
Mini Review
Immun Res. 2016; 3(1): 1025.
Novel Autoantibody and Autoantigen in Sjögren’s Syndrome
Nozawa K* and Uomori K
Department of Rheumatology, Juntendo University Faculty of Medicine, Tokyo, Japan
*Corresponding author: Kazuhisa Nozawa, Department of Rheumatology, Juntendo University Faculty of Medicine, Tokyo, Japan
Received: August 29, 2016; Accepted: September 19, 2016; Published: September 22, 2016
Abstract
Over the past more than 30 years since the identification of the now classic anti-SS-A/Ro and anti-SS-B/La autoantibodies in Sjögren’s syndrome, there have been a few new and interesting autoantibodies including antifodrin, anti-M3 muscarinic receptor, and other autoantibodies reported to be closely linked to this disease. We have recently identified a novel autoantibody predominantly recognized in Sjögren’s syndrome specifically reacting with NA14 (nuclear autoantigen 14 kDa)/SSNA1 (Sjögren Syndrome Nuclear Autoantigen 1). This review describes the current data available for anti-NA-14 antibody in their usefulness of diagnosis and the nature of the autoantibody production in Sjögren’s syndrome.
Keywords: Autoantigen; Autoantibody; Systemic autoimmune disease; Sjögren’s syndrome
Abbreviations
NA14: Nuclear Autoantigen 14 kDa; PM/DM; Polymyositis/ Dermatomyositis; RA; Rheumatoid Arthritis; SLE: Systemic Lupus Erythematosus; SS: Sjögren’s Syndrome; SSc: Scleroderma; IIF: Indirect Immunofluorescence; IP-10: Interferon-gamma (IFN-γ)- inducible Protein of 10 kDa; BAFF: B cell-Activating Factor belonging to the tumor necrosis Factor Family
Introduction
Sjögren’s syndrome (SS) is a systemic autoimmune disease in which the immune response is activated to attack and destroy the exocrine glands that produce tears and saliva [1]. The disease may be isolated (primary SS) or it may occur in association with other rheumatic diseases (secondary SS). The etiology of SS remains unknown but the pathogenesis of exocrine cell damage is apparently immuno dysregulation involving aberrant regulation of cytokines production or apoptosis. The central clues of the hypothesis come from the observation that the immune system in SS targets a restricted and highly specific group of intracellular autoantigens [2,3]. The predominant target autoantigens in SS are the ribonucleoprotein autoantigens SS-A/Ro and SS-B/La [3], which are included in the disease diagnostic criteria [4]. Although autoantibodies to SS-A/ Ro and SS-B/La are indicative of SS, the autoimmune response to these autoantigens is not specific for SS since there are patients not having these autoantibodies with some frequency in SS and having autoantibodies to other intracellular autoantigens.
Nuclear autoantigen 14 kDa (NA-14)/Sjögren’s syndrome nuclear antigen-1 (SSNA-1) was originally identified as a novel autoantigen recognized by autoimmune serum from a patient with SS [5]. In the present study, we have reported that the anti-NA-14 autoantibody was frequently recognized in patients with SS compared to patients with other connective tissue diseases [6,7]. In this review, we discuss the characterization of anti-NA-14 antibody, and possible mechanisms for the autoantibody production against in SS.
Characterization of Anti-NA-14 Antibody in Patients with Sjögren’s Syndrome
As shown in Table1, we found that the anti-NA-14 autoantibody was predominantly recognized in patients with SS compared to patients with other connective tissue diseases [6,7]. The prevalence of anti-NA14 antibody were 18/132 (13.6%) primary SS, 0/50 (0%) secondary SS, 2/100 (2%) SLE, 1/43 (2.3%) SSc, 0/54 RA (0%), and 1/29 (3.4%) PM/DM (Table 1). The staining patterns of anti-NA-14 antibodies in IIF microscopy of HEp-2 cells strongly indicated that the cellular distribution of NA-14 was enriched in mitotic-phase cells since staining pattern of anti-NA-14 antibody was almost identical to anti-CENP-F (mitosin) antibody, which is reliable hallmark of the mitotic phase (Figure 1). Therefore, anti-NA-14 antibody can be classified as novel autoantibody reacting with cell cyclerelated autoantigens. In clinical profile, although we could not find involvement of specific organs damage in anti-NA-14 antibodies positive patients with pSS compared to anti-NA-14 negative patients, anti-NA-14 antibodies positive patients with pSS revealed significant elevation of serum IgA level. In contrast to IgA, significant elevation of both serum IgG and IgM levels was not recognized [7].
