulfonyl) phosphatidylethanolamine (Rh-PE) were purchased from Sigma Chemical Co., USA. All the antibodies used for FACS analysis and cytokine ELISA kit (Ready Set Go) were purchased from eBiosciences, San Diego, USA.
Liposome preparation and characterization
Liposomes were prepared as described previously [39, 47]. For preparing liposomes total 20mg lipids [IFL (10mg DPPC, 8mg DOPE, 2mg PG and16 μg MPLA) or CL (16mg Egg PC and 4mg cholesterol)] were dissolved in methanol/chloroform, reduced to a thin dry film in a rotary evaporator, and left to stand for 30 min in a high vacuum desiccator. The film was hydrated, sonicated and lyophilized and then reconstituted with PBS. For preparing antigen entrapped liposomes, the film was hydrated, sonicated, mixed with an equal volume of OVA (2mg/ml) and lyophilized. The free flowing dried powder was then hydrated with distilled water and washed thrice to remove unincorporated antigen. The liposomes were finally suspended in PBS and the amount of antigen entrapped was estimated using a BCA protein assay kit (Pierce, USA). The liposomes were characterized by Transmission Electron Microscopy (TEM) as described previously [5]. To determine fusogenic potential, liposomes (CL or IFL) were fluorescently labeled by incorporating fluorescent probe l-(phosphatidylethanolamine-N-(4- nitrobenzo-2-oxa-1,3-diazole) NBD-PE (5 mol %) and incubated with J774 A1 cells for 2hrs. After washing the fixed macrophages were observed under fluorescence microscope [34]. Fusogenicity of liposomes was further confirmed by Resonance energy transfer (RET assay) as described previously [34]. Lipid mixing was monitored between NBD-PE /Rh-PE (N- (lissamino6o; se rhodamine B sulfonyl) Phosphatidylethanolamine) labeled IFL or CL and unlabeled lipids. Finally, fluorescence was monitored at 520nm and RET efficiency was calculated as previously described [34]. Dequenching assay was performed by mixing NBD probed and unprobed vesicles in a molar ratio of 1:10 in the presence of 5mM Ca2+ and incubating at 370C for varying time periods. Fluorescence was measured spectrofluorometrically and dequenching was calculated as previously described [34].
Evaluation of immune response
Liposome specific innate responses: C57BL/6 mice were injected intramuscularly (i.m., 50μl) or intraperitoneally (i.p., 200μl) with PBS or CL or IFL. Mice were bled and euthanized at various timepoints (0, 1hr, 6hr, 12hr and 24hr) and the peritoneal exudate cells (PECs) were harvested by washing the peritoneal cavity as follows: 2 ml of cold RPMI containing 10% FBS was injected, the abdomen was massaged gently for 30 seconds and 1 ml was extracted. The cells were spun down, washed and stained for flow cytometry. The peritoneal lavage fluid was stored at -200C for analysis of cytokines/ chemokines by ELISA. For the microarray analysis, the PECs (at 24hr time point) were put in RNA later immediately after extraction from the peritoneal cavity and stored at -200C until required for isolation of RNA. Similarly muscle tissues were excised; RNA was isolated later. Antigen specific adaptive response: C57BL/6 mice (5 mice per group) were immunized intraperitoneally at Day 0 and 14 with (1) PBS, (2)20μg OVA in 200 μl of PBS (Free OVA), (3) 20μg OVA entrapped in CL (CL-OVA), (4) 20μg OVA entrapped in IFL (IFLOVA), (5) 20μg OVA mixed with CL, (6) 20μg OVA mixed with IFL. Animals were euthanized on day 14 and 21 and spleens were taken out aseptically. Single-cell suspensions were made and evaluated for proliferation (BrDU incorporation and CFSE), analysis of surface markers (CD80, CD86 and MHCII) and cytokine estimation in culture supernatant after in vitro stimulation with 10μg/ml of free OVA or entrapped in liposomes. The mice were bled at various time points and the serum was analyzed for OVA specific antibodies.
Proliferation assay
Splenocytes (5 ×105) isolated from mice euthanized at day 14 and 21 post immunization with CL-OVA or IFL-OVA were stimulated with varying dose (0.1, 1 and 10μg/ml) of free OVA or entrapped in liposomes in 200μl RPMI for 72hrs at 370C in a humidified atmosphere supplemented with 5% CO2 [39]. The proliferative response was measured by cell proliferation ELISA, BrdU colorimetric kit (Roche Diagnostics, Indianapolis, IN) as per the manufacturer’s protocol. The results are expressed as stimulation index (SI), and the error bars indicate standard deviation from the mean. Proliferation was also assessed by CFSE dilution following standard procedures [48]. Briefly, 106 cells/ml were stained with CFSE (10μM) at 370C for 10 min. Unentrapped dye was quenched with FBS and then cells were washed with RPMI and then stimulated with 10μg/ml antigen. The proliferative response was determined by dye dilution using flow cytometry.
Cytokine analysis
The cytokines CCL2, IL-6, TNF-α, IL-5 in serum and peritoneal lavage and IL-4, IL-10, IFN- γ and IL-2 in culture supernatant was analyzed using the specific Ready-Set-Go cytokine ELISA kit (eBiosciences) following the manufacturer’s instructions.
Flow cytometry
PECs and splenocytes were washed and Fc receptors were blocked with anti-mouse CD16/CD32 antibody in FACS buffer. The cells were kept at 40C during staining. The PECs were stained with anti-mouse CD19 (B cells), CD11c (DCs), CD11b/F4/80 (Monocytes/ Macrophages), CD3/CD4/CD8 (T cells), Ly6G (Neutrophils) and CD49b (NKcells). Splenocytes were stained with CD3/CD4/CD8 to access proliferating cell types and CD80, CD86 and MHCII for analysis of surface markers. 10,000 cells of PEC and 50,000 cells splenocytes were collected on LSRII (BD). All data were analyzed by using FlowJo software (Tree Star Inc.)
