
Research Article
Austin J Infect Dis. 2023; 10(1): 1076.
A Mouse Model of Yellow Fever Virus Infection for Study of Pathogenesis and Development of Vaccines and Therapeutics
Dia M¹, Dieye Y2,3*, Nguer CM3, Bédékélabou AP1, Boye CSB4, Faye O1 and Fall C2
1Pôle de Virologie, Institut Pasteur de Dakar, Sénégal
2Pôle de Microbiologie, Institut Pasteur de Dakar, Sénégal
3Groupe de Recherche Biotechnologies Appliquées & Bioprocédés environnementaux, école Supérieure Polytechnique, Université Cheikh Anta Diop, Dakar, Sénégal
4Centre de Reference IST/VIH,Centre Hospitalier Universitaire Aristide le Dantec, Dakar, Sénégal
*Corresponding author: Dieye Y Pôle de Microbiologie, Institut Pasteur de Dakar, 36 Avenue Pasteur, BP220, Dakar, Sénégal
Received: November 16, 2022; Accepted: January 04, 2023; Published: January 10, 2023
Abstract
Yellow Fever (YF) is a mosquito-borne viral disease that is endemic in several African and South American countries. YF Virus (YFV) causes subclinical infections with mild and non-specific symptoms, to severe, potentially lethal illness. Despite the existence of efficient vaccines, epidemics continue to occur, mostly in Africa. One major drawback of the available YF vaccines is their method of preparation that is fastidious and have limits to produce high volumes of doses needed to respond to recurring epidemics. The best available animal models for YFV are Non-Human Primates (NHP) in which it causes a disease similar to human infection. However, the cost of NHP studies is a limit to preclinical studies. There are a few mouse models of YF. However, these models consist of genetically deficient rodents that are not the best for evaluating new vaccines or therapies. We have developed a mouse model of YFV infection based on the Swiss Webster out bred strain. We have tested several epidemic isolates and identified two strains that, when administrated by the intraperitoneal route, caused an acute infection leading to death. Interestingly, these YFV strains are lethal only when prepared from mouse brain and not when cultured on cell lines. We used this model to test the efficacy of the 17D YFV vaccine strain in protecting mice against lethal challenge showing that the model can be used to evaluate new YF vaccines and therapies.
Keywords: Yellow Fever; Mouse Model; Vaccine
Abbreviations: BSA: Bovine Serum Albumin; CPE: Cytopathic Effect; FBS: Fetal Bovin Serum; IACUC: Institutional Animal Care and Use Committee; L15: Leibovitz; NHP: Non-Human Primate; PFU: Plaque Forming Unit; PS: Porcine Stable Kidney; SW: Swiss Webster; WHO: World Health Organization; YF: Yellow Fever; YFV: Yellow Fever Virus
Introduction
Yellow fever is a mosquito-borne viral disease that is endemic in several African and South American countries. Yellow Fever Virus (YFV) causes subclinical infections with mild and non-specific symptoms, to severe, potentially lethal illness with jaundice, hemorrhage, renal failure [1-3]. Despite the existence of safe and efficient vaccines, epidemics continue to occur, mostly in Africa and South America where the burden of YF is estimated to represent 84,000 to 170,000 severe cases and 29,000 to 60,000 related deaths per year, according to the World Health Organization (WHO) [4].
This high burden is mostly due to a vaccine coverage that greatly varies between African regions and that is low in many areas including certain endemic countries [5]. There are four licensed YF vaccines manufactured by the (i) Institut Pasteur de Dakar, Senegal, (ii) Sanofi Pasteur, France, (iii) Bio Manguinhos, Brazil and (iv) the Institute of Poliomyelitis and Viral Encephalitidis, Russia. These vaccines are all derivatives of a live attenuated strain that was first developed in 1937 [6,7]. They are currently being used in vaccination programs in endemic countries and for travelers visiting these regions. They provide a protective immunity against all known genotypes of YFV despite very rare cases of serious adverse effects [8]. The management of YF is becoming an issue of high importance. After a relatively long period of good control of the disease thanks to vaccination and efficient vector control programs, YF is today in a state of reemergence, with recent large outbreaks and appearance of the disease in new, previously unaffected areas, because of the introduction of mosquito vectors into these lands [9]. The development of large, densely populated cities in endemic countries favors the possibility of spillover and of spread of the disease in cases of epidemics. This concern is compounded by an incertitude regarding the YF vaccine-induced protection. This protection was believed for long to be long-lasting, possibly lifelong, in all individuals [10] resulting in a position paper from the WHO recommending a single immunization without the need of a boost [11]. However, several recent evaluations showed that YF vaccine-induced protection starts to decrease after a few years and could vary between individuals [12-14]. Additionally, there are significant differences between individuals living in endemic regions compared to those from unaffected areas, with the formers showing an impaired response to YF vaccination [11]. Following these new findings, many experts in the field advice boosting immunization to be administered with YF vaccination at least every 10 years [15].
