
Special Article – Cancer Immunology
J Immun Res. 2015; 2(2): 1021.
Maturation of Mouse Bone Marrow Dendritic Cells Induced by Saposhnikovia Divaricate Polysaccharides (SDPs)
Minyu Wang1, Hua Wen3, Zijian Li1, Cuihuan Sun4, Xinghua Gao2* and Fengping Shan1*
1Department of Immunology, School of Basic Medical Science, China Medical University, Shenyang, China
2Department of Dermatology, No.1 Hospital, China Medical University, Shenyang, China
3Department of Pulmonary Medicine, Institute of Respiratory Diseases, No.1 Hospital, China Medical University, Shenyang, China
4Department of Natural Product, Liaoning Institute of Microbiological Science, Chaoyang, China
*Corresponding author: Fengping Shan, Department of Immunology, School of Basic Medical Science, China Medical University, Shenyang, China Xinghua Gao, Department of Dermatology, No.1 Hospital, China Medical University, Shenyang, China
Received: June 12, 2015; Accepted: July 06, 2015; Published: July 08, 2015
Abstract
SDPs, acidic polysaccharides, identified by Japanese in 1989, come from unstemming dried root of Saposhnikovia divaricate (Trucz) Schischk. Every increasing document has proven that SDPs could exert significant effect on up- regulation of immune cells and suppression of tumor growth. In this study we investigated the effect of SDPson phenotypic and functional maturation of murine bone marrow dendritic cell (BMDCs) systematically. The phenotypic maturation of BMDCs was checked by transmission electron microscopy (TEM), flow cytometry (FCM), and functional maturation of BMDCs was confirmed by FITC-dextran test for phagocytosis, measurement of acid phosphatase (ACP) activity, and enzyme linked immunosorbent assay (ELISA) for production of cytokines. We discovered that SDPs up-regulated the expression of key signal molecules of CD80, CD83, CD86,CD40 and MHC II on the surface of BMDCs, down-regulated phagocytosis activity of BMDCs, increased secretion of IL-12 and TNF-α by BMDCs. It is therefore summarized that SDPs could effectively boost maturation of BMDCs. Our results suggest that SDPs could be used as an effective booster in immune handicapped cases such as cancer patients and in the field of vaccine preparation as an adjuvant.
Keywords: Saposhnikovia divaricate polysaccharides; Bone marrow derived dendritic cells; Maturation; Immunoregulation
Abbreviations
SDPs: Saposhnikovia Divaricate Polysaccharides; BMDCs: Bone Marrow Derived Dendritic Cells; MACS: Magnetic Activated Cell Sorting; ACP: Acidic Phosphatase; LPS: Lipopolysaccharide; APCs: Antigen Presenting Cells; TEM: Transmission Electron Microscopy; DAB: 3, 3′-diaminobenzidine; MTT: 3-(4,5-Dimethylthiazol-2-yl)- 2,5-diphenyltetrazolium bromide; FCM: Flow Cytometry; CTL: Cytotoxic Lymphocyte
Introduction
Saposhnikovia divaricate (Turcz) Schischk. [SD, syn. Ledebourielladivaricata (Turcz); Umbelliferae]. Siler, is a perennial herb of the carrot family. Its dried root is known as Fang Feng in traditional Chinese medicine (TCM) for thousands of years. It is often used in poly-herb formulae to treat various kinds of diseases, including anti-proliferative, antioxidant [1], relief of rheumatoid arthritis (RA) [2], spasm alleviation, pain relief, fever removing, [3] anti-tumor [4] and up-regulation of phagocytic function of macrophage [5]. Modern researches have also shown that polysaccharides have significant relationship with the bioactivities and pharmacological properties of the herb, such as: activation of immune system and anti-inflammation [6]. SDPs were first separated and purified bio-macromolecules by the way of biochemistry in 1989. Further identification proves that SDPs composed of D-galacturonic acid, L-rhamnose, L-arabinose and D-galactose in a molar ratio of 27:7:8:8 [7] is acidic polysaccharides. The average molecular weight is around 73KD. The data from early researchers indicated that SDPs could upregulate function of immune cells in vitro and in vivo, such as induction of cytokines production [8].
In late stage of nineteenth century, Dendritic cells (DCs) were first described by Paul Langerhans (Langerhans cells). However, it was until 1973 that DCs was covered as part of immune cells through Ralph M. Steinman and Zanvil A. Cohn. The great discovery represents a milestone of immunology and three scientists mentioned above won the 2011 Nobel Prize in physiology or medicine.
