
Research Article
J Immun Res. 2018; 5(1): 1031.
The Level of Brain-Derived Neurotrophic Factor and Tyrosine Kinase B Expression in Peripheral Blood of the Patients with Dermatomyositis
Tian B1, Zhu Z1, Yang C2, Wang J1, Zhao S1 and Yang P1*
1Department of Rheumatology and Immunology, First Affiliated Hospital, China Medical University, Shenyang 110001, People’s Republic of China
2Department of First Cancer Institute, First Affiliated Hospital, China Medical University, Shenyang 110001, People’s Republic of China
*Corresponding author: Pingting Yang, Department of Rheumatology and Immunology, First Affiliated Hospital, China Medical University, Shenyang 110001, People’s Republic of China
Received: June 24, 2018; Accepted: August 03, 2018; Published: August 10, 2018
Abstract
Objective: Brain-derived neurotrophic factor (BDNF) plays a well-established role in neuronal development and plasticity. Recently it has been speculated that BDNF may be involved in the pathogenesis of immune system diseases, because T lymphocytes can secrete BDNF and express its specific receptor tyrosine kinase B (TrkB). Our aim is to detect the serum BDNF level and the expression of TrkB on peripheral blood T cells in patients with dermatomyositis (DM) and their contribution to the disease pathogenesis.
Methods: Twenty-six patients with DM and 30 age- and sex-matched healthy controls were enrolled. The serum BDNF level was measured by enzyme-linked immunosorbent assay (ELISA). Flow cytometry was used to measure the percentage of TrkB expression on the surface of CD3+CD4+ or CD3+CD8+T cells. Erythrocyte sedimentation rate (ESR), C-reactive protein(CRP), blood lymphocyte count, platelet count, fibrinogen, D-dimer(D-D), IgG, IgM, IgA and T cell subsets were acquired from the standard laboratory procedure.
Results: Compared to the controls, the serum BDNF level of patients with DM was significantly decreased (16012.8±6012.5 pg/ml vs. 33116.5±7146.5 pg/ml, p<0.001). In addition, the serum BDNF level was significantly lower in the DM patients with interstitial lung disease (ILD) than in those without ILD (14586.6±5120.5 pg/ml vs. 20455.1±6484.5 pg/ml, p=0.028). Compared to the controls, the percentage of TrkB expression on CD3+CD4+ and CD3+CD8+ T cells was increased significantly (1.85%±1.81% vs. 0.65%±0.36%, p=0.008; 6.8%±5.27% vs. 3.0%±2.74%, p=0.007, respectively). There was no significant difference in the expression of TrkB on CD3+CD4+ or CD3+CD8+ T cells between DM patients with and without ILD.
Conclusion: The serum BDNF level and TrkB expression on T cells may reflect the disease activity of DM and could be used as serological markers of DM. Especially, a serious decline in serum BDNF level may imply a pulmonary involvement of DM.
Keywords: Brain-derived neurotrophic factor; Immune system diseases; T lymphocytes
Introduction
Dermatomyositis (DM) is an autoimmune disease of unknown etiology and poor prognosis, characterized by involved striated muscle, inflammatory cell infiltration, muscle fiber degeneration and necrosis, multiple skin lesions [1,2]. Chronic proximal and pharyngeal muscle weakness are the clinical features of DM, and DM is frequently complicated with interstitial lung disease (ILD), which is one of the main causes of death. Muscle biopsy in DM patients shows that CD4+T cells, macrophages and a small amount of B cells are the main components of inflammatory cells, suggesting that cellular immune response mediated by T cells plays an important role in the pathogenesis of DM [3]. However, the detailed mechanisms remain unclear. Because muscle biopsy is inconvenient in clinic, serological markers with high sensitivity and specificity are still required for the diagnosis and research of DM [4]. Therefore, studying the features of peripheral blood T cells and relevant serological factors in DM may contribute to a better understanding about the pathogenesis and clinical characteristics of DM.