The recommendation of YF boosting immunization significantly increases the demand for YF vaccine. However, the current production method of the available vaccines cannot satisfy this demand. Licensed YF vaccines are produced from culture on embryonated eggs, a fastidious and lengthy process that limits the capacity to yield large doses of vaccine stocks needed to respond to recurring outbreaks, and to prepare for potential major epidemics. The shortage of YF vaccine is a pressing issue. The WHO recommends the use of fractional doses as a shortterm response in cases of outbreak [16]. Several studies have evaluated the ability of doses lower than those currently used to provide immune protection. A recent clinical trial in Brazil showed that a 1/5 fraction of the 17DD vaccine provided a sero conversion rate and a seropositivity after eight years similar to those conferred by the reference full dose [13,17]. Currently, two ongoing non-inferiority clinical trials are testing the efficacy of fractional doses of the four licensed YFV in children and adults. In these trials, the responses of different branches of the immune system are compared among individuals who received full and fractional doses including the induction of IgG and of neutralizing antibodies, and the cellular immune response [18]. The re-emerging status of YF calls for sustained researches that will better elucidate the pathogenesis of wild type YFV. Actually, most of what is known on the pathogenesis of YFV has been obtained from vaccine strains that are attenuated. Studying wild type lineages could yield new knowledge that can be used to generate control tools including therapeutics and vaccines.
Materials and Methods
Virus Strains
Virus strains analyzed in this study (Table 1) were provided by the WHO Collaborating Center for arboviruses and viral hemorrhagic viruses at the Institut Pasteur de Dakar (IPD), Senegal. These strains were isolated from mosquito (Table 1).
YFV isolates
Hosts
Year
Location
ArM T7
Aedesafricanus
1973
Ivory Coast
ArD 114989*
Stegomyametallicus
1995
Koungheul, Senegal
ArD 114988*
Diceromyiafurcifer
1995
Koungheul, Senegal
ArD 114891*
Stegomyaaegypti
1995
Koungheul, Senegal
YFV, yellow fever virus; *, virus isolated during an outbreak in Koungheul, Senegal in 1995.
Table 1: YFV isolates analyzed in this study.
Cells lines
We used three cell lines for virus cultivation and titration. C6-36 cells from Aedes albopictus were grown in L15 (Leibovitz) medium supplemented with 10% fetal bovin serum (FBS), 1% penicillin-streptomycin, 1% glutamine, 10% tryptose phosphate and 0.05% amphotericin B, and incubated at 28°C without CO2. Vero cells (African green monkey kidney epithelial cells) were grown in medium 199 supplemented with 10% FBS, 1% penicillin- streptomycin, 1% glutamine and 25 mMHepes 25, and incubated at 37°C in the presence of 5% CO2. PS (Porcine Stable kidney) cells (American Type Culture Collection, Manassas, USA) were grown at 37°C without C02 in L15 medium supplemented with 10% of FBS.
Preparation of Virus Stocks from Cells Lines
Viral stocks were prepared from C6-36 and Vero cell-lines as previously described [19]. Briefly, cells were grown in cell culture flask (T75 cm²) until they reached 70% of confluence. The medium was removed and the cells infected with 500 μl of viral suspension. The flasks were gently rocked every 15mn during incubation to enhance viral infection. After 1h, appropriate medium was added and the incubation continued up to 7 days, until a Cytopathic Effect (CPE) was observable. The supernatant was harvested, aliquoted and stored at -80°Cuntil needed.