DCs are the most powerful antigen-presenting cells (APCs) discovered so far. DCs process and present antigen on the surface that makes it recognized by T cell to initiate T cell response [9,10]. So DCs are critically important for the induction of primary immune responses as well as the induction of adaptive immune system [11,12]. Immature DCs migrate in almost every non-lymphoid tissue and organ, with a highly developed capacity to capture antigens, but with a poor antigen-presentingability and T cell-stimulatory activity. Immature DCs also express low content of surface co-stimulating molecules for signaling transduction, such as CD40, CD80, CD83, CD86 and MHC II. Upon maturation, both phenotype and function of DCs will develop into the stage that has opposite features to the immature ones [13].
However, so far there is no published reports on the impact on DCs by SDPs, especially no published article on detailed changes of DCs induced by SDPs and due to the increased clinical application of SDP as immune enhancer for immune handicapped cases, therefore we conducted the following research to investigate the impact on DCs by SDP.
Materials and Methods
Reagents
SDPs were prepared by Liaoning institute of microbiological science, with purity >98%. The impact of SDPs at a range of concentrations from 1μg/ml to 200μg/ml on the growth of BMDCs in vitro was evaluated, and the most suitable concentration of 40μg/ml was found. So the 40 μg/ml was used for this exploration. Recombinant murine IL-4 and GM-CSF were brought from Pepro Tech Inc. LPS used as positive control in current work was a product of Sigma-Aldrich and 1μg/ml was used for the work. The mAbs for this study were PE-anti-CD83, FITC-conjugated anti-CD40, PE-anti- CD80, PE-anti-CD86 and PE-anti-MHC-II, which were all products of eBioscience or BD Pharmingen. ELISA testing kits for cytokines measurement were bought from R&D System. Other experimental reagents in our laboratory were all made in Sigma-Aldrich or BD Pharmingen.
Induction of bone marrow cells to get BMDCs
All mice for present experiment were treated kindly in accordance with the guide for the care and use of laboratory animals of China medical university. BMDCs were gotten from mice by referring to a method documented previously [14]. Simply the bone marrow cells from the femurs and tibias of 20 female C57BL/6 mice were removed of red cells and about107/ml living cells (3 batches of cells in replication) were grown in a 24-well culture plate filled with 2 ml of RPMI 1640 supplemented with 10% fetal bovine serum, 10ng/ml GM-CSF, 10ng/ml IL-4, 2 mM L-glutamine, 100units/ml penicillin, 100μg/ml streptomycin. After incubation for 4h the cells sticky to flask wall were detained. The culture was kept up to 7th day and LPS was added to the culture for overnight to accumulate more cells for purification with CD11c-MACS magnet beads (Miltenyi Biotec). Finally the CD11c+ BMDCs were selected out using anti-CD11ccoated magnetic beads and the auto- MACS system. The purity of the purified cells was confirmed by FCM with > 90%.
Detailed changes of BMDCs boosted with SDPs under TEM
The BMDCs in the presence of SDPs were cultured for 48h, followed by centrifugation and re-suspending in 0.5 ml 0.05M pH 7.2 phosphorus buffer solutions. The cells were then fixed in 2.5% glutaraldehyde, by adding 1% osmium teroxide for overnight, dehydrated in ethanol and embedded in epon resin. Sections were made on a Reiehert-Jung Ultra cut E, stained with uranyl acetate and lead citrate. Finally the sample was checked under TEM (JEOL JEM- 1200EX) for changes inside BMDCs.
Identification of elevated key surface signals molecules by FCM
The BMDCs boosted with 40μg /ml SDPs for 48h were further analyzed for the expression of key surface signals molecules by FCM. The BMDCs post treatment with SDPs was combined with anti-CD83, anti-CD40, anti-CD80, anti-CD86, and anti-MHC II antibodies at 4°C for 20 min. After washing, the reacted cells underwent FACS Calibur (Becton Dickinson, San Diego, CA) to locate percentage of changes of these surface molecules.
Confirmation of phagocytosis process by FCM
The phagocytosis process of BMDCs post boosting with 40μg / ml SDPs for 48h was tested. Exactly 100μl FITC-Dextran (40,000 D) 28-30 was added to the treated BMDCs culture at 4°C for 2h, and the culture was kept at 37°C for 1h. At last the instant process of phagocytosis in BMDC was located with FACS Calibur (Becton Dickinson, San Diego, CA).