Brain-derived neurotrophic factor (BDNF) is one of the representative members of the neurotrophin family, which plays an important role in the survival, differentiation and function of the neurons [5,6]. Previously, BDNF was considered to be existed only in the central nervous system and synthesized by astrocytes. In recent years, it has been found that the lymphocytes can also synthesize and secrete BDNF [7], and express its specific receptor: tyrosine kinase B (TrkB) [8,9]. Both BDNF secretion and TrkB expression are increased in activated T and B cells [7]. The combination of BDNF and TrkB can promote proliferation of T cells and affect the differentiation of T cells into Th1 and Th2 [10], and is involved in the maturation, proliferation and activation of B cells [11-13]. In BDNF- deficient mice, the total number of thymic T cells decreased, and the development of B cells stopped at the pre BII stage [14]. Furthermore, BDNF-deficient mice displayed an attenuated immune response in the acute phase of experimental autoimmune encephalomyelitis (EAE) [15].
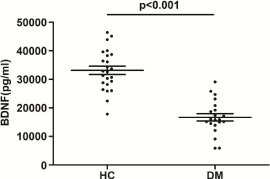
Figure 1: The serum BDNF level in DM patients and healthy controls. Mean
and SD were indicated. P<0.001, Student’s t test.
Significant changes of BDNF level have been reported in sera or tissues of the patients with some autoimmune diseases, such as systemic sclerosis [16], multiple sclerosis [17], Crohn's disease [18], sarcoidosis [19], primary Sjogren syndrome [20] and systemic lupus erythematosus [21-23]. Although the results of BDNF examination are inconsistent across these previous reports, they highlight the potential role of BDNF in the pathogenesis of autoimmune diseases. In this study, we examined the level of BDNF in the sera of DM patients and the expression of TrkB on the peripheral T cells. Our results for the first time suggest that the BDNF and TrkB may relate to the cellular immune response in DM.
Methods
Subjects
We collected 26 newly diagnosed DM patients (including three clinically amyopathic dermatomyositis, CADM) between Oct 2017 and Mar 2018 as well as 30 healthy controls from the Department of Rheumatology of the First Affiliated Hospital of China Medical University. All patients fulfilled the diagnosis criteria of Bohan and Peter [24] and the modified Sontheimer’s definition [25]. We only selected the patients that had a disease duration shorter than one month and had not received immunosuppressive therapy. Exclusion criteria included presence of other immune diseases, current infections, tumor, depression, psychosis and chronic renal disease or stroke. This study was in line with the standards of the ethics committee of our hospital. Informed consent was obtained from all individual participants included in the study.
Clinical and laboratory data
Complications of interstitial lung disease (ILD) in DM patients were diagnosed by at least two rheumatologists based on high-resolution computed tomography (HRCT), pulmonary function test and clinical presentation. Laboratory data such as lymphocyte count, platelet count, erythrocyte sediment rate (ESR), C-reactive protein (CRP), immunoglobulin (IgG, IgA, IgM), complements (C3 and C4), fibrinogen (Fib) and D-dimer (D-D) levels were measured at the time of enrollment and recorded for further analysis. Additional peripheral blood samples were divided into two parts: one was centrifuged shortly after clot formation to obtain serum, and were frozen at -80°C within 2 hours to assay the BDNF level within one month; the other was anticoagulated to obtain peripheral blood mononuclear cells (PBMC).
DM(n=26)
HC(n=30)
Age(years)
45.4(13.5)
46.5(15.6)
Age range(years)
20-67
22-68
Male/Female
8/18
9/21
Duration of disease (months)
3.0
--
LY(109/L)
1.1(0.6)**
2.1(0.4)
PLT (*109/L)
218.1(87.7)
262.1(46.6)
ILD
16
--
Values are mean (SD), LY, lymphocyte; PLT, platelet; ILD, interstitial lung disease. **: p<0.01, compared to HC, Student’s t test
Table 1: Demographic and partial clinical profile of DM patients and healthy controls.
Measurement of serum BDNF level by ELISA
The serum BDNF level were measured by ELISA kits according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN, USA). All assays were performed in duplicate. The data were presented as pg/ml.