Humoral response
Detection of OVA specific antibodies in the sera from immunized mice was evaluated by the Kinetic Enzyme-Linked Immunosorbent Assay (KELA) as described previously [37, 38]. In short, 96-well MaxiSorp plates were coated with 100μl of OVA (10μg/ml in carbonate buffer, 0.1 M, pH 9.6) at 4oC overnight. The plate was washed with 0.1M PBS containing 0.05% Tween 20 (PBST) and subsequently blocked with 200μl/well of 1% BSA for 2 hrs at RT. After washing, 100μl of serum diluted 1:200 in PBST was added to each well and incubated for 1 h at 37°C in a humid chamber. The plate was washed and incubated with 100 μl of a 1:2,000 dilution of peroxidase labeled goat anti-mouse IgG (KPL) or peroxidase labeled anti-mouse IgG1 and IgG2a (Santa Cruz Biotech) for 30 min at RT. After extensive washing, 100μl of TMB ready to use substrate was added to each well. The plate was read three times at 1-min intervals at 650nm on an ELISA reader. The results were calculated by the KELA computer program and expressed as KELA units (KU).
CTL assay
The cytotoxicity assay was performed using the LDH method as described previously [39, 49]. Briefly, 3×107 spleen cells were cultured with 5×105 mytomycin C treated (50μg/ml for 45 min) EG.7 cells in 10 ml of RPMI plus 10% FBS containing 0.2 ng/ml IL-2, in 25-cm2 tissue culture flasks kept upright. After 5 days at 370C in a humidified atmosphere supplemented with 5% CO2, the non-adherent cells were recovered from the flask and used as effector cells. The cells were counted and incubated with EG.7 (specific target) or EL-4 (nonspecific target). The
reaction mixture was set up with varying E:T ratios (10:1, 25:1, 50:1) for 5 h at 370C in a humidified atmosphere supplemented with 5% CO2 and lysis of target cells was determined using a nonradioactive cytotoxicity assay kit (Cytotox 96, Promega) following the manufacturer’s instructions. Specific target cell lysis by CTLs was calculated as the percentage of total LDH activity of target cells as follows: % specific release = (experimental release- spontaneous release)/(maximum release-spontaneous release)
RNA isolation and microarray analysis
RNA was isolated by using combination of Trizol and RNA easy mini kits (Qiagen) to enhance yield and purity. Briefly, muscle tissues (fine chopped) and PECs were put in 1ml Trizol and bead beated for 1 min. 500μl chloroform was then added and centrifuged at 10,000rpm for 15 min. The clear supernatant was then passed through RNA easy column and purified according to manufacturer’s protocol. RNA concentration and quality was assessed using nanodrop Spectrophotometer and Agilent Bioanalyzer 2100. Total RNA (20μg) from each animal was used as templates in RT reactions. Synthesis of cDNA and Cy3/Cy5 dye labelling was performed following the protocols previously described [50]. Labeled cDNAs were extensively purified to remove unincorporated dyes, combined, and concentrated using Microcon 30 spin concentrators. Microarrays were scanned using Agilent G2565BA scanner. Data was then extracted using Agilent’s feature extraction image analysis software v 10.2. Resulting data files was then loaded to Gene Spring GX v7.1 (Agilent Technologies, CA). The net intensities were log2 transformed, within chip normalized using lowess algorithm, and quantile normalized across all chips using the Genespring Gx v7.1. The mean normalized log2 ratio and standard deviation was calculated from all replicates. All subsequent data analyses were performed using Microsoft Excel and Genespring Gx v7.1. The paired 2-sample t-test was performed to identify differentially expressed genes. Significantly up- and down-regulated genes in each comparison group were identified by applying a P value of <0.05. Selected differentially expressed genes from microarray results were validated by qRT-PCR. Differentially expressed genes were further analyzed using Ingenuity Pathway Analysis (IPA) (Ingenuity Systems, Redwood City, CA, USA) to enable identification of functionally related and interacting transcripts in pathways and networks. IPA organizes genes into networks based on relationships and interactions extracted from peer-reviewed scientific literature.
Statistical analysis
The statistical analysis was performed by using the Student’s t test with Excel software. A p value of <0.05 was considered statistically significant.
Results
Characterization of liposomes
The IFL varied in size with average size of 241±117 nm whereas CL was of average size of 147±29nm. The antigen entrapment efficiency of IFL was 56.2±2.1 % whereas CL had efficiency of 61.4±4.2 %. The fusion potential of the liposomes was confirmed by monitoring the transfer of fluorescent probe from liposome to the living cells (J774 A1). The results demonstrated that interaction with CL with J774 A1 followed an endocytosis mode and resulted in a punctate type of fluorescence, while IFL fused with target cells leading to fluorescence being associated with the membrane (Figure 2A). The fusogenic potential was further assessed by monitoring the mixing of lipids as determined by Resonance Energy Transfer (RET) and Dequenching assays. Our results demonstrate that unlike CL, IFL caused a significant reduction (p < 0.05) in RET with time (Figure 2B). The dequenching assay further validated the RET assay, where around 54% dequenching in IFL was observed after 60 min suggesting that vesicles have undergone fusion (Figure 2C).
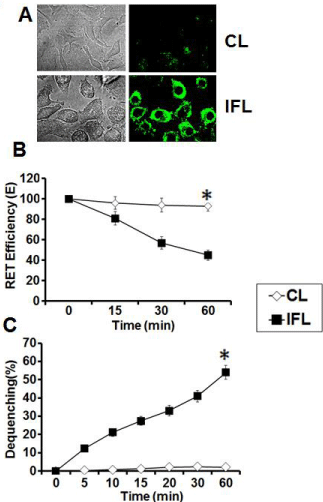
Figure 2: Fusogenic potential of liposomes. (A) Phase contrast and
fluorescence light micrograph of interaction of NBD –PE labeled CL and
IFL with J774A.1 cells (B) The efficiency of energy transfer between NBD
and Rhodamine incorporated on the surface of various liposomes (CL and
IFL) upon mixing with unlabeled probe as assessed by Resonance energy
transfer (RET) assay. (C) Interaction of various NBD- labeled liposomes
with their unlabeled counterparts as assessed by Dequenching assay. The
data represent mean of three determinations ± S.D and are representative of
three independent experiments with similar observations. *indicates p value
is <0.03.