ACP activity determination
ACP activity in BMDCs boosted with SDPs for 48h was detected.1×106/ml BMDCs were reacted with the phenol-4-AAP (amino anti-pyrine), plus ACP testing kit (Jiancheng Bio-engineering institute of the South). The OD number at 520nm (A520) was measured to represent ACP activity. The concrete procedures were included in instruction manual in ACP testing kit.
Confirmation of production of IL-12 and TNF-α by ELSA
The BMDCs co-cultured with 40μg /ml SDPs for 48h were collected for determination of production of IL-12 and TNF-α separately according to manual in ELISA kit. The absorbance at 450 nm (A450) could reflect the amount of cytokine production and corresponding OD number was measured using a bi-chromatic micro plate reader (BIO-TEK, USA).
Statistical analysis
All data were analyzed using statistical program SPSS (Statistical Package for Social Sciences, Version 16.0) for Windows and variables were showed as mean± SE. The p value was set and p<0.05 indicated statistical significance when evaluated by ANOVA.
Results
Detailed changes of BMDCs boosted with SDPs observed under TEM
Commonly immature BMDC is powerful in swallowing antigen and with more phagosomes. Upon the process of maturation, the number of phagosomes will reduce. This means BMDC will terminate phagocytosis and digestion to antigen gradually. Accompanying this process, the elevated expression of key surface signals molecules will happen to enable BMDCS to present antigen to activate T cells. Figure 1 was a vision picture of BMDCs before and after treatment with SDPs under TEM, showing that there were clearly decreased phagosomes.
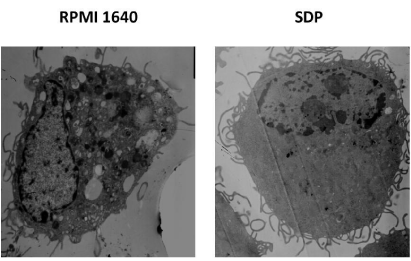
Figure 1: Phagosomes in BMDC boosted with SDPs under TEM. Prepared
sample of BMDC boosted with SDPs was observed under TEM. Compared to
BMDC in control clear reduction of the number of pinocytotic vesicles inside
BMDC treated with SDPs for 24 h was confirmed.
Identification of elevated key surface signals molecules by FCM
With the process of BMDCs’ maturation, up-regulation of key surface signal molecules on BMDCs would happened such as higher expression of molecules of CD40, CD83, CD80, CD86 and MHCII, which would serve as co-signals with antigen to initiate T cell response. The concrete changes could be reflected in Figure 2.
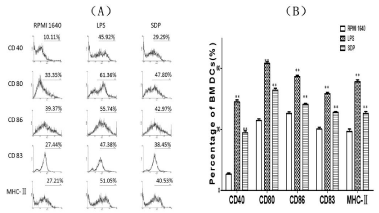
Figure 2: Changes of key surface signals molecules on the BMDCs. With the
process of maturation of BMDCs treated by SDPs, the elevated expression
of key surface signals molecules happened. FCM analysis confirmed the
increase of each different molecule at different ratio. A showed mean intensity
of increment of key surface molecules by FCM and B showed the percentage
of each key molecule on expanded BMDCs.
Confirmation of phagocytosis by FCM
Using FITC-dextran as antigen, the ability of phagocytosis of BMDCs post treatment with SDPs was further confirmed. SDPs stimulated maturation of BMDCs with decreased ability of swallowing the labeled dextran as compared with that of BMDCs without treatment, as shown in Figure 3.
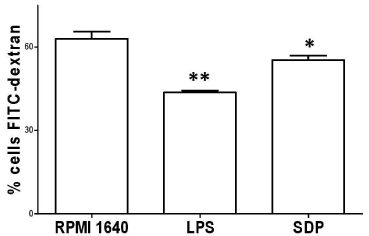
Figure 3: Phagocytosis confirmation. The BMDCs stimulated with SDPs
for 24h decreased in phagocytosis. FITC-dextran used as antigen was put
into the culture of BMDCs for checking this phagocytosis by FCM. The
average percentage showed that mature BMDCs decreased in phagocytosis
markedly, when compared with that in RPMI 1640 group.
ACP activity analysis
After the BMDCs were treated with 40μg/ml SDPs for 48h, immature BMDCs developed into maturation with a marked decrease of ACP activity, which was a symbol of gradual termination of phagocytosis. ACP activity of BMDCs responded with a value of OD250nm (A520). The data was shown in Figure 4.