Detection of TrkB on the surface of T cells by flow cytometry
Blood specimens were disposed with the use of lymphocyte separation medium (Ficoll-Paque™ PLUS, Amersham Biosciences) and then centrifuged to get PBMCS. The PBMCs were stained with fluorochrome-conjugated mAbs by a 30min incubation at 4°C. Stained samples were acquired by using FACSCan and Cell Quest software (BD Biosciences). The results were analyzed by FlowJo v10 software (Tree Star, Ashland, OR, USA). The expression of TrkB were calculated after gating on CD3+CD4+ or CD3+CD8+ lymphocytes. The antibodies used for the surface staining were as follows: PE -conjugated CD3 antibody (clone HIT3a; BD Biosciences); FITC-conjugated CD4 antibody (clone RPA-T4; BD Biosciences); PE-Cy7-conjugated CD8 antibody (clone RPA-T8; BD Biosciences); APC-conjugated TrkB antibody (Mouse IgG1 Clone; R&D Systems) and their Isotype controls.
Statistical analysis
Data were presented as means ± standard deviation (SD). Student’s t test, One-way analysis of variance (ANOVA) and multiple comparison (Tukey’s test) were used to examine the differences between the controls and the DM patients with ILD or without it. Pearson regression was used to examine the correlation between various laboratory measures and BDNF level. Analysis was performed with the SPSS 17.0 software. Statistical tests with two-tailed p values less than 0.05 were considered significant.
Results
Clinical characteristics of DM patients
Demographic and partial clinical profile of DM patients and healthy controls were summarized in Table 1. From the given data, 61.5% DM patients complicated with ILD. DM patients had a significant decrease in lymphocyte count compared with the controls (1.1±0.6 vs. 2.1±0.4, p<0.001, Student’s t test).
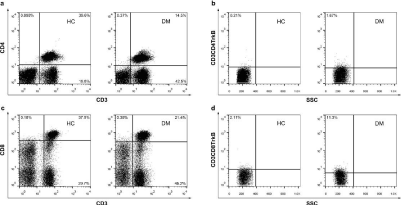
Figure 2: TrkB expression on CD3+CD4+ and CD3+CD8+T cells in DM patients and controls. (a) Fluorescence activated cell sorter (FACS) dot-plots of CD3-CD4. (b) FACS dot–plots of SSC- CD3+CD4+TrkB+. (c) FACS dot–plots of CD3-CD8. (d) FACS dot–plots of SSC- CD3+CD8+TrkB+.
Parameter
BDNF
CD3+CD4+TrkB+
CD3+CD8+TrkB+
r
p
r
p
r
p
WBC (109/L)
0.467*
0.028
0.069
0.765
0.203
0.376
LY (109/L)
0.504**
0.009
-0.108
0.641
0.34
0.131
PLT (109/L)
0.491*
0.011
0.256
0.262
0.006
0.979
ESR(mmH2O/h)
-0.135
0.57
-0.043
0.853
-0.17
0.461
CRP (mg/l)
-0.331
0.132
-0.125
0.589
-0.102
0.66
D-D (ug/ml)
-0.459*
0.018
-0.054
0.816
0.098
0.673
Fib (g/L)
-0.08
0.737
-0.247
0.294
-0.468*
0.033
IgG (g/L)
0.171
0.484
0.12
0.603
0.014
0.952
IgA (g/L)
0.028
0.91
0.157
0.498
-0.13
0.575
IgM (g/L)
0.315
0.189
0.263
0.249
-0.054
0.816
CD4+T
0.245
0.328
0.033
0.89
0.383
0.096
CD8+T
0.184
0.466
0.001
0.996
-0.029
0.904
CD3+T
0.244
0.33
0.018
0.939
0.203
0.391
WBC, white blood cell; LY, lymphocyte; PLT, platelet; ESR, erythrocyte sedimentation rate; CRP, C- reactive protein. D-D, D-dimer; Fib, fibrinogen; IgG, A, M, immunoglobulin G, A, M; CD4+T, CD4+T lymphocyte; CD8+T, CD8+T lymphocyte; CD3+T, CD3+T lymphocyte. *: p<0.05, **: p<0.01, compared to HC, Student’s t test.
Table 2: Correlation between laboratory data and serum BDNF level or percentage of TrkB+ on T cell subsets.
Serum BDNF level in DM patients and healthy controls
The serum BDNF level in DM patients showed a significantly lower level compared with the healthy controls (16012.8±6012.5 pg/ml vs. 33116.5±7146.5 pg/ml, p﹤0.001, Student’s t test) (Figure 1).
