
Special Article – Cancer Immunology
Cells. J Immun Res. 2019; 6(1): 1033.
Impact of Oxidized Low Density Lipoprotein on Cytokine Production and Immune Dialogue between Mononuclears and Colon Carcinoma Cells
Ganelin-Cohen E1,3, Djaldetti M2,3* and Bessler H2,3
1Institute of Pediatric Neurology, Schneider Children’s Medical Center of Israel, Petah Tikva, Israel
2Laboratory for Immunology and Hematology Research, Rabin Medical Center Hasharon Hospital, Petah-Tiqva, Israel
3The Sackler School of Medicine, Tel-Aviv University, Ramat Aviv, Israel
*Corresponding author: Djaldetti M, Laboratory for Immunology and Hematology Research, Rabin Medical Center, Hasharon Hospital, 7, Keren Kayemet St, Petah Tiqva, Israel
Received: May 12, 2019; Accepted: July 15, 2019; Published: July 22, 2019
Abstract
Background: Cholesterol is carried out to the cells via Low (LDL) - and High (HDL) Density Lipoproteins. In its oxidized state (Ox-LDL) it possesses immunomodulatory properties and is capable to promote carcinogenesis. The aim of the study was to examine its effect on the proliferation of human Peripheral Blood Mononuclear Cells (PBMC) and cells from two colon carcinoma cell lines. Cytokine production by PBMC incubated without and with cancer cells in the presence of Ox-LDL was evaluated.
Methods: PBMC were incubated with 1μg/ml, 2μg/ml and 5μg/ml Ox- LDL and the secretion of TNFα, IL-1β, IL-2, IL-6, IL-10, IL-1ra and IFNγ was evaluated. The production of cytokines by PBMC stimulated with cells from HT- 29 or HuCC lines was detected upon the effect of various doses of Ox-LDL.
Results: Ox-LDL stimulated the proliferation of PBMC and HuCC cells but not on that of HT-29. Ox-LDL increased the production of IL-6, IFNγ and IL-10 by non-stimulated PBMC and that of TNFα, IL-1β and IFNγ by mitogen stimulated cells. Ox-LDL added to co-cultures of PBMC and HT-29 cells inhibited all cytokines, except for IL-2 that augmented with the highest concentration of Ox- LDL. PBMC incubated with HuCC cells in the presence of Ox-LDL produced decreased production of TNFα, while the secretion of the remaining cytokines was not affected.
Conclusions: Ox-LDL promotes PBMC to produce a part of the cytokines hereby examined. The inhibited secretion of all cytokines by PBMC incubated with HT-29 cells in the presence of Ox-LDL may clarify the immunomodulatory role of Ox-LDL in carcinogenesis.
Keywords: Oxidized LDL; Mononuclear Cells; Colon Cancer Cells; Cytokines; Immune Dialogue
Introduction
The importance of cholesterol for the course of normal body functions has been decisively established. Its numerous activities at cellular and organismal levels as a precursor of steroid and other hormones’ production, its status as a basic substance for vitamins’ creation and the role it plays as a supplementary ingredient for regulation of numerous metabolic events have been masterly reviewed by Cortes et al. [1]. The traffic of cholesterol to the cells is carried out by two lipoproteins i.e. high- and low density lipoprotein, designated shortly as HDL and LDL respectively. In fact, their existence is the reason that transforms the biological properties of cholesterol to a double edge sword. While HDL is accepted as a protector against heart and brain noxious events, the LDL is blamed for development of vascular diseases and increased risk for heart disorders [2]. Low density lipoprotein, in its oxidized state (Ox- LDL) infiltrates the arterial wall, promotes monocyte migration and is therefore tightly involved in inflammatory conditions serving as a basis for atherogenesis. Macrophages mobilized at the affected vessel wall remove Ox-LDL, transform to foam cells and became activated for inflammatory cytokine production resulting in exacerbation of atherosclerosis [3]. On the other hand, clinical trials to reduce formation of Ox-LDL by administration of antioxidants showed disappointing results [4]. The components and the biological activities of the Ox-LDL and its different properties from the native LDL have been detailed by Parhasarathy et al. [5]. It has been observed that lowering LDL levels in patients with familial hypercholesterolemia ensued in decreased intracellular lipid accumulation with augmented anti-inflammatory activity of the circulating monocytes [6,7]. Considering the role of the classically activated pro-inflammatory M1 monocytes in promoting inflammation, one would expect that following their interaction