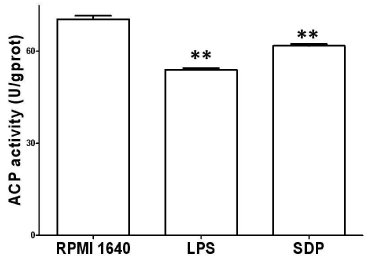
Figure 4: Change of ACP activity. ACP activity was determined after
treatment with SDPs for 24h by the phenol-4-AAP method in conjunction with
ACP testing kit. Results represent the mean ± SE of six samples. **p< 0.01 or
*p< 0.05 vs. that in RPMI 1640.
Cytokine assay of IL-12 and TNF-α
After the immature BMDCs were stimulated with 40μg /ml for 48h, the cultured BMDCs grew with simultaneous maturation in morphology. Besides decreased phagocytosis and higher expression of key surface molecules, the BMDCs also produced extra IL-12 and TNF-α, which represent functional maturation of BMDCs. At the same time, IL-12 and TNF-α were also responsible for triggering a chain of immune responses in both innate and adaptive immune systems. These results were shown in Figure 5A, Figure 5B.
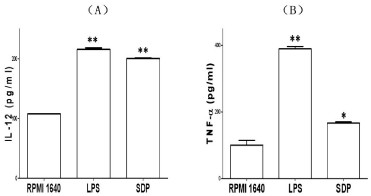
Figure 5: Effects of SDP on production of IL-12p70 (A), and TNF-α (B).
After treatment with SDPs the supernatant of cell culture was collected and
secreted cytokines were detected by ELISA. Similar results were obtained
in three independent experiments and a representative graph was shown.
Results represent the mean ± SE of six samples. **p< 0.01 or *p< 0.05 vs.
that in RPMI 1640.
Discussion
SDPs are separated from Saposhnikovia divaricate (Trucz). Schischk and dried root is widely used as medicinal herb. SDPs could up-regulate the proliferation of T, B lymphoncytes and ratio of CD4+ CD8+ T lymphocytes in the spleen of mice [15]. It is also able to enhance the scavenging effect of hydroxyl radical (*OH), superoxide anion (O2-*), 1, 1-diphenyl-2-picrylhydrazyl radical (DPPH*) inside macrophage [16]. Moreover, the phagocytic ability of macrophages
was enhanced [17], while S180 transplanted tumor was suppressed in mice [4]. These results reveal that SDPs have in oxidizability antiproliferation and immune enhancing activity.
Our current results confirmed the impact of SDPs on BMDCs as evidence by the following:
(1) Post treatment with SDPs, BMDCs developed into maturation with distinctive disappearing of quite some of phagosomes, which are responsible for digestion of antigen. (2) SDPs up-regulated the expression of CD40, CD80, CD83, CD86, and MHC II molecules, which are working as co-signals to serve with antigen to activate T cells. (3) SDPs induced functional maturation of BMDCs, as characterized by the decreased ACP activity, which usually indicates the degree of maturation of BMDCs and corresponds to antigen phagocytosis by BMDCs. (4) SDPs also up-regulated the levels of IL-12 and TNF-α produced by BMDCs. These cytokines would be involved in interaction among immune network [18].
It is known to all that immune system is a pretty sophisticated and diverse network. All of immune cells are interconnecting with each other. Therefore the maturation of BMDCs depends largely on the maturation signals offered by other immune cells as well as the influence by ontogeny and environmental modifiers. T lymphocytes provide signal 1, who sereceptor-recognizing antigen peptides bounded to MHC molecules inducing regulatory T cellgeneration. Signal 2 is a co-stimulatory signal, which will induce T lymphocytes responses when combined with signal 1. The T lymphocytes proliferate and differentiate into memory or effector cells. Furthermore, signal 2 improves antigen-specific immunity significantly by binding CD80 or CD86 on DCs to its receptor [19].
Nowadays, polysaccharides are more often used as adjuvant. Some polysaccharides such as delta in ulin were developed through the National Institutes of Health’s adjuvant development program [20,21,22]. Most of the polysaccharides used as adjuvantin current experiment can stimulate the proliferation of CD4+ and CD8+ T lymphocytes. They can also enhance memory effect of T lymphocytes [23]. Polysaccharides provide signals, which the innate immune system vigorously reacts to. Therefore, antigen co-administrated with the adjuvant can be swallowed as well as be processed and presented in a more effective manner by the activated DCs [24,25]. The documents above-mentioned can contribute to the better understanding of SDPs’ modulating effects on immune system as well as the better understanding of the complicated mechanisms at molecular level [26].