TrkB expression on CD3+CD4+ and CD3+CD8+ T cells in DM patients and healthy controls
Flow cytometry analysis showed that the percentage of TrkB expression on the CD3+CD4+ and CD3+CD8+ T cells (described as CD3+CD4+TrkB+/CD3+CD4+, CD3+CD8+TrkB+/CD3+CD8+) was increased significantly when compared to the controls. Figure 2 showed an example of fluorescence activated cell sorter (FACS) dot-plots for CD3-CD4, CD3-CD8, CD3CD4TrkB-SSC, CD3CD8TrkB-SSC, respectively.
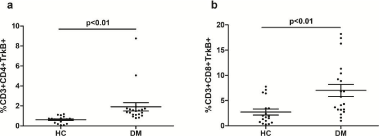
Figure 3: Percentage of TrkB expression on CD3+CD4+ and CD3+CD8+ T cells in DM patients and controls. (a) Mean percentage of CD3+CD4+TrkB+ cells in two groups. (b) Mean percentage of CD3+CD8+TrkB+ cells in two groups. p < 0.01, Student’s t test.
Statistical analysis revealed a of CD3+CD4+TrkB+ and CD3+CD8+TrkB+ T cells in DM patients compared to healthy controls (1.85%±1.81% vs. 0.65%±0.36%, p=0.008; 3.0%±2.74% vs. 6.8%±5.27%, p=0.007 respectively, Student’s t test) (Figure 3(a) and (b)).
Association between the serum BDNF level, TrkB expression and laboratory data
Table 2 presents the results of Pearson regression between laboratory data and serum BDNF level or percentage of TrkB on T cell subsets. The serum BDNF level in DM patients was negatively correlated with D-D (r=-0.464, P=0.018), positively correlated with lymphocyte and PLT count (r=0.505, P=0.009; r=0.503, P=0.011). The percentage of CD3+CD8+TrkB+ T cells was negatively correlated with Fib (r=0.-0.468, p=0.033).
The serum BDNF level and interstitial lung disease
The serum BDNF level in DM patients with ILD was significantly lower than that in the patients without ILD (14586.6±5120.5 pg/ml, n=16 vs. 20455.1±6484.5 pg/ml, n=10, p=0.028 (Figure 4). There was no significant difference in TrkB expression on CD3+CD4+ or CD3+CD8+ T cells between DM with and without ILD (1.81%±1.95% vs. 1.93%±1.58%, p=0.886 ; 6.49%±5.12% vs. 7.60%±6.04%, p=0.674 respectively).
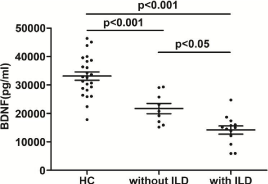
Figure 4: The serum BDNF level in healthy controls, DM patients with ILD (n=16) and without ILD (n =10), p < 0.05, p < 0.001, ANOVA and Tukey’s test.
Discussion
In this study, we, for the first time, examined the serum BDNF level and TrkB expression on T cells in DM patients. We found that the serum BDNF level in DM patients was significantly lower than that in healthy controls, while the TrkB expression on the CD3+CD4+ and CD3+CD8+T cells in peripheral blood was significantly higher. It has been well established that BDNF has potential therapeutic value for some central nervous system degenerative diseases, such as Alzheimer's, Parkinson's and Huntington's diseases [26-28]. Recently, accumulating evidence indicates that neurotrophins play a certain role in immune system diseases, supporting the neuron-immune network theory [29]. Lymphocyte is one of the main sources of BDNF in peripheral blood. TrkB is the specific receptor of BDNF. The binding of BDNF and TrkB can promote the differentiation of T cells into TH1 and TH2, and play an important role in the proliferation and survival of T cells, as well as have anti T cell apoptotic effect [30-32]. Previous studies have suggested that the low BDNF level attenuates the proliferation and differentiation of inflammatory cells and reduces the extension of immune response in autoimmune diseases.
In this study, we found that the serum BDNF level was decreased in DM patients. We speculated that this may be attributable to three factors. Firstly, lymphocyte is one of the main sources of serum BDNF, thus, the low BDNF level might be associated with a marked decrease of lymphocyte count in DM patients. It is well known that DM is often accompanied by lymphopenia [33]. Our data did show a positive correlation between the BDNF level and lymphocyte count.