Conventional and immunostimulatory-fusogenic liposomes induced distinct innate immune signals at the site 24hrs post injection
The innate immune response/signals induced 24hrs post injection by conventional (CL) or Immunostumulatory-Fusogenic Liposomes (IFL) was investigated at the site of injection. Peritoneal exudate cells (PECs) were collected at various time points from animals receiving liposome injection in the peritoneum. PECs were analysed by surfacestaining for markers of different cell types. Both CL and IFL induced similar pattern of recruitment of B cells, T cells and DCs at early time points (<6hrs) after injection, however IFL induced higher number B cells at later time point ( 12 and 24 hrs). IFL also induced recruitment of significantly high (p < 0.05) number of monocytes and neutrophils than CL at later time points (Figure 3A, 3B). In naïve mice (before adjuvant injection, t=0hrs), significant number of B cells, T cells and DCs were present in the peritoneal cavity but their numbers rapidly declined after injection of the CL or IFL. Both CL and IFL induced similar recruitment of NK cells however their numbers were higher in latter at 24hs post injection (Figure 3A, 3B). We evaluated the kinetics of the serum cytokine responses in animals injected with liposomes in muscles. Cytokines generated at the injection site can diffuse to other sites. Thus, Liposome-induced cytokine proteins could be detected even if their mRNAs are not detected in the blood. The level of evaluated cytokines (IL-5, IL-6, CCL2, TNF-α) increased rapidly following injection of both CL and IFL and level peaked at 6hrs (Figure 3C). However level induced by IFL was significantly higher (p < 0.05). IFL induced robust CCL2 and TNF-α when injected intramuscularly however this level was low when injected intraperitoneally. The serum cytokine levels were robust in animal injected i.m. with IFL as compared to levels in animals receiving i.p. injection where the response was delayed and low except IL5.
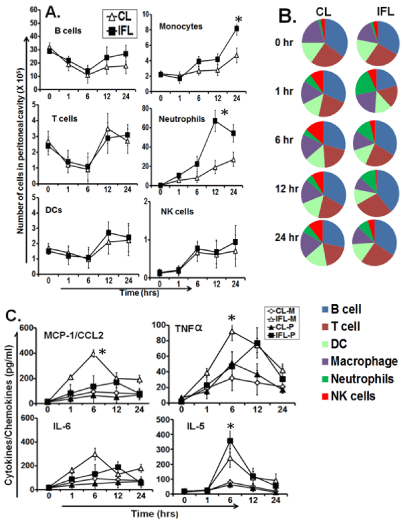
Figure 3: Adjuvant specific immune response after intramuscular or intraperitoneal injection of liposomes. (A) Kinetics of lymphocytes in peritoneal exudate
cells (PECs) at the indicated time-points after intraperitoneal (i.p.) injection of either CL or IFL as analyzed by flow cytometry. (B) Pie chart showing percent fraction
of lymphocytes accessed at various time points after liposome injection. (C) Cytokines/chemokines levels measured in serum and peritoneal lavage at indicated
time points after liposome injection using sandwich ELISA kits from eBiosciences (USA). *indicates p value is <0.05.
Microarray analysis revealed differences in gene expression profile induced by CL and IFL adjuvants
We also investigated the expression of genes for cytokines/ chemokines, their receptors, MyD88, TRIF, B and T cells and antigen processing and presentation in a whole genome microarray analysis on mRNA extracted from the muscle and PECs of mice injected with the CL or IFL 24 hr previously (Figure 4). This single time-point was chosen to make sure that enough number of cells was recruited at site of injection and significant number of genes activated which gives confidence to do microarray analysis on individual mice. Microarray analysis shows that a significant change in expression of immune related genes was observed 24 hrs after liposome injection. The most strongly induced transcripts included cytokines/chemokines, cytokine receptors and signaling molecules, and molecules involved in complement and antigen presentation pathways. A total of 157 genes have been selected with an average log2 ratio 3 compared with PBS control and a P value <0.05 calculated on the three replicates. Out of these 157, there were 36 hypothetical genes while 121 were annotated during the immune response. Among the treatments IFL had the most impact on expression of more than 82 genes (Figure 4A). IFL induced the expression of several granulocyte and monocyte chemoattractant and activators (Ccl3, Ccl4, Ccl7 and Ccl12), range of NK and T cell attracting C-X-C chemokines (CXCL9, CXCL10, CXCL11 and the C-C chemokine CCL5. IFL also induced significant number and high expression TRIF dependent genes (ifit3, irf7, mx1, oasl1, oasl2) and few MyD88 dependent genes (irg1). In contrast CL induced only few genes related to chemokines (CXCL9, CXCL10 and CXCL13), cytokine receptor (TNFRSF13c) and TRIF pathway (GBP3, IFIT, IRF7) and their level was significantly lower than IFL (Figure 4B). IFL induced enhanced expression of T and B cell recruiting chemokines. Th1 cells recruiting chemokines, CXCL9 and CXCL10 were highly upregulated in muscle of IFL treated mice. There was enhanced expression of B cell recruiting chemokine gene CXCL13. T cells and APCs chemokine receptors (CCR2, CCR5 and CSF3R) were also highly induced by IFL. Genes involved in antigen processing and presentation (B2M, C3AR1, FCGR1, H2-K1, H2- M3, H2-T22, TAP1) were also upregulated by IFL. In contrast CL induced at very low levels or failed to induce genes related to antigen processing and presentation (Figure 4B). The majority of immune response related gene upregulated strongly after IFL injection at muscles induced at low levels or failed to induce at peritoneum at 24hr time point analysis. Few genes related to TRIF (IFIT3, IRF7, OASL2), cytokine receptor (CXCR2, ILIF9, IL13RA2), cytokines/ chemokines genes (CCL12, CXCL5, IL1RN, SPP1, TNF) and antigen processing (FCGR1) were induced by IFL at peritoneum. Pathway analysis revealed that IFL induced significant upregulation of genes related to five biologically important pathways viz Inflammatory response, T cell receptor signaling, Cytokine and Inflammatory response, IL-6 signaling and TNF-α and NFкь signaling at least in mouse muscle 24hrs after injection (Figure 4C). The observed effect of the adjuvants was not caused by the injection procedure and related tissue injury because injection of physiological saline did not induce cell recruitment (data not shown). In order to confirm some of the array results we performed RT-PCR for five genes namely TLR-1, CCR5, CCL4, CXCL10 and IL-6. Four genes (TLR-1, CCR5, CCL4, CXCL10) out of five displayed expression levels comparable to those of the microarray. One gene (IL-6) whose expression was not detected by microarray was detected through RT-PCR.