with Ox-LDL these cells will became the active player in atherogenesis. Unexpectedly, it has been observed that following such an exposure, the alternatively activated M2 antiinflammatory monocytes are more prompt to foam cell formation, increased production of the pro-inflammatory cytokines IL-6 and IL-8 [8,9] and to decreased secretion of the anti-inflammatory cytokines TGFβ and IL-10 [10]. The association of dyslipidemia and cancer hazard is a topic of interest. Hypercholesterolemia and obesity have been found to increase the risk of colorectal cancer [11]. Observations on a mouse model of breast cancer have indicated that cholesterol enhances tumor development and aggressiveness [12]. Moreover, since plasma cholesterol level declined with tumor progression, it has been suggested that malignant cells over utilize cholesterol concomitant with their growth. Although epidemiological studies support the role of cholesterol in tumor development, this linkage remains controversial [13]. The same goes as for the role of LDL in carcinogenesis. In a meta-analysis on the subject Tian et al. [14] have found that high LDL levels were positively linked with predominance of colorectal adenoma, but not with colorectal neoplasm. In a series of 122 patients with advanced ovarian cancer the survival for those with normal LDL level was 27 months compared to 12 months for patients with elevated LDL [15]. Studies in vitro have showed that LDL stimulates breast cancer cell proliferation and metastasis via Akt induced Epithelial Mesenhymal Transition (EMT) [16]. On the other hand no association has been found between cholesterol, LDL and HDL and the risk of prostate cancer recurrence [17]. Based on the LDL attribute to act as an immunomodulator and as a possible carcinogenesis promoter, the aim of the present study was twofold - to evaluate the capacity of Ox-LDL for cytokine production by human Peripheral Blood Mononuclear Cells (PBMC) and to examine its effect on the cross-talk between immune and colon cancer cells from two human lines.
Materials and Methods
Cell preparation
The study was approved by the Ethics Committee of Rabin Medical Center. Blood Bank donors provided a written informed consent containing an agreement that components of their blood not needed for therapeutic purposes could be used for medical research. Peripheral Blood Mononuclear Cells (PBMC) were separated from venous blood by Lymphoprep-1077 (Axis-Shield PoC AS, Oslo, Norway) gradient centrifugation. The cells were washed twice in Phosphate Buffered Saline (PBS) and suspended in RPMI-1640 medium (Biological Industries, Beith Haemek, Israel) containing 1% penicillin, streptomycin and nystatin, 10% Fetal Calf Serum (FCS), and was designated as Complete Medium (CM).
Colon cancer cell lines
HT-29 and HuCC human colon cancer cell lines were obtained from the American Type Cultural Collection, Rockville, MD. The cells were maintained in CM containing Mc-COY’S 5A medium and Modified Eagle Medium (MEM- Biological Industries Co, Beth-Haemek, Israel) respectively, supplemented with 10% Foetal Bovine Serum (FBS), 2mM L-glutamine and antibiotics (penicillin, streptomycin and nystatin-Biological Industries Co, Beth-Haemek, Israel). The cells were grown in T-75 culture flasks at 37°C in a humidified atmosphere containing 5% CO2.
Human oxidized low density lipoprotein (Ox-LDL)
Low Density Lipoprotein (LDL) from human plasma, oxidized by copper-mediated process was purchased from Fisher Scientific, Israel, as a solution of 2.5mg/ml, stored at 4°C and protected from light. Further dilutions were prepared in CM. Ox-LDL was added at the onset of the cultures at final concentrations of 1μg/ml, 2μg/ml and 5μg/ml.
Effect of Ox-LDL on cell proliferation
The effect of Ox-LDL on PBMC, HT-29 and HuCC proliferation was detected using XTT proliferation assay kit (Biological Industries, Beith Haemek, Israel). In short, 0.1ml aliquots of PBMC or HT- 29 and HuCC cells obtained after trypsinization and suspended in appropriate CM at 105/ml were added to each one of 96 well plates and incubated for 24hrs in the absence or presence of Ox-LDL added at the onset of cultures at concentrations as indicated. At the end of the incubation period the cells were stained according to the manufacturer’s instructions. The plates were incubated for 3hrs at 37°C in a humidified incubator containing 5% CO2 and the absorbance was measured at 450nm using ELISA reader.
