
Research Article
J Immun Res. 2018; 5(1): 1032.
Effect of Fingolimod on the Frequency of Regulatory T Cells in Patients with Relapsing-Remitting Multiple Sclerosis
Sedaghat N1,5, Motedayyen H2, Etemadifar M3,5, Zarkesh H4, Kianpour F1, Vestri E6 and Alsahebfosoul F1,5*
1Department of Immunology, Faculty of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran
2Department of Laboratory Sciences, School of Allied Medical Sciences, Kashan University of Medical Sciences, Kashan, Iran
3Department of Neurology, Faculty of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran
4Department of Biology, Faculty of Sciences, University of Isfahan, Isfahan, Iran
5Isfahan Multiple Sclerosis (MS) and Neuroimmunology Research Center, Isfahan, Iran
6Department of Pediatric Surgery, Palermo University of Medicine and Surgery Polyclinic “Paolo Giaccone”, Palermo, Italy
*Corresponding author: Alsahebfosoul F, Department of Immunology, Faculty of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran
Received: June 27, 2018; Accepted: August 03, 2018; Published: August 10, 2018
Abstract
Multiple sclerosis (MS) likely results from an imbalance between pathogenic and regulatory T cells which lead to multifocal demyelination and axonal loss. Fingolimod, an immunomodulator, inhibits egress of lymphocytes from lymph nodes and their recirculation, approved for the treatment of MS. In this study, we investigated whether fingolimod affects immune imbalance occurred in MS patients through regulatory T (T reg) cells. Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood obtained from 20 relapsing-remitting MS (RRMS) patients and 10 healthy subjects using Ficoll density centrifugation. Then, RRMS patients were treated by fingolimod (0.5 mg/day). After one month, blood samples were obtained from patients and PBMCs were isolated. The frequency of T reg cells in PBMCs from healthy subjects and MS patients before and after fingolimod therapy was measured by flow cytometry. Our data showed that the percentage of circulating T reg cells was significantly higher in MS patients than healthy subjects. Fingolimod had a potent effect on increasing T reg cell number in MS patients which this effect declined with age. These findings suggest that fingolimod can be considered as an effective therapeutic approach for modulating abnormal immune responses and restoring a balance between pathogenic and regulatory T cells in patients with MS.
Keywords: Multiple sclerosis; Regulatory T cell; Fingolimod; Therapeutic approaches
Abbreviations
PBMCs: Peripheral Blood Mononuclear Cells; MS: Multiple Sclerosis; RRMS: Relapsing-Remitting MS; T reg: Regulatory T cell; BBB: Blood-Brain Barrier; CNS: Central Nervous System; IFN- β: Interferon-β; S1P: Sphingosine-1-Phosphate; S1PR: Sphingosine-1-phosphate Receptor; GITR: Glucocorticoid-induced TNF Receptor; CTLA-4: Cytotoxic T Lymphocyte Antigen-4; RA: Rheumatoid Arthritis; TGF-β1: Transforming Growth Factor; IL-10: Interleukin-10; IL-35: Interleukin-35; PBS: Phosphate Buffered Saline; FITC: Fluorescein Isothiocyanate; PE/Cy5: Phycoerythrin/Cyanine 5; PE: Phycoerythrin; SEM: Standard Error Of Mean; EDSS, Expanded Disability Status Scale; DMT: Disease-Modifying Treatment.
Introduction
Multiple sclerosis (MS) is an inflammatory disease of the brain and spinal cord, which leads to multifocal demyelination and axonal loss [1,2]. The etiology of MS is not fully understood so far, but several distinct genetic and environmental factors have been proposed to disease susceptibility [3-5]. This disorder is largely associated with the breakdown of peripheral tolerance to self-antigens such as myelin antigens and to lesser extent axon antigens, which leads to T cells specific to antigens, especially T helper type 1 (Th1) and Th17 cells, migrate across the blood-brain barrier (BBB) and mediate central nervous system (CNS) tissue injury [1,6,7]. The presence of T cells and their cytokines in the brain lesions of MS patients have provided convincing evidence that autoreactive T cells are the main effector cells responsible for the central neurons damage and their myelin sheaths and axons, however more recent studies have shown that autoreactive B cells also contribute to the pathogenesis of disease, through autoantibody production, antigen presentation, or cytokine secretion [1,8-10]. Several therapeutic approaches for MS patients have been proposed to modulate abnormal immune responses such as interferon-β (IFN-β), natalizumab, glatiramer acetate and fingolimod (FTY720) [11-13]. The specific aim of these treatments are mainly focused at reducing the number of relapses and halting the progression of neurologic disability, although they have not been uniformly successful and are useful in arresting the disease in approximately 30 % of relapsing-remitting (RR) MS patients, which is the most common form of MS [1,11,14].