Secondly, endothelial cells and vascular smooth cells of capillaries and arteries also secret BDNF and express TrkB. BDNF plays an important role in vascular development and survival of vascular endothelial cells [34,35], and can enhance vascular flow and regulate revascularization of ischemic tissues [36]. DM patients often have impaired
endothelial function and inflammation of vascular wall, manifested as skin vacuities, vascular lesions of muscle and pulmonary vacuities. As one of the sources of BDNF, endothelial impairment may also contribute to the decrease of serum BDNF level in DM patients. Moreover, D-D is a sensitive marker of vascular wall damage and acute thrombosis. The more severe of the endothelial cells are damaged, the higher the concentration of D-D is. Our results showed that BDNF and D-D were negatively correlated, suggesting that low BDNF might be significant in judging the damage of vascular wall. Consistent with this, previous reports also found a decrease in the serum BDNF level in systemic sclerosis patients, which has been related to microvascular disease and impaired endothelial function in systemic sclerosis [16].
Thirdly, muscle biopsy of DM patients indicates that there are amount of CD4+T cells infiltrating in the skin rash, muscle tissue and lung [3]. Thus, there is a possibility that a large amount of BDNF accumulates in tissues of DM patients and then is consumed locally. This is required to be further examined using pathological and immunohistochemical methods.
Our results showed that the serum BDNF level of DM patients combined with ILD was the lowest. Endothelial function of DM patients with ILD were more seriously impaired, and muscle biopsy of DM patients indicates that there were amount of CD4+T cells infiltrating in the lung [3]. In addition, Immunoblots revealed that fibroblastic foci in idiopathic pulmonary fibrosis (IPF)/usual interstitial pneumonitis (UIP) strongly was stained for BDNF and TrkB [37–39]. Lung tissue may be the target of CD3+CD4+TrkB+ cells infiltration in DM patients. Those above supported our result that the serum BDNF level of DM with ILD was lower.
It has been reported that the BDNF level in platelet-poor plasma was lower. Platelets may have a non-releasable pool of BDNF or the released BDNF binds to a recognition site on the platelet surface and is internalized. Thrombin and collagen can induce a rapidly amount of release of BDNF from platelets. Our result that the BDNF level was positively correlated with PLT count was accordant with the report above. In contrary, the specific BDNF receptor, TrkB, was not detected in platelets. No BDNF mRNA was detected by Northern blotting in platelets [40].
Until now we do not know the reason of the increase of TrkB expression on T cell surface in DM patients. Though TrkB expression could be up-regulated in response to the decrease of BDNF level, our data does not show a clear negative correlation between serum BDNF level and percentage of TrkB. This issue is worthy of further
investigation to reveal the signal transduction pathways initiated by the binding of BDNF to TrkB, and the interaction between BDNF and other inflammatory factors.
In summary, the present findings provide the first evidence for a role of BDNF level as a marker of disease activity and poor prognosis in DM, especially for the DM which is complicated with ILD. This study raise the possibility, which should be investigated by further studies with larger patient cohorts, that by measuring BDNF at the time of diagnosis we might obtain more information about the disease and the risk of ILD accompanied.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Funding Statement
This work was supported by the following grants: foundation from clinical medical research center of Shenyang, Liaoning, China (18_009-4-03 to PT.Y.), foundation from the Major State Research Development Program of Liaoning, China (No. 2017225024 to PT.Y.), foundation from the Project for Construction of Major Discipline Platform in Universities of Liaoning province, China (2017001 to PT.Y.), the Program of the Distinguished Professor of Liaoning Province, Rheumatology (2017 to PT.Y.).
References
- Lazarou and P Guerne. “Classification, diagnosis, and management of idiopathic inflammatory myopathies.” The Journal of rheumatology. 2013; 40: 550-564.
- BL Adler and L Christopher-Stine. “Triggers of inflammatory myopathy: insights into pathogenesis.” Discovery medicine. 2018; 25: 75-83.
- MC Dalakas. “Muscle biopsy findings in inflammatory myopathies.” Rheumatic diseases clinics of North America. 2002; 28: 779-798.
- M Fujimoto. “Dermatomyositis and Autoantibodies.” Brain and nerve. 2018; 70: 427-438.