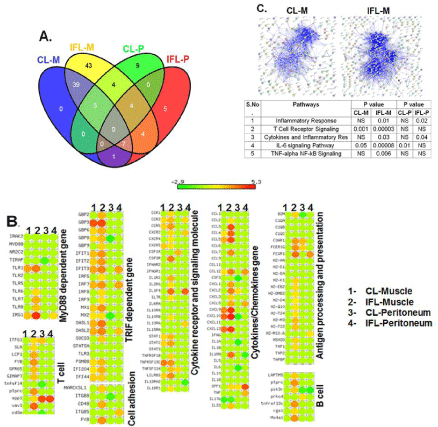
Figure 4: Microarray analysis of liposome specific immune response in muscle and peritoneum 24 hrs. Post injection. (A) Venn diagram showing distribution
of genes modulated by CL and IFL in muscle and peritoneum where CL-M and IFL-M indicates muscle injected with conventional and Immunostimulatoryfusogenic
liposomes respectively. CL-P and IFL-P indicates peritoneum injected with these liposomes. (B) Differentially expressed genes in muscle and PECs
filtered on fold change (3.0, p < 0.05) 24 h post injection. The genes significantly up-regulated after injections of CL or IFL are represented in a clustered heat map
based on the average gene-expression intensities of three individual mice per group. The heat map is colored according to Z-scores of log-transformed intensity
values, with red indicating Z-scores > 0, green indicating < 0 and yellow indicating ∼0. (C) Pathway analysis indicating upregulated processes and the interaction
network among the differentially expressed genes.
CL and IFL induced quantitatively and qualitatively distinct antigen specific adaptive immune response
To test whether differences in innate adjuvant activity at injection site correlated to antigen specific adaptive response we analyzed both humoral and cell mediated immune responses against the model antigen ovalbumin (OVA) delivered in CL or IFL. The antibody response analyzed at day 14 and 21 shows significantly higher levels of IgG were generated in animals immunized with IFL-OVA (p < 0.05). Both CL-OVA and IFL-OVA induced IgG1 but levels were significantly higher in latter (Figure 5A). IgG2a was only detected in animals immunized with IFL-OVA. Animals immunized with a simple mixture of OVA mixed with CL or IFL did not develop significant levels of IgG or any IgG isotype as compared to controls (data not shown). Splenocytes from animals immunized with IFLOVA exhibited significantly higher (p < 0.05) levels of proliferation as compared to CL-OVA as revealed by BrDU incorporation. This was confirmed using CFSE proliferation assay which showed that significantly higher (p < 0.05) numbers of both CD4 and CD8 T cells population from animals immunized with IFL- OVA expanded in response to in vitro stimulation with OVA at Day 14 and 21 (Figure 5B). Both the liposomes induced similar level of Th2 cytokines (IL-4, IL-10) into the culture supernatant, however Th1 cytokine (IFN-γ, IL-2) was only induced by IFL-OVA (Figure 5C). Immunization with IFL-OVA also caused activation and maturation of APCs as revealed by expression of costimulatory molecules (CD80, CD86) and maturation marker (MHCII) on spleen macrophages/monocytes (Figure 5D). Our results further show that higher target cell (EG.7) lysis (∼45%) was demonstrated by CTLs obtained from animals immunized with IFL-OVA as compared to CL-OVA (<10%) at the highest E: T ratio (Figure 5E). Effectors obtained from both CL-OVA and IFL-OVA were not able to lyse non-specific target (EL-4 cells), further confirming the specificity of CD8 T cells (CTLs). Free OVA or OVA physically mixed with CL or IFL induced insignificant or very low level of adaptive immune response (data not shown). Thus the two liposomal adjuvants induced qualitatively and quantitatively distinct immune responses.
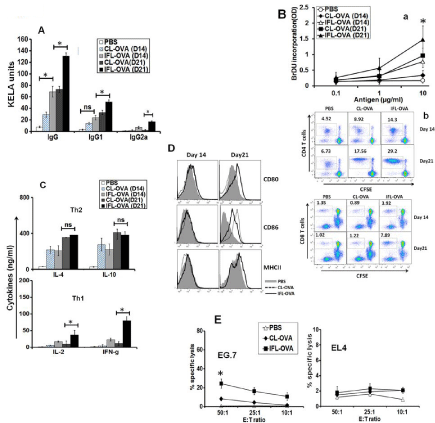
Figure 5: Antigen specific adaptive response. (A) Antibody response- The serum collected on day 14 and 21 from various groups was evaluated for IgG, IgG1
and IgG2a by KELA as described in materials and methods (B) T cell proliferation- (a) Proliferation as measured by the incorporation of bromodeoxyuridine (BrdU)
using cell proliferation ELISA and expressed as SI as described in material and methods (b) Proliferation as measured by dilution of CFSE dye. (C) Cytokine
estimation-The culture supernatants were collected after 72 hrs and analyzed for cytokines (IL-2, IL-4, IL-10, IFN-γ) using specific Ready-Set-Go ELISA kit
according to the manufacturer’s instructions. (D) Analysis of co-stimulatory molecules- Cells were recovered and stained with FITC labeled CD80, CD86 or
MHCII antibodies, acquired on LSRII and then analyzed by FlowJo. (E) Cytotoxicity (CTL assay)- Effectors generated in vitro from splenocytes isolated from various
groups were added to specific (EG.7) or nonspecific (EL-4) targets in varying E: T ratios. Cytotoxicity was measured using a Cytotox kit (LDH method) following
the manufacturer’s instructions. *indicates p value is <0.05.