Effect of Ox-LDL on cytokine production
1.0ml of PBMC (2x106/ml of CM) was incubated without or with LPS (50ng/ml) for TNFα, IL-1β, IL-6, IL-10, and IL-1ra production, or with PMA 1μg/ml and ionomycin 0.5μg/ml for IL-2 and IFNγ secretion. In another set of experiments, 0.5ml of PBMC (4x106/ml of CM) was incubated with 0.5ml of CM or with one of the colon cancer cell lines (4x105/ml) suspended in appropriate CM. Ox-LDL was added at the onset of cultures at concentrations as described. Cultures without Ox-LDL served as controls. The cultures were maintained for 24hrs at 37°C in a humidified atmosphere containing 5% CO2. At the end of the incubation period the cells were removed by centrifugation at 250g for 10min., the supernatants were collected and kept at -70°C until assayed for cytokines content.
Cytokine content in the supernatants
The concentration of TNFα, IL-1β, IL-6, IFNγ, IL-10, IL-1ra and IL-2 in the supernatants was tested using ELISA kits specific for these cytokines (Biosource International, Camarillo, CA) as detailed in the guide-line provided by the manufacturer. The detection levels of these kits were: 15pg/ml for IL-6, and 30pg/ml for the remaining ones.
Statistics
A linear mixed model with repeated measures and the assumption of Compound Symmetry (CS) was used to assess the effect of different concentrations of Ox-LDL on cytokine secretion by non-stimulated PBMC or cells stimulated by LPS, PMA/ionomycin or by colon cancer cells. SAS vs 9.4 was used for this analysis. Paired t-test was applied to compare between the level of cytokines produced with various concentrations of Ox-LDL and that found in control cultures. Probability values of p‹0.05 were considered as significant. The results are expressed as mean ± SEM.
Results
Effect of Ox-LDL on cell proliferation
Incubation of PBMC or HuCC cells for 24hrs with Ox-LDL at concentrations as indicated caused a significant increase in cell proliferation tested by XTT test (p‹0.001 and p=0.02, respectively), whereas the proliferation of HT-29 cells was not affected (p=0.066). At Ox-LDL concentrations of 1μg/ml, 2μg/ml and 5μg/ml the proliferation rate of PBMC was 3.24, 3.3 and 2 times higher respectively, than that of cells incubated without Ox-LDL. The effect of Ox-LDL on HuCC cell proliferation was two times higher at 1μg/ ml only (Figure 1).
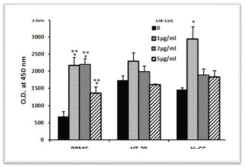
Figure 1: Effect of Ox-LDL on cell proliferation. 0.1ml aliquots of PBMC,
HT-29 and HuCC (105/ml of appropriate CM) were added to each one of
96 well plates and incubated for 24 hrs at 37°C in a humidified incubator
containing 5% CO2 in the absence or presence of Ox-LDL at concentrations
as indicated. At the end of the incubation period XTT test was performed as
described in Materials and Methods. Each column represents the mean of 6
experiments, bars SEM and asterisks-statistically significant difference from
cells incubated without Ox-LDL (*p‹0.05, ***p<0.001).
Effect of Ox-LDL on cytokine production
No detectable concentrations of any of the cytokines tested could be found in supernatants obtained from 24 hrs cultures of either HT-29 or HuCC cells incubated at the above mentioned conditions without or with Ox-LDL added at concentrations between 1-5μg/ml (data not shown).