Fingolimod is a structural and functional analog of the natural serum lipid sphingosine-1-phosphate (S1P) which leads to inhibit the exit of lymphocytes from lymph nodes [15,16]. A numerous studies have reported that fingolimod causes peripheral blood lymphopenia through down-regulation of sphingosine-1-phosphate receptor (S1PR) expression on activated lymphocytes [12,17]. Extensive data from the literature have demonstrated effectiveness of fingolimod in reducing the migration of autoreactive lymphocytes from lymph nodes to brain lesions in MS patients and slowing the progression of disease [16,18,19]. Moreover, several studies have reported that fingolimod exert some central mechanisms such as S1PR modulation on neurons and glia to reduce inflammation, relapse rates and the risk of disability progression and support structural restoration of the brain lesions [12,16,18,20,21].
Regulatory T (T reg) cell is a key component in the maintenance of immunologic tolerance to self-antigens [22]. These cells comprise 5 to 10% of the peripheral blood CD4+ T cells which are characterized by the expression of some markers such as Glucocorticoid-induced TNF receptor (GITR), cytotoxic T lymphocyte antigen-4 (CTLA- 4), CD25 marker, and Foxp3 transcription factor. T reg cells play a pivotal role in preventing the development of autoimmune diseases through the control all aspects of the immune response [23]. Previous studies have reported that reduced number and impaired function of T reg cells participate in the development of various autoimmune diseases such as MS, allergy, and rheumatoid arthritis (RA) [24]. It has been shown that the inhibitory function of T reg cells is mediated by cell-cell contact, consumption of IL-2, and the production of immunosuppressive mediators such as transforming growth factor (TGF-β1), interleukin-10 (IL-10), and interleukin-35 (IL-35) [25-27].
Regarding the fact that fingolimod therapy effectively produces peripheral blood lymphopenia, we investigated whether fingolimod therapy can affect the percentage of T reg cells in peripheral blood of RRMS patients. Furthermore, we evaluated how the age of patients can influence the effect of fingolimod administration on the frequency of circulating T reg cells in RRMS patients.
Materials and Methods
Study population
A total of 20 RRMS subjects were recruited among those referred to the clinic of autoimmune diseases of Alzahra hospital, Isfahan, Iran from May 2017 to December 2017 (Table 1). RRMS disease was diagnosed by specialist according to the Mac Donald’s diagnostic criteria for MS disease [28,29]. The sampling from RRMS patients was performed at least 1 week (baseline) before any immunosuppressive modalities, including natalizumab, glatiramer acetate, interferonbeta (TFN-β), and fingolimod therapy and four weeks after initiation of fingolimod therapy (0.5 mg/day). RRMS patients experienced mild MS relapses, but severe MS relapses have also been observed following fingolimod initiation. 10 healthy volunteers were also participated in this study (Table 1). The study was approved by the Ethics Committee of Isfahan University of Medical Sciences, Isfahan, Iran. The informed consent was provided from all participants before entering the study.
Patient group (n = 20)
Control group (n=10)
Age, year (range of age)
33.7±6.0 (27-47)
28.75±2.9 (25-56)
Gender (male/female)
4/16
2/8
Age is shown as mean±standard deviation (SD).
Table 1: Specifications of the studied subjects.
Isolation of peripheral blood mononuclear cells (PBMCs)
Heparinized blood samples (10ml) were obtained from RRMS patients and healthy individuals. PBMCs were isolated from whole blood using Ficoll gradient centrifugation according to the manufacturer’s guideline (Lymphodex, Germany). The isolated cells were washed three times with phosphate buffered saline (PBS). The cells were counted with a haemocytometer. Cell viability was also determined using trypan blue dye exclusion.