It should be further noted that activation of T cell will mount a series of immune responses. For example, activated CD4+Th1 cells will secret interferon-γ to activate macrophage, which will secret a spectrum of cytokines serving immune network. Also interferon-γ can activate NK cells, which are powerful non-specific killer cells in innate immune system and play a critical role in killing mutated cells. Thus SDPs actually activates whole immune system both directly and indirectly.
Overall, the data in this study indicate that SDPs induce the phenotypic and functional maturation of BMDCs significantly and increase understanding of detailed working mechanisms of SDPs at sub-cellular or molecular level. SDPs are good supplement for people with poor immunity, such as those with chronic wasting disease, cancer, and aging. The improvement of immunity through SDPs can be achieved by oral, intramuscular and venous administration [27].
Finally, the current results contribute to the better understanding of SDPs’ modulating role in improving immune system and to highlight the clinical significance of SDPs, which would also play a vital role in combating tumor. According to current data, we look forward to figuring out more evidence about SDPs, so that SDPs can be used as an adjuvant in vaccine to treat people with other infectious diseases.
Conclusion
We believe that it is the first time that published article elucidates that SDPs at used concentration could induce the phenotypic and functional maturation of BMDCs. The BMDCs induced by SDPs are with distinctive characteristic of higher expression of key surface signals molecules like: CD40, CD83, CD80, CD86 and MHC II, plus more secretion of IL-12 and TNF-α. As consequence of this, maturation BMDCs would initiate specific T cell response as well as other cellular immune response.
Acknowledgement
This work was supported by the funding from China Liaoning province supporting construction of discipline platform in universities and received funding from China Liaoning science foundation (2009225008-7, 2012225016).
References
- Tai J, Cheung S. Anti-proliferative and antioxidant activities of Saposhnikovia divaricata. Oncol Rep. 2007; 18: 227-234.
- Wang Z, Chen Z, Yang S, Wang Y, Huang Z, Gao J, et al. Berberine ameliorates collagen-induced arthritis in rats associated with anti-inflammatory and anti-angiogenic effects. Inflammation. 2014; 37: 1789-1798.
- Kong X, Liu C, Zhang C, Zhao J, Wang J, Wan H, et al. The suppressive effects of Saposhnikoviadivaricata (Fangfeng) chromone extract on rheumatoid arthritis via inhibition of nuclear factor-? B and mitogen activated proteinkinases activation on collagen-induced arthritis model. J Ethnopharmacol. 2013; 148: 842-850.
- Kuo YC, Lin YL, Huang CP, Shu JW, Tsai WJ. A tumor cell growth inhibitor from Saposhnikovae divaricata. Cancer Invest. 2002; 20: 955-964.
- Zhao B, Yang XB, Yang XW, Liu JX. Biotransformation of prim-O-glucosylcimifugin by human intestinal flora and its inhibition on NO production and DPPH free radical. J Asian Nat Prod Res. 2012; 14: 886-896.
- Tan W, Yu KQ, Liu YY, Ouyang MZ, Yan MH, Luo R, et al. Anti-fatigue activity of polysaccharides extract from Radix Rehmanniae Preparata. Int J Biol Macromol. 2012; 50: 59-62.
- Shimizu N, Tomoda M, Gonda R, Kanari M, & Kubota A. 1989. An acidic polysaccharide having activity on the reticuloendothelial system from the roots and rhizomes of Saposhnikoviadivaricata. Chem Pharm Bull. 1989; 37: 3054-3057.
- Cui HT, Bian YH, Wang L. Progress of immunoregulation of Saposhnikovia Divaricate Polysaccharides. Modern Chinese Medicine. 2013; 15: 286-289.
- Liu J, Chen W, Meng J, Lu C, Wang E, Shan F. Induction on differentiation and modulation of bone marrow progenitor of dendritic cell by methionine enkephalin (MENK). Cancer Immunol Immunother. 2012; 61: 1699-1711.
- Xue M, Zhu L, Meng Y, Wang L, Sun H, Wang F, et al. Detailed modulation of phenotypes and functions of bone marrow dendritic cells (BMDCs) by interferon-gamma (IFN-î³). Int Immunopharmacol. 2013; 17: 366-372.