- MS Spagnuolo, A Donizetti, L Iannotta, et al. “Brain-derived neurotrophic factor modulates cholesterol homeostasis and Apolipoprotein E synthesis in human cell models of astrocytes and neurons.” Journal of cellular physiology. 2018; 233: 6925-6943.
- Chen, LJ Xiong, Y Tongand M Mao. “The neuroprotective roles of BDNF inhypoxic ischemic brain injury.” Biomedical reports. 2013; 1: 167-176.
- M Kerschensteiner, E Gallmeier, L Behrens, et al. “Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: a neuroprotective role of inflammation?” The Journal of experimental medicine. 1999; 189: 865-870.
- EJ Huang and LF Reichardt. “Trk receptors: roles in neuronal signal transduction.” Annual review of biochemistry. 2003; 72: 609-642.
- CR Rose, R Blum, B Pichler, A Lepier, KW Kafitz and A Konnerth. “Truncated TrkB-T1 mediates neurotrophin-evoked calcium signalling in glia cells.” Nature. 2003; 426: 74-78.
- Besser M, Wank R. “Cutting edge: clonally Restricted Production of the Neurotrophins Brain-Derived Neurotrophic-3 mRNA by Human Immune Cells and Th1/Th2-Polarized Expression of Their Receptors.” The Journal of immunology. 1999; 162: 6303-6306.
- M Torcia, G De Chiara, L Nencioni, et al. “Nerve growth factor inhibits apoptosis in memory B lymphocytes via inactivation of p38 MAPK, prevention of Bcl-2 phosphorylation, and cytochrome c release.” The Journal of biological chemistry. 2001; 276: 39027-39036.
- R Barouch, E Appel, G Kazimirsky, A Braun, H Renz, C Brodie. “Differential regulation of neurotrophin expression by mitogens and neurotransmitters in mouse lymphocytes.” Journal of neuroimmunology. 2000; 103: 112-121.
- M D’Onofrio, U de Grazia, S Morrone, et al. “Expression of neurotrophin receptors in normal and malignant B lymphocytes.” European cytokine network. 2000; 11: 283-291.
- B Schuhmann, A Dietrich, S Sel, Hahn C, Klingenspor M, et al. “A role for brain-derived neurotrophic factor in B cell development.” Journal of neuroimmunology. 2005; 163: 15-23.
- RA Linker, DH Lee, S Demir, et al. “Functional role of brain-derived neurotrophic factor in neuroprotective autoimmunity: therapeutic implications in a model of multiple sclerosis.” Brain. 2010; 133: 2248-2263.
- MC Lise, A Sparsa, I Marie, et al. “Serum Neurotrophin Profile in Systemic Sclerosis.” PloS One. 2010; 5: e13918.
- ER Frota, DH Rodrigues, EA Donadi, DG Brum, DR Maciel and AL Teixeira. “Increased plasma levels of brain derived neurotrophic factor(BDNF) after multiple sclerosis relapse.” Neuroscience Letters. 2009; 460: 130-132.
- M Steinkamp, N Schulte, U Spaniol, C Pflüger, C Hartmann, et al. “Brain derived neurotrophic factor inhibits apoptosis in enteric glia during gut inflammation.” Medical science monitor. 2012; 18: 117-122.
- C Dagnell, J Grunewald, M Kramar, et al. “Neurotrophins and neurotrophin receptors in pulmonary sarcoidosis - granulomas as a source of expression.” Respiratory research. 2010; 11: 156.
- L Fauchais, A Boumediene, F Lalloue, et al. “Brain-derived neurotrophic factor and nerve growth factor correlate with T-cell activation in primary Sjogren's syndrome.” Scandinavian journal of rheumatology. 2009; 38: 50-57.
- LF Tamashiro, RD Oliveira, R Oliveira, et al. “Participation of the neutrophin brain-derived neurotrophic factor in neuropsychiatric systemic lupus erythematosus.” Rheumatology. 2014; 53: 2182-2190.
- Ikenouchi, R Yoshimura, N Ikemura, K Utsunomiya, MJ Mitoma. “Plasma levels of brain derived-neurotrophic factor and catecholamine metabolites are increased during active phase of psychotic symptoms in CNS lupus: a case report.” Progress in neuro-psychopharmacology & Biological Psychiatry. 2006; 30: 1359-1363.