Primary SS
Secondary SS
SLE
SSc
RA
PM/DM
NHC
Number of patients
132
50
100
43
54
29
58
Number of anti-NA14 antibody positive patients
18
0
2
1
0
1
0
Frequency of positive anti-NA14 antibody
13.7%
0%
2%
2.3%
0%
3.4%
0%
Statistical analysis (vs primary SS
P=0.0041
P=0.001
P=0.0281
P=0.0029
P=0.11
P=0.002
Patient’s sera from rheumatic diseases were analyzed for reactivity against NA14 recombinant protein.
The cut off value designing a positive reaction was the mean OD of NHC sera +5 standard deciation.
Secondary SS consisted of SS in association with other rheumatic diseases including SLE (n=27), RA (n=15), SSc (n=2), PM/DM (n=3), PN (n=1), and APS (n=2). Statistical analysis was performed by chi square test. This table is sited from our previous report (Nozawa K et al. Front. Biosci. (Landmark Ed). 2009.14: 3733–3739).
Table 1: Prevalence of anti-NA14 autoantibody in patients with rheumatic diseases.
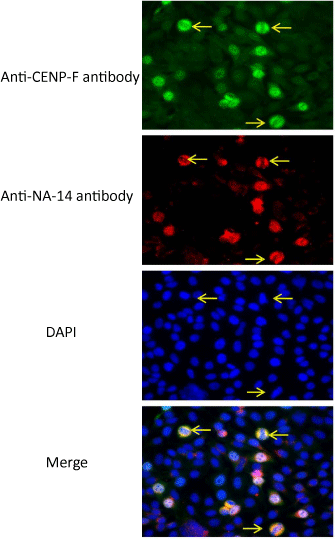
Figure 1: Immunolocalization of NA14 in mitotic cells.
Double staining with anti-NA-14 monoclonal antibody in combination with
anti-NA-14 anti-CENP-F polyclonal antibody on HEp-2 substrates was
performed using indirect immunofluorescence (IIF) microscopy. DAPI staining
image and merged picture are also shown. Cells stained by the anti-NA-14
serum antibodies were co localized with staining by anti-CENP-F polyclonal
antibody, as indicated by arrows. This figure is sited from our previous report
[7].
Possible Mechanisms of Anti-NA-14 Antibody Production in Patients with Sjögren’s Syndrome
Nuclear autoantigen 14 kDa (NA14) was originally identified as a novel coiled-coil autoantigen recognized by an autoimmune serum from a patient with SS [5]. This is interesting since we and others reported a class of coiled-coil proteins that are recognized as intracellular autoantigens in systemic autoimmune diseases [8,9]. The mitotic organelles are also known to be associated with coiled-coil rich autoantigens, including NuMA [10] and centromere associated protein CENP-E [11]. Although it is not obvious why coiled-coil rich protein can selectively elicit autoimmune response, this protein structure may be easily to promote the induction and production of autoantibodies in certain diseases states such as SS. Moreover, it is important to note that the immune response is not merely directed at cross-reactive coiled-coil structure, because the human autoimmune response to coiled-coil proteins appears to be highly specific in our study [12,13].
The autoimmune pathogenic model of Sjögren’s syndrome is based on the existence of altered immune system incapable of discriminating between foreign and self antigens. The abnormal autoimmune response may be initiated by the self antigens expressed at the epithelium of exocrine glands or other organs through a specific combination of intrinsic and exogenous factors. The abnormal responses of both T and B cells against autoantigens may contribute to the pathogenesis of SS. In this context, dysregulation of cytokine production (e.g., elevated IFN-α/β, IFN-γ, and BAFF production) and chronic hyperactivity of B cells are consistent and prominent immunoregulatory abnormalities in SS [1]. In our previous study [7], we found that serum levels of interferon-gamma (IFN-γ)-inducible protein of 10 kDa (IP-10) and B cell-activating factor belonging to the tumor necrosis factor family (BAFF) were elevated, particularly in patients with pSS having anti-SSNA-1/NA-14 antibodies (Figure 2). IP-10 is known to be secreted by several cell types in response to IFN-γ. BAFF is known to B‑cell maturation, proliferation, and survival. Moreover, BAFF release is primarily induced by IFN-α/β and IFN-γ [14]. Therefore, we believe that anti-NA-14 antibody production is associated with BAFF-mediated response via enhanced IFN-gamma response in SS.