TNFα, IL-1β and IL-6
24 hrs of incubation of PBMC with Ox-LDL at concentrations between 1 and 5μg/ml had no effect on the production of TNFα and IL-1β by non-stimulated cells (F3,24=1.06, p=0.39; F3,24=2.87, p=0.07, respectively), whereas that of IL-6 was significantly increased (F3,24=8.75, p=0.0014) and was higher by 2.13 (p=0.001) at Ox- LDL concentration of 1μg/ml. At 2μg/ml and 5μg/ml of Ox-LDL, the secretion of IL-6 by non-stimulated PBMC was increased, but was not statistically significant. The production of TNFα by LPSstimulated PBMC was elevated following incubation with the above mentioned concentrations of Ox-LDL (F3,24=3.87, p=0.03) and was higher by 26% (p=0.03) at 5μg/ml of Ox-LDL. At the same culture conditions IL-6 secretion was not affected (F3,24=1.83, p=0.184), whereas that of IL-1β was enhanced (F3,24=5.9, p=0.0072) and was increased by 37% at Ox-LDL concentrations of 1 and 5μg/ml (p=0.01). The production of TNFα by PBMC stimulated with HT-29 and HuCC colon cancer cells was reduced following incubation with Ox-LDL (F3,24=10.16, p=0.0007 and F3,24=5.93, p=0.007, respectively). At Ox-LDL concentrations of 1, 2, and 5μg/ml TNFα secretion by PBMC induced by HT-29 cells was inhibited by 28%, 32% and 35%, respectively (p‹0.01) and that induced by HuCC was reduced by 9% (p=0.07), 17% and 15%, respectively (p‹0.05). IL-1β and IL-6 production induced by HuCC was not affected upon incubation with the above mentioned concentrations of Ox-LDL (p=0.7 and p=0.09, respectively). However, the secretion of both cytokines induced by HT-29 cells was reduced (F3,24=5.06, p=0.0128 and F3,24=8.5, p=0.0016, respectively). At Ox-LDL concentration of 2μg/ml and 5μg/ml IL-1β secretion was lowered by 27% and 22%, respectively (p‹0.05) and that of IL-6 was reduced by 26% and 36% at Ox-LDL concentrations of 1μg/ml and 2μg/ml, respectively (p‹0.01) (Figures 2-4).
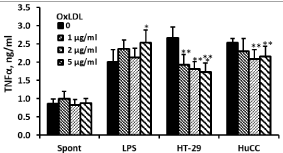
Figure 2: Effect of OX-LDL on TNFα production. Non-stimulated PBMC or
cells stimulated with LPS, or with one of the colon cancer cell lines HT-29 or
HuCC were incubated for 24hrs without (0) or with Ox-LDL at concentrations
as indicated. The level of TNFα in the supernatants was tested by ELISA.
Each column represents the mean of 6 experiments (6 different donors),
bars SEM and asterisks represent statistically significant difference from cells
incubated without Ox-LDL (*p‹0.05, **p‹0.01, ***p‹0.001).
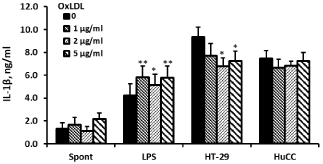
Figure 3: Effect of OX-LDL on IL-1β production. Non-stimulated PBMC or
cells stimulated with LPS, or with one of the colon cancer cell lines HT-29 or
HuCC were incubated for 24hrs without (0) or with Ox-LDL at concentrations
as indicated. The level of IL-1β in the supernatants was tested by ELISA.
Each column represents the mean of 6 experiments (6 different donors),
bars SEM and asterisks represent statistically significant difference from cells
incubated without Ox-LDL (*p‹0.05, **p‹0.01).
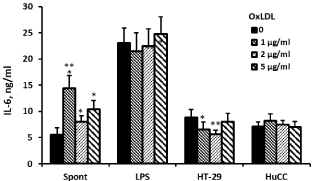
Figure 4: Effect of OX-LDL on IL-6 production. Non-stimulated PBMC or cells
stimulated with LPS, or with one of the colon cancer cell lines HT-29 or HuCC
were incubated for 24hrs without (0) or with Ox-LDL at concentrations as
indicated. The level of IL-6 in the supernatants was tested by ELISA. Each
column represents the mean of 6 experiments (6 different donors), bars SEM
and asterisks represent statistically significant difference from cells incubated
without Ox-LDL (*p‹0.05, **p‹0.01, ***p‹0.001).
IL-2 and IFNγ
Supernatants collected from non-stimulated PBMC incubated without or with Ox-LDL did not contain detectable amounts of IL- 2. The production of IL-2 by PMA/ionomycin stimulated PBMC incubated with Ox-LDL at 1-5μg/ml was similar to that found in supernatants obtained from control cultures incubated without Ox- LDL. IFNγ secretion by non-stimulated PBMC or cells induced by PMA/ionomycin was enhanced following incubation with Ox-LDL (F3,24=4.78, p=0.016 and F3,24=3.73, p=0.034, respectively). Statistically significant increased production of IFNγ by 58% (p=0.003) was observed when non-stimulated PBMC were incubated with 2μg/ml of Ox-LDL only and 20% enhancement was found upon incubation of PMA/ionomycin stimulated PBMC with 2 and 5μg/ml (p‹0.05). Incubation of HT-29-stimulated PBMC cells with Ox-LDL revealed enhanced production of IL-2 (F3,24=8.65, p=0.004) that was higher by 23% (p=0.0019) at 5μg/ml of Ox-LDL, while that of IFNγ was decreased (F3,24=7.22, p=0.0032) and was 32% lower at 1μg/ml (p=0.0032). HuCC induced IL-2 and IFNγ secretion by PBMC was not affected by incubation with Ox-LDL (F3,24=4.7, p=0.0166 and F3,24=1.08, p=0.388, respectively). Although ANOVA calculations revealed statistically significant value, none of Ox-LDL concentrations showed any significant effect on HuCC-induced IL-2 production (Table 1).