- Meng Y, Wang Q, Zhang Z, Wang E, Plotnikoff NP, Shan F. Synergistic effect of methionine encephalin (MENK) combined with pidotimod(PTD) on the maturation of murine dendritic cells (DCs). Hum Vaccin Immunother. 2013; 9: 773-783.
- Steinman R M, & Cohn Z A. Pillars Article: Identification of a Novel Cell Type in Peripheral Lymphoid Organs of Mice. I. Morphology, Quantitation, Tissue Distribution. J. Exp. Med. 1973. 137: 1142–1162. JImmunol. 2007; 178: 5-25.
- Li X, Xu W, Chen J. Polysaccharide purified from Polyporus umbellatus (Per) Fr induces the activation and maturation of murine bone-derived dendritic cells via toll-like receptor 4. Cell Immunol. 2010; 265: 50-56.
- Segovia M, Cuturi MC, Hill M. Preparation of mouse bone marrow-derived dendritic cells with immunoregulatory properties. Methods Mol Biol. 2011; 677: 161-168.
- Hua L, Tian JM, Sun L, Bai XM, Li JJ, Jia TJ. Reaction of Macrophage and Lymphocytes subsets in normal mice to Radix Saposhnikoviae Polysaccharide. Journal of Clinical Rehabilitative Tissue Engineering Research. 2008; 12: 3475-3478.
- Zhang ZQ, Tian YJ, Zhang J. [Studies on the antioxidative activity of polysaccharides from radix Saposhnikoviae]. Zhong Yao Cai. 2008; 31: 268-272.
- Tang RJ, Min ZH, Xu CY. [Pharmacologic studies on the root of Saposhnikovia divaricata (Turcz.) Schischk]. Zhong Yao Tong Bao. 1988; 13: 44-46, 64.
- Tugues S, Burkhard SH, Ohs I, Vrohlings M, Nussbaum K, Vom Berg J, et al. New insights into IL-12-mediated tumor suppression. Cell Death Differ. 2015; 22: 237-246.
- Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol. 2003; 3: 984-993.
- Cooper PD, Petrovsky N. Delta inulin: a novel, immunologically active, stable packing structure comprising î²-D-[2 -> 1] poly(fructo-furanosyl) î ±-D-glucose polymers. Glycobiology. 2011; 21: 595-606.
- Honda-Okubo Y, Saade F, &Petrovsky N. Advax™, a polysaccharide adjuvant derived from delta inulin, provides improved influenza vaccine protection through broad-based enhancement of adaptive immune responses. Vaccine. 2012; 30: 5373-5381.
- Layton RC, Petrovsky N, Gigliotti AP, Pollock Z, Knight J, Donart N, et al. Delta inulin polysaccharide adjuvant enhances the ability of split-virion H5N1 vaccine to protect against lethal challenge in ferrets. Vaccine. 2011; 29: 6242-6251.
- Guo L, Wang D, Hu Y, Zhao X, Wang Y, Yang S, et al. Adjuvanticity of compound polysaccharides on chickens against Newcastle disease and avian influenza vaccine. Int J Biol Macromol. 2012; 50: 512-517.
- Cohen N, Stolarsky-Bennun M, Amir-Kroll H, Margalit R, Nussbaum G, Cohen-Sfady M, et al. Pneumococcal capsular polysaccharide is immunogenic when present on the surface of macrophages and dendritic cells: TLR4 signaling induced by a conjugate vaccine or by lipopolysaccharide is conducive. J Immunol. 2008; 180: 2409-2418.
- Giagulli C, Noerder M, Avolio M, Becker PD, Fiorentini S, Guzman CA, et al. Pidotimod promotes functional maturation of dendritic cells and displays adjuvant properties at the nasal mucosa level. Int Immunopharmacol. 2009; 9: 1366-1373.
- Rhule A, Rase B, Smith JR, Shepherd DM. Toll-like receptor ligand-induced activation of murine DC2.4 cells is attenuated by Panax notoginseng. J Ethnopharmacol. 2008; 116: 179-186.
- Zheng, Y. The therapeutic effects of rehmannia oral liquid for the syndrome of heat accumulation with Yin consumption in esophagus cancer patients undergoing radiotherapy--a report of 60 cases. Journal of traditional Chinese medicine by All-China Association of Traditional Chinese Medicine, Academy of Traditional Chinese Medicine. 2007; 27: 248-254.