Preparation of Virus Stocks from Brain Homogenates
For preparation of brain homogenates, 2-day old newborn Swiss Webster (SW) mice were infected by intracranial inoculation of 20 μL of virus suspension and followed for eight days. The brains of ill mice were recovered, homogenized in phosphate buffer saline containing 0.2% endotoxin-free Bovine Serum Albumin (BSA) and aliquots stored at -80°C until used.
Virus Titration
The viral stocks were titrated on PS cells by using a modified version of the assay previously described by De Madrid and Porterfield [20]. Briefly, 4.105 PS cells were seeded in each well of a 24-well plate (Corning/Costar). Ten-fold serial were done and 200 μL of each dilution were tested in duplicate. After an incubation period of 4h at 37 °C, an overlay medium (1.6% carboxymethyl cellulose in L15-3% FBS) was added to all the wells and the plates were incubated for 4 days at 37°C. Cells were observed daily and once a cytopathic effect is detected, the wells were washed with PBS. Afterwards, the wells were stained with Amido Black dye (Sigma-Aldrich, Germany) for 30 minutes at room temperature. The Plaque Forming Units (pfu) caused by lysis of infected cells were counted and the titers calculated according to the Reed and Munch method [21].
Mouse Experiments
SW mice were bred at the Pasteur Institute farm. Experimental infections were carried out at the animal facility of the WHO Collaborating Centre for Arboviruses and Hemorrhagic Fever that is accredited for routine diagnostic, surveillance and animal research, according to IACUC [22]. Upon arrival at the animal facility, mice were kept during one week for acclimatization. For infection, groups of 5-8-week old mice were administered with different doses of virus in PBS supplemented with 0.2% BSA by the intraperitoneal route. Infected animals were monitored daily for first symptoms of encephalitis (hunching, lethargy, eye closure, or hind legs paralysis) and death throughout the follow-up period. Terminally ill mice were humanly euthanized by chloroform inhalation and considered as killed by the infection. For secondary infection of survivors, a second viral administration was performed four weeks after the first one and the mice followed as described above. For testing of YF human vaccine, each mouse was immunized with a dose corresponding to 86,536 pfu of 17D IPD vaccine. Vaccinated mice were followed for four weeks, at which time point they were infected with a lethal dose of ArMT7 and then monitored as described above.
Results
Yellow Fever Virus Strains from Brain Homogenates But Not From Cell-Lines Cause Lethal Infection in Swiss Webster Mice
To develop a mouse model of YFV infection, we tested four strains available at the WHO Collaborating Centre for Arbovirus and Haemorrhagic Fever, IPD (Table 1). Repetitive infections with viral stocks prepared from VERO and C6-36 cell lines failed to cause mortality in SW mice after intraperitoneal administration (Table 2). Since in our laboratory we also use viral stocks prepared from infected brain of newborn mice, we asked whether viral particles from brain homogenates could cause a lethal infection. Intraperitoneal administration of brain homogenates showed one strain, DakArAmt7 [22] (will be referred to as ArMT7 thereafter), which killed all mice at a dose of 2,500 pfu while all the other strains failed to cause lethality at doses of 2,000 or 5,000 pfu (Table 2). Additionally, brain homogenates from another strain, ArD 114989, killed mice at high doses of 2 X 105 pfu or 106 pfu (Table 2). We could not test higher doses of the two other strains because we could not prepare stocks above 5,000 pfu per ml of these viruses. As a control for these experiments, we administered virus-free brain homogenates to mice and, as expected, all these rodents survived without developing any sign of illness. Strain ArMT7 was isolated from Aedes africanus mosquito in 1973 in Ivory Coast [23]. To investigate its pathogenicity in SW mice further, we infected groups of six rodents with various doses of this virus. As a control, we infected similar groups of mice with various doses of strain ArD 114989. The results showed that ArMT7 could kill mice after ip infection of doses as low as 10 pfu (Figure 1). In contrast, only one mouse out of 24 succumbed to a dose of 2,000 pfu of ArD 114989 with the possibility of this being an unspecific death since it occurred after 20 days following the infection and we did not observe death at this dose in previous experiments with this strain. Overall, these results show that YFV from brain homogenate can cause lethal infection in SW mice and this could be used as a model to study the pathogenicity of this virus.