Ox-LDL
0
1.0µg/ml
2.0µg/ml
5.0µg/ml
IL-2, ng/ml
Non-stimulated
ND
ND
ND
ND
PMA/iono-stimulated
25.9±10.5
26.9±4.0
27.0±4.5
25.4±6.7
HT-29-stimulated
1.45±0.03
1.49±0.06
1.62±0.04
1.78±0.03**
HuCC-stimulated
1.62±0.08
1.44±0.08
1.72±0.04
1.53±0.06
IFNγ, ng/ml
Non-stimulated
1.18±0.17
1.60±0.08
1.87±0.24**
1.41±0.15
PMA/iono-stimulated
35.88±4.31
33.31±2.61
42.56±2.41*
43.57±2.79*
HT-29-stimulated
2.74±0.08
1.87±0.21**
2.55±0.09
2.57±0.13
HuCC-stimulated
2.47±0.08
2.84±0.21
2.83±0.17
2.76±0.19
Table 1: Effect of Ox-LDL on IL-2 and IFNγ production by PBMC. Non-stimulated PBMC (spontaneous), or cells stimulated with PMA or with one of the colon cancer cell lines HT-29 or HuCC were incubated for 24hrs without (0) or with Ox-LDL at concentrations as indicated. The level of cytokines in the supernatants was tested by ELISA. The results are expressed as Mean ± SEM of 6 experiments (6 different blood donors). ND-not detected level of IL-2 in the supernatant. Asterisks represent statistically significant difference from cells incubated without Ox-LDL (*p‹0.05, **p‹0.01).
IL-10 and IL-1ra
The secretion of IL-10 or IL-1ra by non-stimulated PBMC or by cells stimulated with LPS was not affected following incubation with Ox-LDL at concentrations between 1-5μg/ml (F3,24=2.38, p=0.11; F3,24=1.54, p=0.245 for IL-10 and F3,24=1.97, p=0.162; F3,24=1.8, p=0.189 for IL-1ra, respectively). The secretion of both cytokines induced by HuCC was not affected (F3,24=0.25, p=0.86; F3,24=0.47, p=0.70, respectively). However, Ox-LDL caused reduced secretion of IL-10 and IL-1ra by PBMC stimulated with HT-29 cells (F3,24=38.5, p‹0.0001 and F3,24=7.19, p=0.0032, respectively) that was lowered by 20% at Ox-LDL concentrations of 1 and 2μg/ml (p‹0.03) (Table 2).
Ox-LDL
0
1.0µg/ml
2.0µg/ml
5.0µg/ml
IL-10, pg/ml
Non-stimulated
0.37±0.04
0.63±0.11
0.45±0.06
0.7±0.2
LPS-stimulated
2.66±0.45
2.91±0.53
2.87±0.54
2.96±0.56
HT-29-stimulated
0.97±0.05
0.77±0.05***
0.79±0.04***
0.97±0.06
HuCC-stimulated
0.79±0.10
0.82±0.04
0.77±0.02
0.77±0.04
IL-1ra, ng/ml
Non-stimulated
0.75±0.02
0.82±0.02
0.74±0.03
0.74±0.04
LPS-stimulated
0.86±0.03
0.75±0.04
0.79±0.04
0.79±0.03
HT-29-stimulated
1.20±0.07
0.99±0.12*
0.96±0.11*
1.19±0.13
HuCC-stimulated
10.4±0.13
1.06±0.14
1.09±0.11
1.05±0.12
Table 2: Effect of Ox-LDL on anti-inflammatory cytokine production by PBMC. Non-stimulated PBMC or cells stimulated with LPS or with one of the colon cancer cell lines HT-29 or HuCC were incubated for 24hrs without (0) or with Ox-LDL at concentrations as indicated. The level of cytokines in the supernatants was tested by ELISA. The results are expressed as Mean ± SEM of 6 experiments (6 different blood donors). Asterisks represent statistically significant difference from cells incubated without Ox-LDL (*p‹0.05, ***p‹0.001).