Discussion
Being associated with several limitations Alum is the only approved adjuvant since several decades, hence there is urgent need for development of new and improved vaccine adjuvants [51-55]. Freund’s adjuvants (FA) although are very potent, the mineral oil cannot be metabolized and the mycobacterial elements cause severe systemic reactions and local ulcerations. Further, the immune response to protein antigens emulsified in FA may be lowered or suppressed [56]. Liposomes have advantage over FA as they are biodegradable, non-toxic and induce strong immune response for longer period of time. While liposomes have initially reached the market as drug carriers, their potential as potent vaccine adjuvant has been demonstrated against various infectious and life threatening diseases, indicating that liposomes hold high promise in becoming vaccine adjuvants of future [5-7, 57, 58]. With an increased understanding of the type of immune response required to combat various diseases it has become possible to retro-engineer adjuvants to a particular disease. Although much success has been shown by liposomes, the mechanism of their stimulating effect on innate immunity has been studied inadequately. In particular their global effects on gene transcription and the complex regulatory machinery in the cell that leads to enhanced immune responses are poorly understood. Liposomes are considered to be sensitive adjuvants. Small changes in their properties (lipid composition, size, charge) may induce radically different immune responses. Thus, availability of immunological profile of a liposome with a particular charge, size and lipid composition would enable rational retro- design of liposomal vaccine adjuvants.
In the current study we have used microarray based gene transcription profiling which is an attempt towards this approach. Microarray based gene expression by DOTAP (1,2-dioleoyl-3- trimethylammoniumpropane) liposome treatment was first study of liposome induced gene expression profiling [43]. Recently Korsholm et al have exploited microarray to compare immune response induced by DDA/MPL liposomes (Th1 adjuvant) with Th2 adjuvant, alum [42]. Thus development of a common database for the immunoprofile of liposomes would provide researchers an essential tool for the retroengineering of liposomal adjuvants. Our previous studies have demonstrated that liposomes composed of fusogenic and immunostimulatory lipids derived from non-pathogenic bacteria are efficient adjuvants capable of inducing both antibody and cell mediated immunity (CMI) correlating to protection against infection in animal model [37-39]. Here we compared innate immune pathways activated by immunostimulatory and fusogenic liposomes (made of fusogenic lipid PE and incorporating immunostimulatory adjuvant monophosphoryl lipid A MPLA) to non-stimulatory conventional liposomes to understand how this innate response at site of injection correlates to adaptive response. Conventional PC liposomes containing MPLA have shown considerable potency and safety in human trials with a variety of candidate vaccines [57]. In the current study MPL containing fusogenic liposomes (IFL) induced significantly higher amounts of pro-inflammatory mediators and the influx of large numbers of various types of immune cells whereas the conventional liposomes (CL) induced a response which was associated with the recruitment of lower number of these cells at the site of injection. Although IFL induced recruitment of B cell, T cell and DCs in significantly higher number as compared to CL after 6hrs post injection, their number rapidly declined within 6 hrs of injection in both CL and IFL. This phenomenon is difficult to explain; however it is likely that trafficking of liposomes to draining lymph nodes have led to migration of these cells from peritoneum. Some previous studies which reported the similar observations after adjuvant injection have not discussed the mechanism involved in rapid decline of these cells [42, 59]. The cluster of genes modulated by both CL and IFL named “liposome core response genes” was characterized by upregulation of cytokine, chemokines and adhesion molecules suggesting that liposome adjuvanticity is partly associated with nonpathogenic inflammatory process. Microarray analysis further revealed that innate response was robust at muscle injection site whereas it was lower and much delayed within the peritoneum. The kinetics and magnitude of the overall alterations in gene expression differed between the two liposomes (CL and IFL). While IFL induced a change in expression of large number of immune related genes at 24hrs post injection the response to CL was more modest and transient, with significantly lesser number of genes modulated at the same time point. However, not much difference in the level of gene expression induced by between CL and IFL at 24hrs post injection was observed in the peritoneum indicating that response is delayed or these genes might have expressed at later time points (excluded from analysis). IFL induced enhanced expression of TRIF dependent gene which is in agreement with previous reports of MPLA being TRIF biased TLR agonist [60]. IFL induced enhanced expression of T and B cell recruiting chemokines. Th1 cells recruiting chemokines, CXCL9 and CXCL10 were highly upregulated in muscle of IFL treated mice which is in agreement with similar TLR agonist adjuvant GLA inducing these chemokines [41]. There was enhanced expression of B cell recruiting chemokine gene CXCL13 by IFL in muscles indicating that IFL can induce both B and T cell responses. In addition, Monocyte and macrophage recruiting chemokines (CCL3, CCL4, CCL5, CCL7 and CCL12) were also induced by treatment with IFL. IFL induced expression of STAT (signal transducer and activator of transcription) genes coding family of proteins that are key players in the regulation of many immune processes. These proteins mediate cytokine signaling and are critically involved in the differentiation of both activated T and B cells [61]. IFL induced significantly higher level expression of genes related to important immune pathways like inflammatory response, T cell receptor and cytokine signaling. It is likely that IFL, being small size particles, can traffic quickly in lymph nodes following injection in muscles and can cause induction of immunostimulatory genes that can promote migration of responding immune cells [62]. Like other adjuvants viz. MF59 and CpG, IFL also activated muscle fiber and caused activation of immune related genes indicating that these liposomes have potential to be used in intramuscularly injected human vaccines [40]. To gain better insight to liposome adjuvanticity we measured adaptive response in C57/BL6 mice against