- Ikenouchi-Sugita, R Yoshimura, N Ueda, Y Kodama, W Umene-Nakano, J Nakamura. “Continuous decrease in serum brain-derived neurotrophic factor (BDNF) levels in a neuropsychiatric syndrome of systemic lupus erythematosus patient with organic brain changes.” Neuropsychiatric disease and treatment. 2008; 4: 1277-1281.
- Anthony Bohan, James B Peter. “Polymyositis and dermatomyositis.” The New England Journal of Medicine. 2010; 292: 344-347, 403-407.
- RD Sontheimer. “Clinically amyopathic dermatomyositis: what can we now tell our patients?” Archives of dermatology. 2010; 146: 76-80.
- L Benussi, G Binetti and R Ghidoni. “Loss of Neuroprotective Factors in Neurodegenerative Dementias: The End or the Starting Point?” Frontiers in Neuroscience. 2017; 11: 672.
- RM Lindsay, CA Altar, JM Cedarbaum, C Hyman, SJ Wiegand. “The therapeutic potential of neurotrophic factors in the treatment of Parkinson's disease.” Experimental Neurology. 1993; 124: 103-118.
- T Zimmermann, F Remmers, B Lutz, J Leschik. “ESC-Derived BDNF- Overexpressing Neural Progenitors Differentially Promote Recovery in Huntington's Disease Models by Enhanced Striatal Differentiation.” Stem Cell Reports. 2016; 7: 693-706.
- R Hohlfeld. “Neurotrophic cross-talk between the nervous and immune systems: relevance for repair strategies in multiple sclerosis?” Journal of the Neurological Sciences. 2008; 265: 93-96.
- L De Santi, L Cantalupo, M Tassi, D Raspadori, C Cioni, P Annunziata. “Higher expression of BDNF receptor gp145trkB is associated with lower apoptosis intensity in T cell lines in multiple sclerosis.” Journal of the Neurological Sciences. 2009; 277: 65-70.
- D Azoulay, N Urshansky, A Karni. “Low and dysregulated BDNF secretion from immune cells of MS patients is related to reduced neuroprotection.” Journal of Neuroimmunology. 2008; 195: 186-193.
- M Maroder, D Bellavia, D Meco, et al. “Expression of trKB neurotrophin receptor during T cell development. Role of brain derived neurotrophic factor in immature thymocyte survival.” Journal of Immunology. 1996; 157: 2864-2872.
- M Viguier, S Fouéré, P de la Salmonière, et al. “Peripheral blood lymphocyte subset counts in patients with dermatomyositis clinical correlations and changes following therapy.” Medicine. 2003; 82: 82-86.
- MJ Donovan, MI Lin, P Wiegn, et al. “Brain derived neurotrophic factor is an endothelial cell survival factor required for intramyocardial vessel stabilization.” Development. 2000; 127: 4531-4540.
- T Nakahashi, H Fujimura, CA Altar, et al. “Vascular endothelial cells synthesize and secrete brain-derived neurotrophic factor.” FEBS Letters. 2000; 470: 113-117.
- P Kermani, D Rafii, DK Jin, et al. “Neurotrophins promote revascularization by local recruitment of TrkB+ endothelial cells and systemic mobilization of hematopoietic progenitors.” Journal of Clinical Investigation. 2005; 115: 653-663.
- Ricci, L Felici, S Mariotta, et al. “Neurotrophin and Neurotrophin Receptor Protein Expression in the human lung.” American Journal of Respiratory Cell and Molecular Biology. 2004; 30: 12-19.
- Ricci, S Greco, F Amenta, et al. “Neurotrophins and neurotrophin receptors in human pulmonary arteries.” Journal of Vascular Research. 2000; 37: 355-363.
- P Kermani, D Rafii, DK Jin, et al. “Neurotrophins promote revascularization by local recruitment of TrkB+endothelial cells and systemic mobilization of hematopoietic progenitors.” Journal of Clinical Investigation. 2005; 115: 653-663.
- H Fujimura, CA Altar, R Chen, et al. “Brain-derived neurotrophic factor is stored in human platelets and released by agonist stimulation.” Thrombosis and Haemostasis. 2002; 87: 728-734.