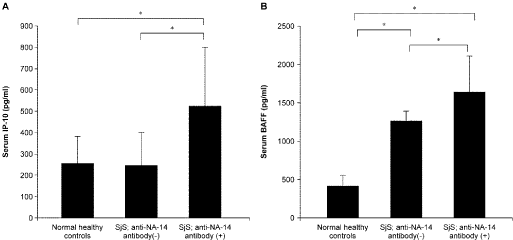
Figure 2: Serum levels of IP-10 and BAFF.
Serum levels of IP-10 and BAFF were measured by ELISA in pSS patients
with/without anti-NA-14 antibodies and NHCs (Figure 2A, 2B). Bars indicate
standard deviations (SDs). Statistical analysis was performed using paired
t-tests, and p-values of less than 0.05 (*) were considered statistically
significant. This figure is sited from our previous report [7].
Conclusion
We propose that anti-NA-14 antibody could be classified as novel autoantibodies reacting with mitosis-related autoantigens predominantly observed in patients with SS. Moreover, interferon-γ played a central role in the production of anti-NA-14 autoantibody as patients with SS having anti-NA-14 antibody exhibited increased serum levels of IP-10 and BAFF.
References
- Fox RI. Sjögren's syndrome. Lancet. 2005; 366: 321-331.
- Rosen A, Casciola-Rosen L. Altered autoantigen structure in Sjögren's syndrome: implications for the pathogenesis of autoimmune tissue damage. Crit Rev Oral Biol Med. 2004; 15: 156-164.
- Chan EK, Andrade LE. Antinuclear antibodies in Sjögren's syndrome. Rheum Dis Clin North Am. 1992; 18: 551-570.
- Rasmussen A, Ice JA, Li H, et al. Comparison of the American-European Consensus Group Sjogren's syndrome classification criteria to newly proposed American College of Rheumatology criteria in a large, carefully characterised sicca cohort. Ann Rheum Dis. 2014; 73: 31-38.
- Ramos-Morales F, Infante C, Fedriani C, Bornens M, Rios RM. NA14 is a novel nuclear autoantigen with a coiled-coil domain. J Biol Chem. 1998; 273: 1634-1639.
- Nozawa K, Ikeda K, Satoh M, et al. Autoantibody to NA14 is an independent marker primarily for Sjogren's syndrome. Front Biosci (Landmark Ed). 2009; 14: 3733-3739.
- Uomori K, Nozawa K, Ikeda K, et al. A re-evaluation of anti-NA-14 antibodies in patients with primary Sjögren's syndrome: Significant role of interferon-γ in the production of autoantibodies against NA-14. Autoimmunity. 2016: 1-10.
- Chan EKL, Fritzler MJ. Golgins: coiled-coil proteins associated with the Golgi Complex. Electronic J Biotechnology. 1998; 1: 1-10.
- Nozawa K, Fritzler MJ, Chan EK. Unique and shared features of Golgi complex autoantigens. Autoimmun Rev. 2005; 4: 35-41.
- L. E. C. Andrade et al. Arthritis Rheum. 1998. 27; 774-779.
- Mack GJ, Rees J, Sandblom O, Balczon R, Fritzler MJ, Rattner JB. Autoantibodies to a group of centrosomal proteins in human autoimmune sera reactive with the centrosome. Arthritis Rheum. 1998; 41: 551-558.
- Nozawa K, Fritzler MJ, von Mühlen CA, Chan EK. Giantin is the major Golgi autoantigen in human anti-Golgi complex sera. Arthritis Res Ther. 2004; 6: R95-102.
- Nozawa K, Casiano CA, Hamel JC, Molinaro C, Fritzler MJ, Chan EK. Fragmentation of Golgi complex and Golgi autoantigens during apoptosis and necrosis. Arthritis Res. 2002; 4: R3.
- Nocturne G, Mariette X. Advances in understanding the pathogenesis of primary Sjögren's syndrome. Nat Rev Rheumatol. 2013; 9: 544-556.