
Short Communication
Austin J Infect Dis. 2023; 10(1): 1080.
Hepatitis C Virus and Hepatitis C Virus-Associated Hepatocellular Carcinoma in Egyptian Patients: Driving Disease Progression
Othman OA1, Abdel-Latif R2*, Ezzat AA1, Mokhtar H3 and Othman EM4,5*
1Department of chemistry (Biochemistry division, Faculty of Science, University of Minia, Egypt
2Department of Pharmacology and Toxicology, Faculty of Pharmacy, University of Minia, Egypt
3Department of Clinical Oncology, Faculty of Medicine, Minia University, Egypt
4Department of Biochemistry, Faculty of Pharmacy, University of Minia, Egypt
5Department of Bioinformatics, Biocenter, University of Wuerzburg, Germany
*Corresponding author: Othman EMDepartment of chemistry (Biochemistry division, Faculty of Science, University of Minia, 61519 Minia, Egypt Department of Bioinformatics, Biocenter, University of Wuerzburg, Germany
Department of Pharmacology and Toxicology, Faculty of Pharmacy, University of Minia, 61519 Minia, Egypt
Received: January 31, 2023; Accepted: March 08, 2023; Published: March 15, 2023
Abstract
Background: Hepatocellular carcinoma is the leading cause of cancer-related death worldwide and it is commonly associated with hepatitis C virus infection. Identifying the key mechanisms and biological markers involved in the hepatitis C virus-associated hepatocellular carcinoma may help in early detection and/or diagnosis of this malignancy. The aim of this study was to evaluate and assess the role of the serum VEGF, NF-kB, and IGF-1 in incidence and development of hepatocellular carcinoma in hepatitis C virus-infected patients.
Methods and Results: VEGF, NF-kB, and IGF-1 were measured by ELISA assay in Egyptian patients with hepatitis C virus infection and hepatocellular carcinoma-associated hepatitis C virus infection, healthy individuals. Serum VEGF, NF-kB, and IGF-1 were significantly elevated in HCV and hepatitis C virus-associated hepatocellular carcinoma patients compared to the control group. Additionally patient with hepatitis C virus-associated hepatocellular carcinoma showed significantly higher serum levels of NF-kB, and IGF-1 compared with hepatitis C virus infection patients.
Conclusions: Our data supports the role of VEGF, NF-kB, and IGF-1 in development of hepatocellular carcinoma during the course of hepatitis C virus infection and the use of these biomarkers in combination with alpha-fetoprotein (AFP) may offer an improved diagnostic tool for hepatocellular carcinoma early detection.
Keywords: HCV; HCC; VEGF; NF-kB; IGF-1
Introduction
Chronic Liver Disease (CLD) and cirrhosis account for 2 million deaths per year worldwide, showing approximately 1 million deaths due to the complications of liver cirrhosis and 1 million deaths due to the viral infection and hepatocellular carcinoma [1,2]. In addition to a high load of special needs and increased healthcare utilization [3], many statistical studies reported the chronic Hepatitis B Virus (HBV), Hepatitis C Virus (HCV), Alcohol-related Liver Disease (ALD), and Non-Alcoholic Fatty Liver Disease (NAFLD) as the most common etiologies of CLD and cirrhosis [3].
Hepatitis C virus (HCV) is a major causative agent of chronic hepatitis worldwide that is step-wisely progressed into hepatic cirrhosis, which increases the risk for Hepatocellular Carcinoma (HCC) development [4,5]. In Egypt, HCC represents the fourth most common cancer associated with an unsatisfactory long-term survival rate [6]. The association between HCC development and HCV infection is well established. Among the risk factors for developing HCC, HCV infection is considered the most frequent one [7,8]. Indeed, more than 60% of HCC is reported to be associated with HCV infection [9]. In Egypt, the prevalence of HCV is the highest in the world, estimated to be around 14% of the general population which causes an immense public health burden.