model antigen OVA entrapped in CL or IFL. The strong innate immune activity of IFL correlated to enhanced antigen specific adaptive immune response. Activation through TLR4 induces TNF-α, cc chemokines (CCL-3, CCL-4, CCL-5) and cxc chemokines like CXCL2 (MIP-2), CXCL9, CXCL10 expression as induced by IFL in the current study that might have led to recruitment of inflammatory monocytes resulting in macrophage differentiation [63-65]. These inflammatory monocytes might differentiate into DCs, thus providing a source of APCs capable of initiating subsequent antigen-specific adaptive responses [66]. The strong fusogenicity of IFL coupled with the ability to enhance expression of genes related to antigen processing and presentation like B2M, FCGR1, H2, TAP correlated to strong T cell proliferation and Cytotoxic T cell response to recall antigen OVA (Figure 5). The ability of CL to induce Th2 and IFL to induce Th1 biased or mixed Th1/Th2 immune responses may partly be attributed to their sizes, as small size liposomes (~100nm) induce humoral response and large size (~400nm) induce cell mediated immunity (CMI) [67]. Furthermore, the ability of IFL to induce both Th1 and Th2 types of immune response simultaneously can be explained on the basis that besides being phagocytosed by APCs and targeted to endosomes a fraction of IFL might also fuse leading to delivery of antigen to cytosol, thereby processing through proteosome machinery and presentation through MHCI molecules leading to activation of CD8T cell response. This is in agreement with several previous studies demonstrating the potential of fusogenic liposomes to target antigen to both the endosomal and cytosolic pathways thereby inducing both CD4 and CD8 T cell responses [36, 39]. IFL also induced enhanced expression of costimulatory molecules (CD80, CD86) and maturation marker (MHCII) on APCs; however these genes were not detected at 24 hr time point in microarray analysis. It is likely that these genes expressed at later time points. Although the number of genes and their expression levels were much lower in peritoneum than muscle 24hrs post IFL injection, the strong adaptive response after ip injection of IFL-OVA indicates that response in peritoneum was delayed and these genes were expressed at later time points. It would have been more appropriate if we would have determined the kinetics of liposome specific innate response by analyzing the gene expression at various time points. The limitations associated with single time point gene expression analysis as presented in the current study was partly compensated by analyzing the response at two injection sites (muscle and peritoneum) and our data clearly indicates that strong innate response activated at site of liposome injection correlated to adaptive immune response. To our knowledge, this is the first study of transcription profiling of liposome adjuvants in mouse muscle and peritoneum. The results provide new insights into understanding the mechanism of action of their adjuvanticity. We expect that our findings together with further analysis of molecular events underlying the adjuvant action of liposomes will pave way for a better understanding of key molecular components of immunomodulation and for the future development of liposome based vaccine formulations.
Acknowledgments
This work was supported partly from Grant No- BT/RLF/2012 (SP003-NIAB) funded by Department of Biotechnology, Ministry of Science and Technology, Government of India and partly from Biotechnology Research and Development Corporation (BRDC),USA. The authors would like to thank Director, National Institute of Animal Biotechnology (NIAB) for providing necessary infrastructural facility for execution of the above study.
Author’s Contribution
Conceived and designed the experiments: SMF, JS and YFC. Performed the experiments SMF and JS. Immunological data analysis: SMF Microarray data analysis: JS. Wrote the manuscript: SMF, JS and YFC.
References
- Medzhitov R, Janeway CA Jr. Innate immunity: the virtues of a nonclonal system of recognition. Cell. 1997; 91: 295-298.
- Fearon DT, Locksley RM. The instructive role of innate immunity in the acquired immune response. Science. 1996; 272: 50-53.
- Sharma SK, Farah D, Misra-Bhattacharya S, Bajpai P, Agarwal A, Mohammad O. Escheriosome entrapped soluble blood stage antigens impart protective immunity against a multi-drug resistant isolate of Plasmodium yoelii nigeriensis in BALB/c mice. Vaccine. 2006; 24: 948-956.
- Alving CR. Immunologic aspects of liposomes: presentation and processing of liposomal protein and phospholipid antigens. Biochim Biophys Acta. 1992; 1113: 307-322.
- Faisal SM, Yan W, McDonough SP, Chang YF. Leptospira immunoglobulin-like protein A variable region (LigAvar) incorporated in liposomes and PLGA microspheres produces a robust immune response correlating to protective immunity. Vaccine. 2009; 27: 378-387.
- Fries LF, Gordon DM, Richards RL, Egan JE, Hollingdale MR, Gross M, et al. Liposomal malaria vaccine in humans: a safe and potent adjuvant strategy. Proc Natl Acad Sci U S A. 1992; 89: 358-362.
- Gregoriadis G. The immunological adjuvant and vaccine carrier properties of liposomes. J Drug Target. 1994; 2: 351-356.
- Lian T, Ho RJ. Trends and developments in liposome drug delivery systems. J Pharm Sci. 2001; 90: 667-680.
- Sakaue G, Hiroi T, Nakagawa Y, Someya K, Iwatani K, Sawa Y, et al. HIV mucosal vaccine: nasal immunization with gp160- encapsulated hemagglutinating virus of Japan-liposome induces antigen-specific CTLs and neutralizing antibody responses. Journal of immunology. 2003; 170: 495-502.
- Ahmad N, Khan MA, Owais M. Liposome mediated antigen delivery leads to induction of CD8+ T lymphocyte and antibody responses against the V3 loop region of HIV gp120. Cell Immunol. 2001; 210: 49-55.
- Akagi T, Ueno M, Hiraishi K, Baba M, Akashi M. AIDS vaccine: Intranasal immunization using inactivated HIV-1-capturing core-corona type polymeric nanospheres. Journal of controlled release : official journal of the Controlled Release Society. 2005; 109: 49-61.
- Chambers MA, Wright DC, Brisker J, Williams A, Hatch G, Gavier-Widen D, et al. A single dose of killed Mycobacterium bovis BCG in a novel class of adjuvant (Novasome) protects guinea pigs from lethal tuberculosis. Vaccine. 2004; 22: 1063-1071.
- Yoshida S, Tanaka T, Kita Y, Kuwayama S, Kanamaru N, Muraki Y, et al. DNA vaccine using hemagglutinating virus of Japan-liposome encapsulating combination encoding mycobacterial heat shock protein 65 and interleukin-12 confers protection against Mycobacterium tuberculosis by T cell activation. Vaccine. 2006; 24: 1191-1204.
- Peek LJ, Middaugh CR, Berkland C. Nanotechnology in vaccine delivery. Adv Drug Deliv Rev. 2008; 60: 915-928.