Discussion
The large number of articles dealing with the qualities of LDL and particularly with its oxidized form [5] indicates the existence of its numerous biological assets and intricate mechanisms by which Ox-LDL affects normal and pathological functions of the organism. The immunomodulatory activity of LDL merits particular attention because it is closely linked to inflammatory events that are the basis of atherosclerosis. Since monocytes and T cells possess capacity to oxidize LDL in vitro it is conceivable that the stimulatory effect of native LDL results from LDL oxidation during incubation with these cells [18]. Macrophages express a few receptors for native LDL; however, they bind and take up Ox-LDL via a specific family of receptors referred as scavengers [19]. It has been shown that exposure to Ox-LDL transforms anti-inflammatory M2 macrophages to pro-inflammatory M1 ones with further aggravation of vascular inflammation leading to atherosclerosis [8]. Furthermore, it has been reported that total human monocyte population did not respond to native LDL by increased cytokine production. Such a response expressed by stimulated secretion of TNFα and IL-6 has been observed only when CD14++CD16+ and CD14+CD16++ monocytes were promoted by minimally modified LDL [10,20]. It has been suggested that Ox-LDL modifies cytokine secretion by inhibition of TNFα-RNA [21]. Removal of Ox-LDL is considered to be one of the most important functions of the macrophage scavenger receptors since it has been stated that Ox-LDL is a contributor for advancement of atherosclerosis [4]. In an editorial on the subject Saggini et al. [22] claim that atherosclerotic lesions contain cytokines capable to activate and transform T-cells to T-helper ones that induce further production of a number of proinflammatory cytokines. In our hands incubation of unstimulated mononuclears with all concentrations of Ox-LDL applied in the study increased the secretion of IL-6, IL-10 and IFNγ, but did not affect that of IL-1β, IL-1ra, IL-2 and TNFα. On the other hand, the same procedure carried out with stimulated PBMC showed elevated production of IL-1β observed with all three concentrations used, and a concentration dependent increase of TNFα and IFNγ. Activation of T-cells by Ox-LDL resulted in an increased production of IL-1β in another work [18]. In our study, the effect of Ox-LDL on cytokine secretion depended probably on the mode of PBMC stimulation and concentrations of Ox-LDL. Since various cytokines are produced by different PBMC types it is conceivable that Ox-LDL may affect immune cell populations in a different way.
The role of chronic inflammation and cancer development has been well recognized [23-25]. Immune cells penetrate the tumor and initiate an immune dialogue that affects tumor development from its very beginning till its spreading. Tumor Associated Macrophages (TAM) are the component of the innate immunity, whereas subpopulations of regulatory T-lymphocytes (Tregs) are a part of acquired immunity. Studies have shown that this immune dialogue can been modified by a variety of substances affecting the capacity of immune cells to produce cytokines [26]. In the study hereby described addition of Ox-LDL to cultures of PBMC activated with HT-29 colon cancer cells caused significant inhibited production of all cytokines examined, except for IL-2 that was increased after application of 5μg/ml of Ox-LDL only. At the same culture conditions HuCC cells were less responsive - PBMC prompted by this type of colon cancer cells showed a decreased generation of TNFα only, while that of the remaining cytokines was not affected. It has been shown that infiltration of TAMs and Tregs in the tumor stroma are negative prognostic factors with a positive correlation between them [27]. The finding that Ox-LDL induced a more selective reduction in Tregs as compared to responder T cells implies that Tregs are more susceptible to the influence of Ox-LDL [28]. We therefore believe that in addition to reduction in the number of Tregs induced by Ox- LDL as described previously, the changes observed in our study in the inflammatory cytokine equilibrium between colon cancer and immune cells caused by Ox-LDL may contribute to the imbalance of the immune response and the outcome in colorectal cancer. It is of interest that Ox-LDL caused a significant increased proliferation of PBMC, while that of HuCC was promoted by the lowest Ox-LDL dose only. Notably the proliferation of HT-29 cells was not affected at all. Significantly increased proliferation of PBMC from healthy individuals induced by Ox-LDL has been observed in other studies [29]. On the other hand it has been reported that LDL enhanced breast cancer cell viability and increased their in vitro tumorigenesis [16]. The influence of elevated LDL level on the survival of patients with cancer has been documented. The level of LDL correlated with prevalence of colorectal cancer [14]. According to Li et al. [15 ] the disease-specific survival of patients with normal LDL was longer than those with elevated LDL.