Although HCV infection is well controlled and treated following the introduction of novel therapies, the risk of HCC development is still high especially in those with higher degrees of liver cirrhosis [10]. Hence, detecting biomolecular events that trigger HCC in HCV-infected patients is vital for early diagnosis and treatment of HCC and enhancing patients’ prognosis.
Alpha-Fetoprotein (AFP) is the most widely used serum biomarker to detect HCC worldwide. However, HCC-early phase detection by serum AFP level is limited by its low sensitivity and poor specificity [11]. Up to 30 % of patients with HCC showed normal AFP serum levels [12]. Therefore, there is an urgent demand to identify a reliable alternative marker for the early detection of HCC. Several studies showed that HCV may directly potentiate the risk of developing HCC by regulating several host pathways including those involved in angiogenesis and inflammation. Vascular Endothelial Growth Factor (VEGF) signaling is the main regulator of both physiological and pathological angiogenesis [13]. VEGF upregulation has been reported in HCV-related HCC patients’ serum and it is correlated with tumor vascularity [14,15]. Although it is suggested that detecting serum VEGF could be useful for the detection of HCC [14], the prognostic value of VEGF in Egyptian patients with HCV-related HCC is still to be clarified.
A clear association has been reported between chronic inflammation and the promotion of carcinogenesis in HCV-HCC patients [16]. The significant increase of proinflammatory cytokines noted in HCV-expressing cells is reported to have a key role in HCC development via dysregulation of cell cycle control and the loss of tumor-suppressor gene functions [17,18]. Therefore, it is of interest to elucidate the role of serum NF-kB, the transcription factor for the expression of the inflammatory genes, in the development of HCC during the HCV infection course. Of importance, previous studies have also shown the potential role of the Insulin-like Growth Factor (IGF) signal transduction pathway in HCC [19-21]. IGF is a hormone synthesized in the liver and has a vital role in cell proliferation, differentiation, and apoptosis. Unbalanced IGF levels can promote tumor proliferation and activate cancer reprogramming in tumor tissues, especially in the liver [22]. Although many studies showed that IGF has been implicated in the maintenance of the transformed hepatocyte phenotype [23] the role of IGF in HCV-related HCC, however, remains to be fully recognized. Therefore, the current study aims to evaluate and track the expression levels of VEGF, NF-kB, and IGF in Egyptian patients with HCV and HCC-associated HCV for early prediction of HCC in HCV-infected patients. In addition, we aim to investigate the role of these different biomarkers in the incidence and development of HCC in HCV-infected patients.
Materials and Methods
Study Design
These is a hospital-based case-control study conducted on 65 matched age and Body Mass Index (BMI) adult patients, and were selected regardless of gender, ethnicity. Patients were divided into three groups. Group 1 (control; n=10), Group 2 (HCV; n= 21), and Group 3 (HCV+HCC; n= 34). Patients were recruited from the Internal medicine department, Minia University Hospital, Minia, Egypt during the period from June to October 2021. The study was approved (Nr.HV22/2020) by the Investigation and Ethics Committee of the Minia University, Minia, Egypt and written consent was obtained from all the patients involved.
The HCC group was diagnosed in compliance with the practice guidelines of the American Association for the Study of Liver Disease (AASLD) by abdominal ultrasonography, serologic test for AFP, and confirmed histopathologically in case of inconclusive imaging diagnosis. HCV without cirrhosis was detected via PCR testing for HCV without any evidence of hepatic nodules presence during the imaging test. All controls were negative PCR for HCV and did not show any clinical or biochemical signs of liver disease. Exclusion criteria included patients with HBV infection and patients with HCC without HCV infection. Patients with HCV or HCC who received previous treatment or antiviral therapy for HCV were also excluded.
Measurement of Serum Biomarkers
5 ml of blood was collected and then centrifuged at 5000rpm for 10 minutes. Serum was collected after and then stored at -800C until used. Serum levels of VEGF, NF-kB, and IGF-1 were measured by a commercially available ELISA kit from (Assay Genie, Dublin, Ireland) and AFP was measured by an ELISA kit from (Elabscience biotechnology, Texas, USA) according to the manufacturer’s instructions
Statistical Analysis
Continuous variables were expressed as mean ±SD, median. Differences in statistical significance were evaluated using a one-way analysis of variance (one-way ANOVA test) followed by a Tukey-Kramer post-analysis test for comparing groups. Statistical significance was presented at p<0.05. Analysis was performed using GraphPad Prism® software (Version 8.1.).