Flow cytometry
To investigate the effects of fingolimod on the percentage of CD4+Foxp3+GITR+cells in peripheral blood of RRMS patients, PBMCs isolated from participants were stained by fluorescein isothiocyanate (FITC) anti-human CD4 (eBiosciences, USA) and phycoerythrin/cyanine 5 (PE/Cy5) anti-human GITR antibodies (Biolegend, USA) for 30 min at 4°C. The relevant isotype control antibodies were used as negative control. Further, the cells were washed twice with PBS and centrifuged at 200×g for 5min. Fixation and permebilization of the cells were performed using Foxp3 Fix/Perm buffer set and cell staining buffer according to the manufacturere’s protocol (Biolegend, USA). The cells were then stained by phycoerythrin (PE) anti-human Foxp3 (Biolegend, USA) for 30 min at 4°C. The percentage of the stained cells was assessed using a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA). In this regard, lymphocyte population was gated using forward and side scatter in order to exclude non-lymphocyte populations or debris from the analysis of CD4+GITR+Foxp3+ cells. The gated population was then analyzed using FlowJo software (v10.1, FlowJo, Ashland, OR, USA). In this experiment, the CD4+GITR+Foxp3+ cells were considered as T reg cells.
Statistical analysis
Data were analyzed by GraphPad Prism 6 (GraphPad software, San Diego, CA) and expressed as mean ± standard error of mean (SEM). Unpaired t test was used to compare two groups with normal distribution and Mann-Whitney test in the case of non-normal distribution. Pearson and Spearman tests were used to determine the correlation coefficients of the data with normal distribution and nonnormal distribution, respectively. P<0.05 was considered statistically significant.
Results
Description of subjects
20 RRMS subjects (aged 33.7±6.0, mean±standard deviation (SD), range 27 to 47 years) participated in the study. The onset of clinical symptoms in patients was varied and occurred from an age of 25 years old to 47 (Table 2). The patients had a wide age range in the diagnosis of the illness, range of 3 years old to 22 (Table 2). The most common clinical manifestations among RRMS patients were difficulties with coordination and balance (ataxia), needles or numbness, and tingling (Table 2). Of the 20 RRMS patients, 12 had difficulties with coordination and ataxia, 10 patients had needles or numbness, seven had tingling, six had diplopia or blurred vision, one had seizure, type II diabetes, hepatitis A infection, and depression (Table 2). The demographic and clinical characteristics of patients and healthy subjects are shown in Table 2.
RRMS patients
Healthy control
Disease duration, year (range of age)
The onset of clinical symptoms, year (range of age)
9.1±4.8 (3-22)
36±7.9 (25-47)
NA
NA
EDSS score
2.0±1.0
NA
Difficulty in moving
12
0
Needles or numbness
10
0
Tingling
7
0
Diplopia or blurred vision
6
0
Seizure
1
0
Type II diabetes
1
0
Hepatitis A
1
0
Depression
1
0
Table 2: Demographic and clinical characteristics of RRMS and healthy subjects.
Effect of fingolimod on the percentage of circulating T reg in RRMS patients
To evaluate fingolimod effect on the number of T reg cells in peripheral blood, the percentage of CD4+GITR+Foxp3+ cell was measured by flow cytometry. This study indicated that there was a significant difference in T reg cell percentage between RRMS patients and healthy subjects (p<0.0001-0.01, Figure 1A and B). The frequency of T reg cells within CD4+ subpopulation was significantly higher in untreated patients than healthy control (p<0.01, Figure 1A and B). Moreover, we found that fingolimod therapy had a positive effect on increasing the number of T reg cells in RRMS patients (p<0.001, Figure 1A and B). Our data showed that the frequency of T reg cells within CD+ subpopulation was significantly higher in fingolimodtreated patients than untreated patients and healthy subjects (p<0.0001-0.001, Figure 1A and B).
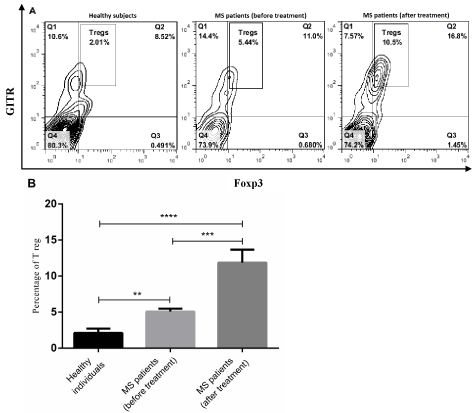
Figure 1: The frequency of peripheral blood T reg cells in MS and healthy subjects. PBMCs obtained from healthy individuals (n=10), MS patients (before
and after treatment with fingolimod) (n=20) were stained with anti-CD4, anti-GITR and anti-Foxp3 antibodies. A) The percentage of T reg cells in PBMCs were
measured by flow cytometry (B) and then analyzed. Each bar in B shows mean±SEM. **p<0.01,* **p<0.001,****p<0.0001.