YFV isolates
VERO
C6-36
Brain homogenate
Dose (pfu)
Survival
Dose (pfu)
Survival
Dose (pfu)
Survival
Ar MT7
5 X 105
6/6
5 X 105
6/6
5 X 102
2/6
2.5 X 106
6/6
2.5 X 106
6/6
2.5 X 103
0/6
ArD 114989
3 X105
6/6
3 X105
6/6
2 X 105
4/6
1.5 X 106
6/6
1.5 X 106
6/6
1 X 106
3/6
ArD 114988
2 X 103
6/6
2 X103
6/6
5 X 103
3/6
ArD 114891
2 X 103
6/6
2 X103
6/6
2 X103
6/6
YFV, yellow fever virus; pfu, plaque-forming unit
Table 2: Mice survival after infection with YFV stocks prepared from cell lines or brain homogenate.
Figure 1: Virulence of strain ArMT7 in Swiss Webster mice. Mice
were infected by the intraperitoneal route with the shown doses
of ArMT7 particles prepared from brain homogenates and survival
was recorded for the following four weeks.
Survivors of Non-Lethal YFV Infection Are Not Protected Against ArMT7 Strain
Since brain homogenate of strain ArD114989 killed mice only at high dose, we asked whether prior administration of a non-lethal dose of this strain could protect rodents against a subsequent infection by ArMT7. Mice that survived ip infection with ArD 114989 from brain homogenates were challenged with a single dose of 1,000 pfu of ArMT7 four weeks later. After a month of follow up, 14 of 23 mice succumbed to infection by ArMT7 corresponding to a survival rate of 39.1% (Table 3). These results suggest that prior exposition to other YFV viruses could provide partial protection against a lethal infection by ArTM7.
First infection = ArD 114989
Second infection = challenge with ArMT7 (1,000 pfu)
Dose (pfu)
Survival
Survival
200,000
6/6
2/6 (33%)
20,000
6/6
3/6 (50%)
2,000
5/6
1/5 (20%)
200
6/6
3/6 (50%)
Table 3: Mice survival to ArMT7 challenge following prior administrative of sub-lethal doses of ArD 114989.
Yellow Fever Vaccine Provides a Partial Protection against Strain Armt7
YF control is mediated by licensed vaccines that are derivatives of a live attenuated strain developed in 1937. Our institute being one of four WHO-prequalified YF vaccine manufacturers [18], we conducted two experiments to test whether our YF vaccine can protect SW mice against ArMT7 infection. In the first experiment, a group of 10 mice was immunized with 86,536 pfu of 17D IPD vaccine corresponding to a dose used for human vaccination. In the second experiment, we immunized two groups of six mice with two doses corresponding to the human dose (86,536 pfu) and its 1/10 dilution (8,653 pfu) respectively. After four weeks, the mice were challenged with a single dose of 1,000 pfu of strain ArMT7. In both experiments, we included a group of six non-immunized control mice. The results show that 20% (Figure 2A) and 50% (Figure 2B) of the mice survived ArMT7 infection. As expected, all the control mice succumbed to the ArMT7 challenge. These results show that YF 17D vaccine used in humans provides only a partial protection against ArMT7 in SW mice.
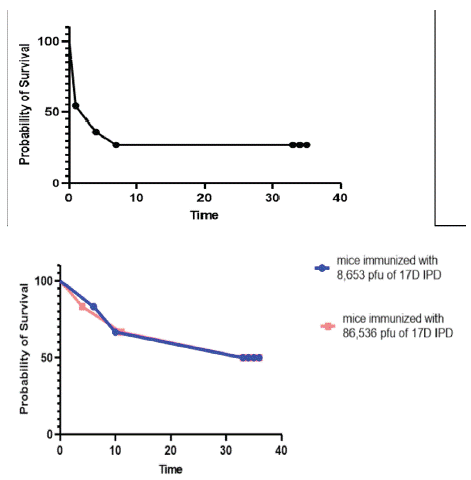
Figure 2: Vaccine-mediated protection of mice against ArMT7. Mice
were immunized by the intraperitoneal route of with a human dose
(86,536 pfu) of 17D vaccine (Figures. 2A and 2B) or 1/10 of this
dose (Figure 2B), and challenged with lethal dose of ArMT7 virus
prepared from brain homogenates. Mouse survival was recorded
for the following four weeks.