Results
Detailed clinical data of all studied groups are shown in (Table 1). The three groups are balanced with respect to gender. The median of the Body Mass Index (BMI) was comparable in the three groups. However, the median of BMI tends to be higher in HCV and HCV+HCC groups. The values of the functional liver enzymes (AST and ALT) were significantly (P-value<0.05) higher in HCV and HCV+HCC groups compared to the control group. However, the value of ALT in the HCV+HCC group was significantly higher than HCV group. Similarly, the Alkaline Phosphatase (ALP) levels were significantly (P-value<0.05) higher in both HCV and HCV+ HCC groups compared to the control group. Additionally, The HCV+HCC group showed a significantly higher level of ALP compared to the HCV group. Serum levels of AFP were significantly higher in the HCV+HCC group compared to the HCV group
Control
HCV
HCV + HCC
Gender
Male
6 (60%)
12 (60%)
20 (58.83%)
Female
4 (40%)
8 (40%)
14 (41.17%)
ALT (U/L)
Mean±SD
22.81
53.79
76.00
±4.73
±31.87*#
Median
22.55
49.27
69.41
AST (U/L)
Mean±SD
31.25 ±6.86
67.59 ± 25.2*
84.72±34.44*
Median
32.24
64.46
78.19
ALP(U/L)
Mean±SD
98.16
167.8± 1.94*
248.8±25.78*#
Median
93.68
167.8
246.2
AFP (ng/ml)
Mean±SD
6.79±1.83
25.26± 10.45
116.8±60.56#
Median
7.05
23.95
99.73
(*): Denotes significant difference in comparison to control group, (#): Denotes significant difference in comparison to HCV group, BMI: Body Mass Index, AST: Aspartate Transaminase, ALT: Alanine Aminotransferase, ALP: Alkaline Phosphatase, AFP: Alpha-Fetoprotein
Table 1: Clinical Data of the Studied Groups.
The levels of studied biomarkers in the different groups were expressed as floating bars. (Figure 1) showed a significant (P-value<0.05) elevation in serum VEGF in both HCV and HCV+ HCC groups compared to the control group (170.4 ± 10.96 and 172.6 ± 31.11 Vs. 88.32 ± 11.14 ng/ml, respectively). However, the values of VEGF were comparable in both HCV and HCV+HCC groups. (Figure 2) depicts a significant (P-value<0.05) increase in serum NF-kB level as a marker of inflammation in both HCV and HCV+HCC groups compared to the control group. Moreover, the HCV+HCC group showed higher levels of NF-kB in the patients' sera compared to the HCV group.
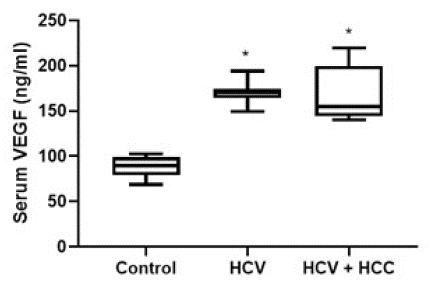
Figure 1: Diagrammatic representation of the serum level of VEGF in HCV patients and HCV-associated HCC patients. Data are represented as mean ±SD. *is significant difference from the control group, respectively, at P<0.05.
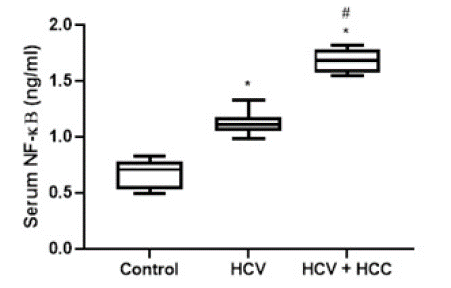
Figure 2: Diagrammatic representation of the serum level of NF-κB in HCV patients and HCV-associated HCC patients. Data are represented as mean ±SD. *and # are significant differences from control and HCV patients’ group, respectively, at P<0.05.