Association of T reg cell number with patient characteristics
The results of Pearson test showed a significant positive correlation between the age of RRMS patients and the number of T reg cells before fingolimod therapy (p<0.01, R=0.5827 95% CI=0.1885-0.8150, Figure 2A), while this significant correlation was inverse after fingolimod therapy (p<0.05, R=-0.450, 95% CI=0.74458-0.00974, Figure 2B). Other results of Pearson and Spearman tests revealed that there was no significant association between sex, disease duration, and EDSS (Expanded Disability Status Scale) score and T reg cell frequency in RRMS patient before and after fingolimod administration.
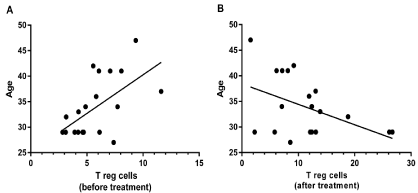
Figure 2: Correlation between the percentage of T reg cells and age of MS patients. A) Result of Pearson test showed a positive correlation between the
number of T reg cells in MS patients (before treatment) and age (p<0.01). B) There was an inverse correlation between T reg cell frequency in MS patients (after
treatment) and age (p<0.05).
Discussion
Fingolimod is a disease-modifying treatment (DMT) which effectively inhibits the migration of lymphocytes from lymph nodes to blood and peripheral tissues, leading to reduce relapse frequency and short-term disability progression in MS patients [15]. In this study, we assessed the number of peripheral blood T reg cells in RRMS patients treated with fingolimod. Our results showed that fingolimod therapy had a potent effect on the increased number of T reg cells in RRMS patients. Moreover, we observed that fingolimod effect on the increased frequency of T reg cells in MS patients declined with age.
Having considered that T reg cells play an indispensible role in preventing and developing the risk of autoimmune disorders through the maintenance of peripheral tolerance to self-antigens [23], the critical question was whether the percentage of T reg cells in RRMS patients differed from healthy subjects. Unexpectedly, the results of study revealed that RRMS patients had a significant increase in T reg cell number in peripheral blood compared to healthy control subjects. Although many reports have indicated a decreased number of T reg cells in peripheral blood of MS patients [30-32], there are several studies pointing to the similar distribution of T reg cell frequency in MS patients and healthy subjects [33]. Moreover, some studies have indicated that the frequency of T reg cells may increase in peripheral blood of chronic MS patients due to the expansion of memory T reg cells [34]. Several studies have reported that the thymic output of naive T reg cells is impaired in MS which can compensated by higher proportions of memory T reg cells, resulting in a stable or higher cell count of the total T reg cell population [34,35]. Therefore, the increased frequency of memory T reg cells is likely a compensatory response to the decreased number or impaired function of T reg cells in MS which participate in development of disease. This hypothesis corresponded well with many studies on MS patients showing the decreased number or reduced suppressive function of T reg cells on the T cell immune response against auto-antigens in MS patients [30,34-36].
In the next step, we evaluated the effect of fingolimod on the frequency of T reg cells in RRMS patients. Our data revealed that the number of circulating T reg cells within CD4+ subpopulation was significantly increased in patients after fingolimod administration. Consistent with this observation, previous studies on patients with MS have also demonstrated that fingolimod increases the frequency of circulating T reg cells [15,32,37]. Moreover, Muls et al (2014) reported that fingolimod had a stimulatory activity on the increased number of CD39-expressing T reg cells in MS patients which exert an anti-inflammatory function through cleave ATP to AMP by CD39 [38]. Several lines of evidence revealed that apart from these effects of fingolimod are due to its effects on lymphocyte egress from lymphoid organs [38,39]. Previous studies also provide possible lines of explanation for these observations. It has been reported that S1PR signaling upon engagement of S1P inhibits Treg cell differentiation through down-regulating SMAD3 activity and subsequently reducing TGF-β receptor-mediated signaling, which is required for T reg cell development [39]. Moreover, in vitro studies have shown that fingolimod stimulates the production of TGF-β in splenocyte cell cultures [40]. Inhibition in S1PR signaling and induction of TGF-β production of immune cells by fingolimod may therefore participate in regulating the balance towards T reg cell development. Consequently, it is likely that fingolimod possess the ability to modulate pathogenic immune responses and restore a balance between pathogenic and regulatory T cells in patients with MS.