Discussion
In this study, we tested four strains of YFV with the goal of developing a mouse model of YF infection. We identified two strains, one of which, ArMT7, killed outbred SW mice at relatively low doses while the other displayed a mild virulence. Interestingly, these viruses kill mice only when prepared from newborn mouse brain and not from cell lines. Additionally, 17D YF vaccine that is used for human vaccination provide only a partial protection against strain ArMT7. The differing virulence of the tested strains demonstrates the involvement of intrinsic genetic determinants. In contrast, we do not know the basis for the virulence of viruses prepared from brain homogenates compared to stocks prepared from cell lines. It is possible that growth in mouse brain makes the virus more fit to develop in the tissue of subsequent host. This may be related to the development of immune escape strategies or to the ability to use metabolites present in mouse organs. Additional studies are needed to elucidate the genetic determinants behind the virulence of strain ArMT7 and the difference of the same virus prepared from cell lines or from brain homogenates.
Our results show that, despite not having the relevance of NHP model, SW mice can be a valuable tool for studying YFV infection and developing vaccines and therapies against this virus. Indeed, the availability of an efficient vaccine and its absence in many regions of the world have limited interest in studying YFV pathogenesis [24,25]. Today the threat of YF is increasing [26] and with it, the needs to better understand the mechanisms of YF development [27]. SW mice can be used to rapidly screen for collection of YFV strains and identify isolates best suited to study YF in mice and other animal models. Furthermore, the SW model can serve to develop novel types of YF vaccine that can be produce at high scales in order to fulfill the current needs. Candidate vaccines can be tested in preclinical studies with SW and the most promising ones moved to further investigations. The Covid crisis has shown that available scientific tools can be rapidly harnessed to respond to a major outbreak by developing vaccines based on different methods [28]. Such an approach could be applied for YF and with the availability of many reagents for dissecting immune response in mouse [29,30] SW model can serve this purpose.
Acknowledgments
We would like to thank the staff of the animal facility of the WHO collaborating Centre for Arboviruses and Hemorrhagic Fever Viruses for their valuable help in experimental infection of mice.
Authors’ Contribution
Conceived and designed the study: YD. YF strains stock preparation: MD. Mouse infection: MD, APB, YD. Results analysis: MD, YD. Wrote the paper: YD. Revised the manuscript: SSBB, OF, CF.
References
- The Lancet null. Yellow fever: a global reckoning. Lancet Lond Engl. 2016; 387: 1348.
- Litvoc MN, Novaes CTG, Lopes MIBF. Yellow fever. Rev Assoc Medica Bras 1992. 2018; 64: 106-13.
- Lopes RL, Pinto JR, Silva Junior GB da, Santos AKT, Souza MTO, Daher EDF. Kidney involvement in yellow fever: a review. Rev Inst Med Trop Sao Paulo. 2019; 61: e35.
- Paules CI, Fauci AS. Yellow Fever - Once Again on the Radar Screen in the Americas. N Engl J Med. 2017; 376: 1397-9.
- Wilder-Smith A. Yellow fever vaccination: estimating coverage. Lancet Infect Dis. 2017; 17: 1109-11.
- Theiler M, Smith HH. THE USE OF YELLOW FEVER VIRUS MODIFIED BY IN VITRO CULTIVATION FOR HUMAN IMMUNIZATION. J Exp Med. 1937; 65: 787-800.
- Barrett AD. Yellow fever vaccines. Biol J Int Assoc Biol Stand. 1997; 25: 17-25.
- Porudominsky R, Gotuzzo EH. Yellow fever vaccine and risk of developing serious adverse events: a systematic review. Rev Panam Salud Publica Pan Am J Public Health. 2018; 42: e75.
- Woodall JP, Yuill TM. Why is the yellow fever outbreak in Angola a « threat to the entire world »? Int J Infect Dis IJID Off Publ Int Soc Infect Dis. 2016; 48: 96-7.
- Hansen CA, Barrett ADT. The Present and Future of Yellow Fever Vaccines. Pharm Basel Switz. 1 sept 2021; 14: 891.
- Vasconcelos PFC, Barrett ADT. Are booster doses of yellow fever vaccine needed? Lancet Infect Dis. déc 2019; 19: 1275-6.