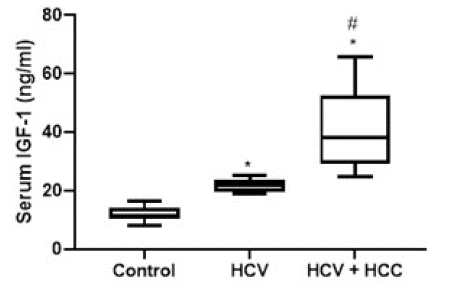
Figure 3: Diagrammatic representation of the serum level of IGF-1 in HCV patients and HCV-associated HCC patients. Data are represented as mean ±SD. *and # are significant differences from control and HCV patients’ group, respectively, at P<0.05.
A significant elevation of serum IGF-1 level was observed in HCV patients compared to the control group (P-value<0.05), similarly, compared to the control group, HCV patients with HCC developed a significant elevation in IGF-1 serum level. Additionally, a significant elevation in IGF-1 serum level was detected in the HCV+HCC group compared to the HCV group (21.95 ± 1.99 vs 40.67 ± 12.74 ng/ml).
Discussion
HCV chronic infection is one of the leading causes of end-stage liver disease including HCC [5]. In most HCV-infected patients, persistent virus replication can be progressed to cirrhosis, and subsequently to HCC [24]. Although the molecular basis for the transformation of HCV into HCC is not yet clear, HCV is suggested to play an integral role in the development of HCC via mechanisms mediated by changes in several cellular signaling pathways. These pathways include stress signaling [25], inflammatory mediators [17,18], and growth factor receptors [14,19].
VEGF signaling is the main regulator of angiogenesis, that has shown an important role in HCC cell growth, invasion, and metastasis [26]. In this study, we found a high statistically significant higher VEGF serum levels in HCV and HCV+HCC patients than in the control group reflecting the proangiogenic state of these patient groups. Several studies reported a positive correlation between HCC and serum level of VEGF [27,28] that could support the use of VEGF as a tumor marker for HCC. Indeed, a study of Mukozu et al., showed that VEGF has a higher sensitivity and accuracy than the other tumor markers in patients with HCC [14]. Although we observed an increase in VEGF serum level in HCC patients with HCV infection, such an increase was not statistically significant than the serum VEGF level measured in the HCV patients' group. This could be explained that our HCV+ HCC patients’ cohort was in the early stage of HCC. Supporting our hypothesis, previous studies showed that the circulating VEGF level is correlated with the stage of HCC and the highest VEGF levels are found in patients with metastasis [28,29]. In agreement with our results, previous studies showed an increase in serum level of VEGF in HCV-infected patients [30,31]. The upregulation of VEGF in HCV patients may affect the inflammatory pattern in these patients. In this regard, VEGF has been shown to be associated with unregulated hepatic inflammation leading to more cirrhosis progression [31]. In the same line, a study by Nage et al (2021) showed a positive correlation between inflammatory mediators and VEGF in HCV-infected patients that supports the intimate link between the processes of inflammation and angiogenesis. Compelling with our results, we reported a significant increase in serum NF-κB, a potent proinflammatory cytokinin, in the sera of HCV-infected patients. Supporting our finding, A significant increase in proinflammatory cytokines was noted in HCV-infected patients [31,32]. Furthermore, chronic inflammation during HCV infection is thought to be responsible for HCC development via increased mutation rate in the regenerating hepatocytes [33]. In the current study, we reported a significant increase in serum NF-κB in HCV+HCC patients compared with the HCV group which supports the role of NF-κB tumorigenesis
IGF-1 has a crucial role in the pathogenesis of chronic liver disease and HCC progression via regulating cell proliferation, angiogenesis, immune evasion, and metastasis [19,20]. Several clinical studies have shown that the levels of IGF-1 serum concentrations were significantly lower in HCV-associated HCC than in healthy subjects [34,35], the detected low serum IGF-1 level may be attributed to the effect of damage to the liver parenchyma, where the IGF ligands are produced [36,37]. On the other hand, other studies documented upregulation of IGF levels in HCV and HCC patients as compared to the control [38]. Consistent with the result of the current study, we found upregulation of serum IGF-1 in HCV and HCV patients with HCC as compared with healthy individuals and higher levels of IGF-1 in the HCV+HCC group than in HCV patients. Data from previous studies showed that HCV core protein could positively regulate IGF transcription and increase its expression [39]. During HCV infection, IGF-1 has an integral role in the persistence of chronic hepatic inflammation through the control of signaling pathways linked to proinflammatory cytokines that subsequently lead to the induction of acute inflammatory reaction [16]. Additionally, IGF was reported to induce angiogenesis in many types of cancers that could explain their role in the growth and metastasis of HCC [32].