Given that it has been reported that the number of human peripheral blood T reg cells increases with age, we investigated whether other patient characteristics can affect the frequency of T reg cells and also the effect of fingolimod on T reg cells in MS patients. The results of Pearson and Spearman tests indicated that the frequency of T reg cells in MS patients before and after treatment with fingolimod was not associated with EDSS score, disease duration, and sex. However, there was a positive association between T reg cell number and patient age which this correlation was disrupted following fingolimod administration. These data suggest that fingolimod had more effective effect on increasing the number of T reg cells in patients with lower age than those of higher age. This unexpected result points to other unknown factors and/ or non-immunologic dependent mechanisms which may be involved in the effects of fingolimod on increasing T reg cell number in MS patients. Nevertheless, it should be noted that additional studies are required to confirm our results and explain the molecular mechanisms involved in the negative effect of the age on the frequency of T reg cells in fingolimod-treated patients.
Conclusion
Taken together, the results of this study provide evidence to show that fingolimod therapy has a potent effect on the increased percentage of peripheral blood T reg cells in patients with RRMS which decline with age. Based on these findings, fingolimod can be considered as one of effective therapeutic approaches for modulating abnormal immune responses and improving peripheral tolerance to self-antigens in patients with MS.
Acknowledgments
The authors would like to thank Felora Fatehi for her careful training of patients in the use of fingolimod therapy and for her excellent nursing care. We also thank Iranian blood transfusion organization and Osveh company for their help and support of the study.
Funding
This study was financially supported by Isfahan University of Medical Sciences (IUMS).
References
- Fletcher JM, Lalor S, Sweeney C, Tubridy N, Mills K. T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin Experi Immunol. 2010; 162: 1-11.
- Frohman EM, Racke MK, Raine CS. Multiple sclerosis—the plaque and its pathogenesis. N Engl J Med. 2006; 354: 942-955.
- Hedström AK, Bomfim IL, Barcellos L, Gianfrancesco M, Schaefer C, Kockum I, et al. Interaction between adolescent obesity and HLA risk genes in the etiology of multiple sclerosis. Neurol. 2014; 82: 865-872.
- Dick G. The etiology of multiple sclerosis. SAGE Publications; 1976.
- Johnson R. The possible viral etiology of multiple sclerosis. Adv Neurol. 1975; 13: 1-46.
- Compston A, Coles A. Multiple sclerosis. Lancet. 2008; 372: 1502-1517.
- McDonald W, Sears T. The effects of experimental demyelination on conduction in the central nervous system. Brain. 1970; 93: 583-598.
- Montalban X, Hauser SL, Kappos L, Arnold DL, Bar-Or A, Comi G, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017; 376: 209-220.
- Genain CP, Cannella B, Hauser SL, Raine CS. Identification of autoantibodies associated with myelin damage in multiple sclerosis. Nat Med. 1999; 5: 170.
- Storch MK, Piddlesden S, Haltia M, Iivanainen M, Morgan P, Lassmann H. Multiple sclerosis: in situ evidence for antibody and complement mediated demyelination. Ann Neurol. 1998; 43: 465-471.
- McDonald CA, Payne NL, Sun G, Moussa L, Siatskas C, Lim R, et al. Immunosuppressive potential of human amnion epithelial cells in the treatment of experimental autoimmune encephalomyelitis. J Neuroinflammation. 2015; 12: 112.
- Cohen JA, Chun J. Mechanisms of fingolimod’s efficacy and adverse effects in multiple sclerosis. Ann Neurol. 2011; 69: 759-777.
- Lorscheider J, Benkert P, Lienert C, Hänni P, Derfuss T, Kuhle J, et al. Comparative analysis of natalizumab versus fingolimod as second-line treatment in relapsing–remitting multiple sclerosis. Multiple Sclerosis J. 2018: 1352458518768433.
- Kappos L, Radue E-W, O’connor P, Polman C, Hohlfeld R, Calabresi P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010; 362: 387-401.
- Sato DK, Nakashima I, Bar-Or A, Misu T, Suzuki C, Nishiyama S, et al. Changes in Th17 and regulatory T cells after fingolimod initiation to treat multiple sclerosis. J Neuroimmunol. 2014; 268: 95-98.
- Brinkmann V, Billich A, Baumruker T, Heining P, Schmouder R, Francis G, et al. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat Rev Drug Discov. 2010; 9: 883-897.
- Chun J, Hartung H-P. Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin Neuropharmacol. 2010; 33: 91.
- Brinkmann V. FTY720 (fingolimod) in multiple sclerosis: therapeutic effects in the immune and the central nervous system. British J Pharmacol. 2009; 158: 1173-1182.
- Cohen JA, Barkhof F, Comi G, Hartung H-P, Khatri BO, Montalban X, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010; 362: 402-415.