Conclusions
The results of the study display the existence of a relationship between Ox-LDL and the immune activity of PBMC expressed by the capacity of Ox-LDL to promote PBMC proliferation and production of certain cytokines hereby examined. Both activities were not observed when Ox-LDL was added to the colon cancer cells from the two lines; moreover, addition of Ox-LDL to co-cultures of PBMC and HT-29 cells caused inhibited secretion of all cytokines, except for IL-2 that was increased only when the highest Ox-LDL was applied. On the other hand, similar experiments carried out with cells from HuCC line resulted in a decreased production of TNFα, while the secretion of the remaining cytokines was not affected. While these observations may clarify the way Ox-LDL may affect carcinogenesis, it is notable that the interrelation between immune and malignant cells might be cell-dependent as it has been in our study.
Declaration Section
Funding sources: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors
Authors ‘contribution: AGC participated in conceiving the research idea and in drafting the MS. HB performed the laboratory experiments, interpretation of the results and writing the MS. MD participated in the design of the study and writing the MS. All authors read and approved the final version of the manuscript.
Acknowledgments
The authors greatly appreciate the assistance of Ms. Tzippy Shochat, MSc, Statistical Consultant, Rabin Medical Center, Beilinson Hospital, in performing the statistical calculations.
References
- Cortes VA, Busso D, Maiz A, Arteaga A, Nervi F, Rigotti A. Physiological and Pathological Implications of Cholesterol. Front Biosci (Landmark Ed). 2014; 19: 416-428.
- Schnitzler JG, Dallinga-Thie GM, Kroon J. The Role of (Modified) Lipoproteins in Vascular Function: a Duet between Monocytes and the Endothelium. Curr Med Chem. 2018.
- Ganjali S, Gotto AM Jr, Ruscica M, Atkin SL, Butler AE, Banach M, et al. Monocyte-to-HDL-Cholesterol Ratio as a Prognostic Marker in Cardiovascular Diseases. J Cell Physiol. 2018; 233: 9237-9246.
- Jiang X, Yang Z, Chandrakala AN, Pressley D, Parthasarathy S. Oxidized Low Density Lipoproteins-Do We know enough about them? Cardiovasc Drugs Ther. 2011; 25: 367-377.
- Parthasarathy S, Raghavamenon A, Garelnabi MO, Santanam N. Oxidized Low-Density Lipoprotein. Methods Mol Biol. 2010; 610: 403-417.
- Bernelot Moens SJ, Neele AE, Kroon J, van der Valk FM, Van den Bossche J, Hoeksema MA, et al. PCSK9 Monoclonal Antibodies Reverse the Proinflammatory Profile of Monocytes in Familial Hypercholesterolaemia. Eur Heart J. 2017; 38: 1584-1593.
- Zhang M, Lu Y, Liu X, Zhang X, Zhang C, Gao W, et al. Relationship Between XspI Site Polymorphisms of LDL-R Gene and Serum IL-2 and IL-10 in Patients with Hypercholesterolemia. J Clin Lab Anal. 2016; 30: 1122-1127.
- van Tits LJ, Stienstra R, van Lent PL, Netea MG, Joosten LA, Stalenhoef AF. Oxidized LDL Enhances Pro-inflammatory Responses of Alternatively Activated M2 Macrophages: a Crucial Role for Krüppel-like Factor 2. Atherosclerosis. 2011; 214: 345-349.
- de la Paz Sánchez-Martínez M, Blanco-Favela F, Mora-Ruiz MD, Chávez- Rueda AK, Bernabe-García M, Chávez-Sánchez L. IL-17-Differentiated Macrophages Secrete Pro-inflammatory Cytokines in Response to Oxidized Low-Density Lipoprotein. Lipids Health Dis. 2017; 16: 196.
- Al-Sharea A, Lee MK, Moore XL, Fang L, Sviridov D, Chin-Dusting J, et al. Native LDL Promotes Differentiation of Human Monocytes to Macrophages with an Inflammatory Phenotype. Thromb Haemost. 2016; 115: 762-772.
- Ulaganathan V, Kandiah M, Shariff ZM. A Case-Control Study on the Association of Abdominal Obesity and Hypercholesterolemia with the Risk of Colorectal Cancer. J Carcinog. 2018; 17: 4.