Conclusion
In conclusion, the findings of the current study support the potential role of VEGF, NF-kB, and IGF-1 in the development of HCC during the HCV infection course. Additionally, the present findings suggested that the serum levels of VEGF, NF-kB, and IGF-1 might offer improved diagnostic performance for the early detection of HCC.
Study Limitation
The major study limitations include the modest number of the patients in a single center study. Therefore, our results require validation in a larger sample population. We did not examine the stages of fibrosis in HCC patients which could help in further justification of the association of serum level VEGF with HCC stage.
As the current study is cross sectional in nature, therefore, the conclusions regarding the causality between VEGF, NF-κB and IGF-1 levels and the development of HCC in HCV patients should be treated with caution. Consequently, the clinical relevance of our findings needs to be validated by subsequent prospective, multicenter-based studies.
Funding
“The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.”
Competing Interests
“The authors have no relevant financial or non-financial interests to disclose.”
Author Contributions
“All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by R. A., A. A. E., and E. M.O. The first draft of the manuscript was written by R. A, and A.A. E. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.”
Ethics Approval
“This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Minia University, Minia, Egypt (HV22/2020).”
Consent to Participate
“Informed consent was obtained from all individual participants included in the study.”
References
- SK Asrani, H Devarbhavi, J Eaton, PS Kamath. Burden of liver diseases in the world. Journal of hepatology. 2019; 70: 151-171.
- E Goldstein, K Yeghiazaryan, A Ahmad, FA Giordano, H Fröhlich, et al. Optimal multiparametric set-up modelled for best survival outcomes in palliative treatment of liver malignancies: unsupervised machine learning and 3 PM recommendations. EPMA Journal. 2020; 11: 505-515.
- AM Moon, AG Singal, EB Tapper. Contemporary Epidemiology of Chronic Liver Disease and Cirrhosis. Clin Gastroenterol Hepatol. 2020; 18: 2650-2666.
- JD Yang, WR Kim, R Coelho, TA Mettler, JT Benson, et al. Cirrhosis is present in most patients with hepatitis B and hepatocellular carcinoma. Clinical Gastroenterology and Hepatology. 2011; 9: 64-70.
- AJ Freeman, GJ Dore, MG Law, M Thorpe, J Von Overbeck, et al. Estimating progression to cirrhosis in chronic hepatitis C virus infection. Hepatology. 2001; 34: 809-816.
- T Akinyemiju, S Abera, M Ahmed, N Alam, MA Alemayohu, et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the global burden of disease study 2015. JAMA oncology. 2017; 3: 1683-1691.
- S Abd-Elsalam, N Elwan, H Soliman, D Ziada, W Elkhalawany, et al. Epidemiology of liver cancer in Nile delta over a decade: a single-center study. South Asian journal of cancer. 2018; 7: 24-26.
- AS Ibrahim, HM Khaled, NN Mikhail, H Baraka, H Kamel Cancer incidence in Egypt: results of the national population-based cancer registry program. Journal of cancer epidemiology. 2014; 2014: 437971.