- O’Connor P, Comi G, Montalban X, Antel J, Radue E, De Vera A, et al. Oral fingolimod (FTY720) in multiple sclerosis two-year results of a phase II extension study. Neurol. 2009; 72: 73-79.
- Choi JW, Gardell SE, Herr DR, Rivera R, Lee C-W, Noguchi K, et al. FTY720 (fingolimod) efficacy in an animal model of multiple sclerosis requires astrocyte sphingosine 1-phosphate receptor 1 (S1P1) modulation. Proc Natl Acad Sci. 2011; 108: 751-756.
- Arumugakani G, Wood PM, Carter CR. Frequency of Treg cells is reduced in CVID patients with autoimmunity and splenomegaly and is associated with expanded CD21lo B lymphocytes. J Clin Immunol. 2010; 30: 292-300.
- Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009; 30: 636-645.
- Strickland DH, Holt PG. T regulatory cells in childhood asthma. Trends Immunol. 2011; 32: 420-427.
- Lim KP, Chun NAL, Ismail SM, Abraham MT, Yusoff MN, Zain RB, et al. CD4+ CD25hiCD127low regulatory T cells are increased in oral squamous cell carcinoma patients. PloS One. 2014; 9: e103975.
- Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008; 9: 239.
- Cretney E, Kallies A, Nutt SL. Differentiation and function of Foxp3+ effector regulatory T cells. Trends Immunol. 2013; 34: 74-80.
- Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018; 17: 162-176.
- Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011; 69: 292-302.
- Venken K, Hellings N, Thewissen M, Somers V, Hensen K, Rummens JL, et al. Compromised CD4+ CD25high regulatory T-cell function in patients with relapsing remitting multiple sclerosis is correlated with a reduced frequency of FOXP3 positive cells and reduced FOXP3 expression at the single-cell level. Immunol. 2008; 123: 79-89.
- Matarese G, Carrieri PB, La Cava A, Perna F, Sanna V, De Rosa V, et al. Leptin increase in multiple sclerosis associates with reduced number of CD4+ CD25+ regulatory T cells. Proc Nat Acad Sci U S A. 2005; 102: 5150-5155.
- Serpero LD, Filaci G, Parodi A, Battaglia F, Kalli F, Brogi D, et al. Fingolimod modulates peripheral effector and regulatory T cells in MS patients. J Neuro Pharmacol. 2013; 8: 1106-1113.
- Feger U, Luther C, Poeschel S, Melms A, Tolosa E, Wiendl H. Increased frequency of CD4+ CD25+ regulatory T cells in the cerebrospinal fluid but not in the blood of multiple sclerosis patients. Clin Experi Immunol. 2007; 147: 412-418.
- Venken K, Hellings N, Broekmans T, Hensen K, Rummens J-L, Stinissen P. Natural naive CD4+ CD25+ CD127low regulatory T cell (Treg) development and function are disturbed in multiple sclerosis patients: recovery of memory Treg homeostasis during disease progression. J Immunol. 2008; 180: 6411- 6420.
- Haas J, Fritzsching B, Trübswetter P, Korporal M, Milkova L, Fritz B, et al. Prevalence of newly generated naive regulatory T cells (Treg) is critical for Treg suppressive function and determines Treg dysfunction in multiple sclerosis. J Immunol. 2007; 179: 1322-1330.
- Buckner JH. Mechanisms of impaired regulation by CD4+ CD25+ FOXP3+ regulatory T cells in human autoimmune diseases. Nat Rev Immunol. 2010; 10: 849.
- Claes N, Dhaeze T, Fraussen J, Broux B, Van Wijmeersch B, Stinissen P, et al. Compositional changes of B and T cell subtypes during fingolimod treatment in multiple sclerosis patients: a 12-month follow-up study. PloS One. 2014; 9: e111115.
- Muls N, Dang HA, Sindic CJ, Van Pesch V. Fingolimod increases CD39- expressing regulatory T cells in multiple sclerosis patients. PloS One. 2014; 9: e113025.
- Liu G, Burns S, Huang G, Boyd K, Proia RL, Flavell RA, et al. The receptor S1P 1 overrides regulatory T cell–mediated immune suppression through Akt-mTOR. Nat Immunol. 2009; 10: 769.
- Sehrawat S, Rouse BT. Anti-inflammatory effects of FTY720 against viralinduced immunopathology: role of drug-induced conversion of T cells to become Foxp3+ regulators. J Immunol. 2008; 180: 7636-7647.