- Staples JE, Barrett ADT, Wilder-Smith A, Hombach J. Review of data and knowledge gaps regarding yellow fever vaccine-induced immunity and duration of protection. Npj Vaccines. 2020; 5: 1-7.
- de Menezes Martins R, Maia M de LS, de Lima SMB, de Noronha TG, Xavier JR, Camacho LAB, et al. Duration of post-vaccination immunity to yellow fever in volunteers eight years after a doseresponse study. Vaccine. 2018; 36: 4112-7.
- Miyaji KT, Avelino-Silva VI, Simões M, Freire M da S, Medeiros CR de, Braga PE, et al. Prevalence and titers of yellow fever virus neutralizing antibodies in previously vaccinated adults. Rev Inst Med Trop Sao Paulo. 2017; 59: e2.
- Campi-Azevedo AC, Costa-Pereira C, Antonelli LR, Fonseca CT, Teixeira-Carvalho A, Villela-Rezende G, et al. Booster dose after 10 years is recommended following 17DD-YF primary vaccination. Hum Vaccines Immunother. 2016; 12: 491-502.
- Roukens AHE, Visser LG. Fractional-dose yellow fever vaccination: an expert review. J Travel Med. 2019; 26: taz024.
- Vannice K, Wilder-Smith A, Hombach J. Fractional-Dose Yellow Fever Vaccination - Advancing the Evidence Base. N Engl J Med. 2018; 379: 603-5.
- Juan-Giner A, Kimathi D, Grantz KH, Hamaluba M, Kazooba P, Njuguna P, et al. Immunogenicity and safety of fractional doses of yellow fever vaccines: a randomised, double-blind, non-inferiority trial. Lancet Lond Engl. 2021; 397: 119-27.
- Digoutte JP, Calvo-Wilson MA, Mondo M, Traore-Lamizana M, Adam F. Continuous cell lines and immune ascitic fluid pools in arbovirus detection. Res Virol. 1992; 143: 417-22.
- De Madrid AT, Porterfield JS. A simple micro-culture method for the study of group B arboviruses. Bull World Health Organ. 1969; 40: 113-21.
- Ramakrishnan MA. Determination of 50% endpoint titer using a simple formula. World J Virol. 2016; 5: 85.
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals [Internet]. 8th éd. Washington (DC): National Academies Press (US); 2011. (The National Academies Collection: Reports funded by National Institutes of Health).
- Stock NK, Laraway H, Faye O, Diallo M, Niedrig M, Sall AA. Biological and Phylogenetic Characteristics of Yellow Fever Virus Lineages from West Africa. J Virol. 2013; 87: 2895-907.
- Hudson NP, Philip CB. INFECTIVITY OF BLOOD DURING THE COURSE OF EXPERIMENTAL YELLOW FEVER. J Exp Med. 1929; 50: 583-99.
- Silva NIO, Sacchetto L, de Rezende IM, Trindade G de S, LaBeaud AD, de Thoisy B, et al. Recent sylvatic yellow fever virus transmission in Brazil: the news from an old disease. Virol J. 2020; 17: 9.
- Jácome R, Carrasco-Hernández R, Campillo-Balderas JA, López- Vidal Y, Lazcano A, Wenzel RP, et al. A yellow flag on the horizon: The looming threat of yellow fever to North America. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. 2019; 87: 143-50.
- Lemos F de O, França A, Lima Filho ACM, Florentino RM, Santos ML, Missiaggia DG, et al. Molecular Mechanism for Protection Against Liver Failure in Human Yellow Fever Infection. Hepatol Commun. mai 2020; 4: 657-69.
- Marian AJ. Current state of vaccine development and targeted therapies for COVID-19: impact of basic science discoveries. Cardiovasc Pathol Off J Soc Cardiovasc Pathol. 2021; 50: 107278.
- Meier KC, Gardner CL, Khoretonenko MV, Klimstra WB, Ryman KD. A Mouse Model for Studying Viscerotropic Disease Caused by Yellow Fever Virus Infection. PLOS Pathog. 2009; 5: e1000614.
- Sun SH, Chen Q, Gu HJ, Yang G, Wang YX, Huang XY, et al. A Mouse Model of SARS-CoV-2 Infection and Pathogenesis. Cell Host Microbe. 2020; 28: 124-133.e4.