- T Yamashita, M Honda, S Kaneko. Molecular mechanisms of hepatocarcinogenesis in chronic hepatitis C virus infection. Journal of gastroenterology and hepatology. 2011; 26: 960-964.
- GN Ioannou, PK Green, LA Beste, EJ Mun, KF Kerr, et al. Development of models estimating the risk of hepatocellular carcinoma after antiviral treatment for hepatitis C. Journal of hepatology. 2018; 69: 1088-1098.
- P Luo, S Wu, Y Yu, X Ming, S Li, et al. Current status and perspective biomarkers in AFP negative HCC: towards screening for and diagnosing hepatocellular carcinoma at an earlier stage. Pathology & Oncology Research. 2020; 26: 599-603.
- F Aksoy, SA Aksoy, HZ Dundar, B Tunca, M Ercelik, et al. Blood-Based Biomarkers in Afp Normal/Stable Hepatocellular Carcinoma: Diagnostic and Prognostic Relevance of Mir-10b for Patients on Liver Transplant List. Transplantation Proceedings. 2022; 54: 1826-1833.
- D Semela, JF Dufour. Vascular endothelial growth factor signaling, Signaling pathways in liver diseases. Springer. 2005; 91-104.
- T Mukozu, H Nagai, D Matsui, T Kanekawa, Y Sumino. Serum VEGF as a tumor marker in patients with HCV-related liver cirrhosis and hepatocellular carcinoma. Anticancer Research. 2013; 33: 1013-1021.
- WS Moon, KH Rhyu, MJ Kang, DG Lee, HC Yu, et al. Tarnawski, Overexpression of VEGF and angiopoietin 2: a key to high vascularity of hepatocellular carcinoma?. Modern Pathology. 2003; 16: 552-557.
- K Ikeda, S Saitoh, Y Arase, K Chayama, Y Suzuki, et al. Effect of interferon therapy on hepatocellular carcinogenesis in patients with chronic hepatitis type C: a long-term observation study of 1,643 patients using statistical bias correction with proportional hazard analysis. Hepatology. 1999; 29: 1124-1130.
- T Yamashita, S Kaneko, SI Hashimoto, T Sato, S Nagai, et al. Serial analysis of gene expression in chronic hepatitis C and hepatocellular carcinoma. Biochemical and biophysical research communications. 2001; 282: 647-654.
- Y Edamoto, A Hara, W Biernat, L Terracciano, G Cathomas, et al. Alterations of RB1, p53 and Wnt pathways in hepatocellular carcinomas associated with hepatitis C hepatitis B and alcoholic liver cirrhosis. International journal of cancer. 2003; 106: 334-341.
- Y Gan, Y Zhang, A Buckels, AJ Paterson, J Jiang, et al. IGF-1R modulation of acute GH-induced STAT5 signaling: role of protein tyrosine phosphatase activity. Molecular Endocrinology. 2013; 27: 1969-1979.
- JG Scharf, T Braulke. The role of the IGF axis in hepatocarcinogenesis. Hormone and Metabolic Research. 2003; 35: 685-693.
- G Xu, J Chu, Y Shi, L Huang, J Fu. The regulation of proliferation and apoptosis in hepatocellular carcinoma via insulin-like growth factor 1 receptor. Growth Hormone & IGF Research. 2022; 66: 101499.
- TT Wu, YH Hsieh, CC Wu, YS Hsieh, CY Huang, et al. Overexpression of protein kinase Ca mRNA in human hepatocellular carcinoma: a potential marker of disease prognosis. Clinica Chimica Acta. 2007; 382: 54-58.
- JA Price, SJ Kovach, T Johnson, LG Koniaris, PA Cahill, et al. Insulin-like growth factor I is a comitogen for hepatocyte growth factor in a rat model of hepatocellular carcinoma. Hepatology. 2002; 36: 1089-1097.
- HB El–Serag, KL Rudolph. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007; 132: 2557-2576.