- White K, Krzych U, Gordon DM, Porter TG, Richards RL, Alving CR, et al. Induction of cytolytic and antibody responses using Plasmodium falciparum repeatless circumsporozoite protein encapsulated in liposomes. Vaccine. 1993; 11: 1341-1346.
- Badiee A, Jaafari MR, Khamesipour A, Samiei A, Soroush D, Kheiri MT, et al. The role of liposome charge on immune response generated in BALB/c mice immunized with recombinant major surface glycoprotein of Leishmania (rgp63). Exp Parasitol. 2009; 121: 362-369.
- Bhowmick S, Ravindran R, Ali N. gp63 in stable cationic liposomes confers sustained vaccine immunity to susceptible BALB/c mice infected with Leishmania donovani. Infect Immun. 2008; 76: 1003-1015.
- Stewart VA, McGrath SM, Walsh DS, Davis S, Hess AS, Ware LA, et al. Pre-clinical evaluation of new adjuvant formulations to improve the immunogenicity of the malaria vaccine RTS,S/AS02A. Vaccine. 2006; 24: 6483-6492.
- Sanchez Y, Ionescu-Matiu I, Dreesman GR, Kramp W, Six HR, Hollinger FB, et al. Humoral and cellular immunity to hepatitis B virus-derived antigens: comparative activity of Freund complete adjuvant alum, and liposomes. Infect Immun. 1980; 30: 728-733.
- Ambrosch F, Wiedermann G, Jonas S, Althaus B, Finkel B, Glück R, et al. Immunogenicity and protectivity of a new liposomal hepatitis A vaccine. Vaccine. 1997; 15: 1209-1213.
- Vandepapelière P, Horsmans Y, Moris P, Van Mechelen M, Janssens M, Koutsoukos M, et al. Vaccine adjuvant systems containing monophosphoryl lipid A and QS21 induce strong and persistent humoral and T cell responses against hepatitis B surface antigen in healthy adult volunteers. Vaccine. 2008; 26: 1375-1386.
- Moore MW, Carbone FR, Bevan MJ. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell. 1988; 54: 777-785.
- Phillips NC, Emili A. Enhanced antibody response to liposome-associated protein antigens: preferential stimulation of IgG2a/b production. Vaccine. 1992; 10: 151-158.
- Thérien HM, Shahum E, Fortin A. Liposome adjuvanticity: influence of dose and protein:lipid ratio on the humoral response to encapsulated and surface-linked antigen. Cell Immunol. 1991; 136: 402-413.
- Martin M, Michalek SM, Katz J. Role of innate immune factors in the adjuvant activity of monophosphoryl lipid A. Infect Immun. 2003; 71: 2498-2507.
- Yoo YC, Yoshimatsu K, Koike Y, Hatsuse R, Yamanishi K, Tanishita O, et al. Adjuvant activity of muramyl dipeptide derivatives to enhance immunogenicity of a hantavirus-inactivated vaccine. Vaccine. 1998; 16: 216-224.
- Davidsen J, Rosenkrands I, Christensen D, Vangala A, Kirby D, Perrie Y, et al. Characterization of cationic liposomes based on dimethyldioctadecylammonium and synthetic cord factor from M. tuberculosis (trehalose 6,6'-dibehenate)-a novel adjuvant inducing both strong CMI and antibody responses. Biochim Biophys Acta. 2005; 1718: 22-31.
- Müller MR, Wiesmüller KH, Jung G, Loop T, Humar M, Pfannes SD, et al. Lipopeptide adjuvants: monitoring and comparison of P3CSK4- and LPS-induced gene transcription. Int Immunopharmacol. 2002; 2: 1065-1077.
- Garçon N, Chomez P, Van Mechelen M. GlaxoSmithKline Adjuvant Systems in vaccines: concepts, achievements and perspectives. Expert Rev Vaccines. 2007; 6: 723-739.
- Baldwin SL, Shaverdian N, Goto Y, Duthie MS, Raman VS, Evers T, et al. Enhanced humoral and Type 1 cellular immune responses with Fluzone adjuvanted with a synthetic TLR4 agonist formulated in an emulsion. Vaccine. 2009; 27: 5956-5963.
- Coler RN, Bertholet S, Moutaftsi M, Guderian JA, Windish HP, Baldwin SL, et al. Development and characterization of synthetic glucopyranosyl lipid adjuvant system as a vaccine adjuvant. PLoS One. 2011; 6: e16333.
- Krishnan L, Dicaire CJ, Patel GB, Sprott GD. Archaeosome vaccine adjuvants induce strong humoral, cell-mediated, and memory responses: comparison to conventional liposomes and alum. Infect Immun. 2000; 68: 54-63.
- Ahmad N, Deeba F, Faisal SM, Khan A, Agrewala JN, Dwivedi V, et al. Role of fusogenic non-PC liposomes in elicitation of protective immune response against experimental murine salmonellosis. Biochimie. 2006; 88: 1391-1400.
- Ahmad N, Masood AK, Owais M. Fusogenic potential of prokaryotic membrane lipids. Implication in vaccine development. Eur J Biochem. 2001; 268: 5667-5675.
- Sharma SK, Dube A, Nadeem A, Khan S, Saleem I, Garg R, et al. Non PC liposome entrapped promastigote antigens elicit parasite specific CD8+ and CD4+ T-cell immune response and protect hamsters against visceral leishmaniasis. Vaccine. 2006; 24: 1800-1810.
- Syed FM, Khan MA, Nasti TH, Ahmad N, Mohammad O. Antigen entrapped in the escheriosomes leads to the generation of CD4(+) helper and CD8(+) cytotoxic T cell response. Vaccine. 2003; 21: 2383-2393.
- Faisal SM, Yan W, McDonough SP, Mohammed HO, Divers TJ, Chang YF. Immune response and prophylactic efficacy of smegmosomes in a hamster model of leptospirosis. Vaccine. 2009; 27: 6129-6136.
- Faisal SM, Yan W, McDonough SP, Chang CF, Pan MJ, Chang YF. Leptosome- entrapped leptospiral antigens conferred significant higher levels of protection than those entrapped with PC-liposomes in a hamster model. Vaccine. 2009; 27: 6537-6545.