- Llaverias G, Danilo C, Mercier I, Daumer K, Capozza F, Williams TM, et al. Role of Cholesterol in the Development and Progression of Breast Cancer. Am J Pathol. 2011; 178: 402-412.
- Kuzu OF, Noory MA, Robertson GP. The Role of Cholesterol in Cancer. Cancer Res. 2016; 76: 2063-2070.
- Tian Y, Wang K, Li J, Wang J, Wang Z, Fan Y, et al. The Association between Serum Lipids and Colorectal Neoplasm: a Systemic Review and Meta- Analysis. Public Health Nutr. 2015; 18: 3355-3370.
- Li AJ, Elmore RG, Chen IY, Karlan BY. Serum Low-Density Lipoprotein Levels Correlate with Survival in Advanced Stage Epithelial Ovarian Cancers. Gynecol Oncol. 2010; 116: 78-81.
- Lu CW, Lo YH, Chen CH, Lin CY, Tsai CH, Chen PJ, et al. VLDL and LDL, but not HDL, Promote Breast Cancer Cell Proliferation, Metastasis and Angiogenesis. Cancer Lett. 2017; 388: 130-138.
- Allott EH, Howard LE, Cooperberg MR, Kane CJ, Aronson WJ, Terris MK, et al. Serum Lipid Profile and Risk of Prostate Cancer Recurrence: Results from the SEARCH Database. Cancer Epidemiol Biomarkers Prev. 2014; 23: 2349-2356.
- Frostegård J, Wu R, Giscombe R, Holm G, Lefvert AK, Nilsson J. Induction of T-cell Activation by Oxidized Low Density Lipoprotein. Arterioscler Thromb. 1992; 12: 461-467.
- Brown MS, Basu SK, Falck JR, Ho YK, Goldstein JL. The Scavenger Cell Pathway for Lipoprotein Degradation: Specificity of the Binding Site that Mediates the Uptake of Negatively-Charged LDL by Macrophages. J Supramol Struct. 1980; 13: 67-81.
- Blanco-Favela F, Espinosa-Luna JE, Chávez-Rueda AK, Madrid-Miller A, Chávez-Sánchez L. Effect of Native and Minimally Modified Low-density Lipoprotein on the Activation of Monocyte Subsets. Arch Med Res. 2017; 48: 432-440.
- Girona J, La Ville AE, Heras M, Olivé S, Masana L. Oxidized Lipoproteins Including HDL and their Lipid Peroxidation Products Inhibit TNF-alpha Secretion by THP-1 Human Macrophages. Free Radic Biol Med. 1997; 23: 658-667.
- Saggini A, Anogeianaki A, Maccauro G, Teté S, Salini V, Caraffa A, et al. Cholesterol, Cytokines and Diseases. Int J Immunopathol Pharmacol. 2011; 24: 567-581.
- Bessler H, Djaldetti M. Role of the Equilibrium between Colon Cancer and Mononuclear Cells in Cytokine Production. Biomed Pharmacother. 2010; 64: 707-711.
- Grivennikov SI, Greten FR, Karin M. Immunity, Inflammation, and Cancer. Cell. 2010; 140: 83-99.
- Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-Related Inflammation and Treatment Effectiveness. Lancet Oncol. 2014; 15: e493-503.
- Djaldetti M, Bessler H. Modulators Affecting the Immune Dialogue between Human Immune and Colon Cancer cells. World J Gastrointest Oncol. 2014; 6: 129-138.
- Waniczek D, Lorenc Z, Śnietura M, Wesecki M, Kopec A, Muc-Wierzgoń M. Tumor-Associated Macrophages and Regulatory T Cells Infiltration and the Clinical Outcome in Colorectal Cancer. Arch Immunol Ther Exp (Warsz). 2017; 65: 445-454.
- Mor A, Luboshits G, Planer D, Keren G, George J. Altered Status of CD4(+) CD25(+) Regulatory T Cells in Patients with Acute Coronary Syndromes. Eur Heart J. 2006; 27: 2530-2537.
- Mottaghi A, Salehi E, Sezavar H, Keshavarz SA, Eshraghian MR, Rezaei N, et al. The in Vitro Effect of Oxidized LDL and PHA on Proliferation and Gene Expression of Regulatory T Cells in Patients with Atherosclerosis. Iran J Allergy Asthma Immunol. 2012; 11: 217-223.