- N Dionisio, MV Garcia-Mediavilla, S Sanchez-Campos, PL Majano, I Benedicto, et al. Hepatitis C virus NS5A and core proteins induce oxidative stress-mediated calcium signalling alterations in hepatocytes. Journal of Hepatology. 2009; 50: 872-882.
- L Zhang, JN Wang, JM Tang, X Kong, JY Yang, et al. VEGF is essential for the growth and migration of human hepatocellular carcinoma cells. Molecular biology reports. 2012; 39: 5085-5093.
- D Matsui, H Nagai, T Mukozu, Y Ogino, Y Sumino. VEGF in patients with advanced hepatocellular carcinoma receiving intra-arterial chemotherapy. Anticancer Research. 2015; 35: 2205-2210.
- A Alzamzamy, H Elsayed, M Abd Elraouf, H Eltoukhy, T Megahed, et al. Serum vascular endothelial growth factor as a tumor marker for hepatocellular carcinoma in hepatitis C virus-related cirrhotic patients. World Journal of Gastrointestinal Oncology. 2021; 13: 600-611.
- K Jin-no, M Tanimizu, I Hyodo, Y Nishikawa, Y Hosokawa, et al. Circulating vascular endothelial growth factor (VEGF) is a possible tumor marker for metastasis in human hepatocellular carcinoma. Journal of gastroenterology. 1998; 33: 376-382.
- J Hengst, CS Falk, V Schlaphoff, K Deterding, MP Manns, et al. Direct-acting antiviral–induced hepatitis C virus clearance does not completely restore the altered cytokine and chemokine milieu in patients with chronic hepatitis C. The Journal of infectious diseases. 2016; 214: 1965-1974.
- IS Naga, AAF Kamel, SA Ooda, HMF Elbab, RM El-Sharkawy. Effect of directly acting anti-viral agents on immunological imprints in chronic HCV-4a patients: interleukin-10 and vascular endothelial growth factor genes expression level. Egyptian Liver Journal. 2021; 11: 1-10.
- A Kalita, S Gupta, P Singh, A Surolia, K Banerjee. IGF-1 stimulated upregulation of cyclin D1 is mediated via STAT5 signaling pathway in neuronal cells. IUBMB life. 2013; 65: 462-471.
- A Bengochea, M De Souza, L Lefrancois, E Le Roux, O Galy, et al. Common dysregulation of Wnt/Frizzled receptor elements in human hepatocellular carcinoma. British journal of cancer. 2008; 99: 143-150.
- WW Su, KT Lee, YT Yeh, MS Soon, CL Wang, et al. Association of circulating insulin-like growth factor 1 with hepatocellular carcinoma: one cross-sectional correlation study. J Clin Lab Anal. 2010; 24: 195-200.
- U Plockinger, D Kruger, A Bergk, V Weich, B Wiedenmann, et al. Hepatitis-C patients have reduced growth hormone (GH) secretion which improves during long-term therapy with pegylated interferon-alpha. Am J Gastroenterol. 2007; 102: 2724-2731.
- H Huynh, PK Chow, LL Ooi, KC Soo. A possible role for insulin-like growth factor-binding protein-3 autocrine/paracrine loops in controlling hepatocellular carcinoma cell proliferation. Cell Growth Differ. 2002; 13: 115-122.
- V Tovar, C Alsinet, A Villanueva, Y Hoshida, DY Chiang, et al. IGF activation in a molecular subclass of hepatocellular carcinoma and pre-clinical efficacy of IGF-1R blockage. J Hepatol. 2010; 52: 550-559.
- A Kasprzak, A Adamek, W Przybyszewska, P Pyda, J Szmeja, et al. Insulin-like growth factor-1 mRNA isoforms and insulin-like growth factor-1 receptor mRNA expression in chronic hepatitis C. World J Gastroenterol. 2015; 21: 3867-3875.
- S Lee, U Park, YI Lee. Hepatitis C virus core protein transactivates insulin-like growth factor II gene transcription through acting concurrently on Egr1 and Sp1 sites. Virology. 2001; 283: 167-177.