- Faisal SM, Chen JW, McDonough SP, Chang CF, Teng CH, Chang YF. Immunostimulatory and antigen delivery properties of liposomes made up of total polar lipids from non-pathogenic bacteria leads to efficient induction of both innate and adaptive immune responses. Vaccine. 2011; 29: 2381-2391.
- Mosca F, Tritto E, Muzzi A, Monaci E, Bagnoli F, Iavarone C, et al. Molecular and cellular signatures of human vaccine adjuvants. Proc Natl Acad Sci U S A. 2008; 105: 10501-10506.
- Lambert SL, Yang CF, Liu Z, Sweetwood R, Zhao J, Cheng L, et al. Molecular and cellular response profiles induced by the TLR4 agonist-based adjuvant Glucopyranosyl Lipid A. PLoS One. 2012; 7: e51618.
- Korsholm KS, Petersen RV, Agger EM, Andersen P. T-helper 1 and T-helper 2 adjuvants induce distinct differences in the magnitude, quality and kinetics of the early inflammatory response at the site of injection. Immunology. 2010; 129: 75-86.
- Yan W, Chen W, Huang L. Mechanism of adjuvant activity of cationic liposome: phosphorylation of a MAP kinase, ERK and induction of chemokines. Mol Immunol. 2007; 44: 3672-3681.
- Klinman DM, Klaschik S, Tomaru K, Shirota H, Tross D, Ikeuchi H. Immunostimulatory CpG oligonucleotides: Effect on gene expression and utility as vaccine adjuvants. Vaccine. 2010; 28: 1919-1923.
- Klaschik S, Tross D, Shirota H, Klinman DM. Short- and long-term changes in gene expression mediated by the activation of TLR9. Mol Immunol. 2010; 47: 1317-1324.
- Kwissa M, Nakaya HI, Oluoch H, Pulendran B. Distinct TLR adjuvants differentially stimulate systemic and local innate immune responses in nonhuman primates. Blood. 2012; 119: 2044-2055.
- Brandl M, Gregoriadis G. Entrapment of haemoglobin into liposomes by the dehydration-rehydration method: vesicle characterization and in vivo behaviour. Biochim Biophys Acta. 1994; 1196: 65-75.
- Lyons AB, Doherty KV. Flow cytometric analysis of cell division by dye dilution. Curr Protoc Cytom. 2004; Chapter 9: Unit 9.
- Korzeniewski C, Callewaert DM. An enzyme-release assay for natural cytotoxicity. J Immunol Methods. 1983; 64: 313-320.
- Janvilisri T, Scaria J, Chang YF. Transcriptional profiling of Clostridium difficile and Caco-2 cells during infection. J Infect Dis. 2010; 202: 282-290.
- Gupta RK. Aluminum compounds as vaccine adjuvants. Adv Drug Deliv Rev. 1998; 32: 155-172.
- Gupta RK, Relyveld EH, Lindblad EB, Bizzini B, Ben-Efraim S, Gupta CK. Adjuvants--a balance between toxicity and adjuvanticity. Vaccine. 1993; 11: 293-306.
- Relyveld EH, Bizzini B, Gupta RK. Rational approaches to reduce adverse reactions in man to vaccines containing tetanus and diphtheria toxoids. Vaccine. 1998; 16: 1016-1023.
- Hem SL, Hogenesch H. Relationship between physical and chemical properties of aluminum-containing adjuvants and immunopotentiation. Expert Rev Vaccines. 2007; 6: 685-698.
- Hunter RL. Overview of vaccine adjuvants: present and future. Vaccine. 2002; 20: S7-12.
- Stewart-Tull DE. The Use of Adjuvants in Experimental Vaccines : II. Water-in-Oil Emulsions: Freund's Complete and lncomplete Adjuvants. Methods Mol Med. 1996; 4: 141-145.
- Alving CR, Rao M, Steers NJ, Matyas GR, Mayorov AV. Liposomes containing lipid A: an effective, safe, generic adjuvant system for synthetic vaccines. Expert Rev Vaccines. 2012; 11: 733-744.
- Kirby DJ, Rosenkrands I, Agger EM, Andersen P, Coombes AG, Perrie Y. Liposomes act as stronger sub-unit vaccine adjuvants when compared to microspheres. J Drug Target. 2008; 16: 543-554.
- Kool M, Soullié T, van Nimwegen M, Willart MA, Muskens F, Jung S, et al. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J Exp Med. 2008; 205: 869-882.
- Mata-Haro V, Cekic C, Martin M, Chilton PM, Casella CR, Mitchell TC. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science. 2007; 316: 1628-1632.
- Eddahri F, Denanglaire S, Bureau F, Spolski R, Leonard WJ, Leo O, et al. Interleukin-6/STAT3 signaling regulates the ability of naive T cells to acquire B-cell help capacities. Blood. 2009; 113: 2426-2433.
- Ryman BE, Jewkes RF, Jeyasingh K, Osborne MP, Patel HM, Richardson VJ, et al. Potential applications of liposomes to therapy. Ann N Y Acad Sci. 1978; 308: 281-307.
- Le Y, Zhou Y, Iribarren P, Wang J. Chemokines and chemokine receptors: their manifold roles in homeostasis and disease. Cell Mol Immunol. 2004; 1: 95-104.
- Zughaier SM, Zimmer SM, Datta A, Carlson RW, Stephens DS. Differential induction of the toll-like receptor 4-MyD88-dependent and -independent signaling pathways by endotoxins. Infect Immun. 2005; 73: 2940-2950.
- Kato S, Yuzawa Y, Tsuboi N, Maruyama S, Morita Y, Matsuguchi T, et al. Endotoxin-induced chemokine expression in murine peritoneal mesothelial cells: the role of toll-like receptor 4. J Am Soc Nephrol. 2004; 15: 1289-1299.
- Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003; 19: 71-82.
- Badiee A, Khamesipour A, Samiei A, Soroush D, Shargh VH, Kheiri MT, et al. The role of liposome size on the type of immune response induced in BALB/c mice against leishmaniasis: rgp63 as a model antigen. Exp Parasitol. 2012; 132: 403-409.