
Review Article
Austin J Infect Dis. 2023; 10(2): 1085.
Clinical Significance of Dermatophytes and Their Pathogenesis
Shyama Datt*; Thakur Datt
Department of Microbiology, UCMS & GTB Hospital, India
*Corresponding author: Shyama Datt Department of Microbiology, UCMS & GTB Hospital, Dilshad Garden, Delhi-95, India. Email: shyama.mathura@gmail.com
Received: May 31, 2023 Accepted: June 24, 2023 Published: July 01, 2023
Abstract
Despite the superfi cial localization of most dermatophytosis, host-fungus relationship in these infections is complex and still poorly elucidated. Though many efforts have been accomplished to characterize secreted dermatophytic proteases at the molecular level, only punctual insights have been afforded into other aspects of the pathogenesis of dermatophytosis, such as fungal adhesion, regulation of gene expression during the infection process, and immunomodulation by fungal factors. However, new genetic tools were recently developed, allowing a more rapid and high-throughput functional investigation of dermatophyte genes and the identifi cation of new putative virulence factors. In addition, sophisticated in vitro infection models are now used and will open the way to a more comprehensive view of the interactions between these fungi and host epidermal cells, especially keratinocytes.
Keywords: Dermatophytes; Pathogenesis; Trichophyton; Microsporum; Ergosterol
Introduction
Dermatophytes
Infections pertaining to mankind particularly those affecting the keratinized tissues are of serious concerns worldwide and are increasing on a global scale. Dermatomycoses are infections of the skin, hair and nail caused as a result of colonization of the keratinized layers of the body. This colonization is brought about by the organisms belonging to the three genera namely Trichophyton, Microsporum and Epidermophyton [1,2]. Due to their high affinity for the keratinized tissues, dermatophytes are responsible for most of superficial mycosis affecting human skin or nails.
Classification
Dermatophytes are fungi that invade and multiply within keratinized tissues (skin, hair, and nails) causing infection [1]. Based upon their genera, Dermatophytes can be classified into three groups: Trichophyton (which causes infections on skin, hair, and nails), Epidermophyton (which causes infections on skin and nails), and Microsporum (which causes infections on skin and hair). Based upon the mode of transmission, these are been classified as anthropophillic, zoophilic, and geophilic. Finally, based upon the affected site, these are been classified clinically into tinea capitis (head), tinea faciei (face), tinea barbae (beard), tinea corporis (body), tinea manus (hand), tinea cruris (groin), tinea pedis (foot), and tinea unguium (nail). Other clinical variants include tinea imbricata, tinea pseudoimbricata, and Majocchi granuloma [3].
Trichophyton: The genus Trichophyton includes 24 species. The colonies on agar media are powdery, velvety or waxy. The predominant spore type is micro conidia with sparse macro conidia [4].
Microsporum: The genus Microsporum includes 16 species. The colony morphology of Microsporum species on agar surface is either velvety or powdery with white to brown pigmentation [4].
Epidermophyton: The genus Epidermophyton includes only 2 species. The colonies are slow-growing, powdery and unique brownish yellow in colour. This genus is devoid of micro conidia. Macro conidia are abundant and produced in clusters [4].
Distribution Frequency of Dermatophytes and Dermatophytosis
All the three genera of Dermatophytes namely Trichophyton, Microsporum and Epidermophyton are worldwide in geographical distribution. The predominant cause of Dermatophytic infections is Trichophyton followed by Epidermophyton and Microsporum. Within the genus Trichophyton, Trichophyton rubrum is the predominant etiological agent accounting for 69.5% followed by Trichophyton mentagrophytes, Trichophyton verrucosum and Trichophyton tonsurans [5-7].
According to the World Health Organization (WHO) survey on the incidence of dermatophytic infection, about 20% the people worldwide present with cutaneous infections [8].
Pathogenesis and Clinical Presentation
The possible route of entry for the Dermatophytes into the host body is injured skin, scars and burns. Infections caused by arthrospores or conidia. Resting hairs lack the essential nutrient required for the growth of the organism. Hence, these hairs not invaded during the process of infection [22].
The pathogen invades the uppermost, non-living, keratinized layer of the skin namely the stratum corneum, produces exo-enzyme keratinase and induces inflammatory reaction at the site of infection [23-26]. The customary signs of inflammatory reactions such as redness (ruber), swelling (induration), are seen at the infection site. Inflammation causes the pathogen to move away from the site of infection and take residence at a new site. This movement of the organism away from the infection site produces the classical ringed lesion [27].
The infections caused by Dermatophytes commonly referred to as “tinea” or “ring-worm” infections due to the characteristic ringed lesions [9]. Based on the site of infection, the tinea infections are referred to as tinea capitis (scalp), tinea corporis or tinea circinata (non-hairy, glaborous region of the body), tinea pedis (“Athletes’ foot”; foot), tinea ungium (“Onychomycosis”; nail), tinea mannum (hands) (Figure 3), tinea barbae (“Barbers’ itch”; bearded region of face and neck), tinea incognito (steroid modified), tinea imbricata (modified form of tinea corporis), tinea gladiatorial (common among wrestlers’) and tinea cruris (“Jocks’ itch”; groin) [10].
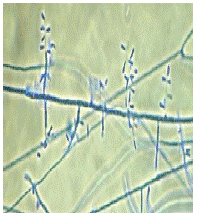
Figure 1: Keratinophylic fungi.
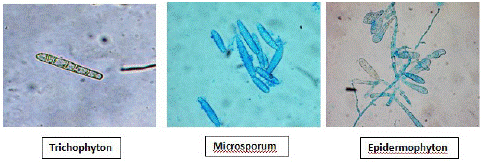
Figure 2:

Figure 3:
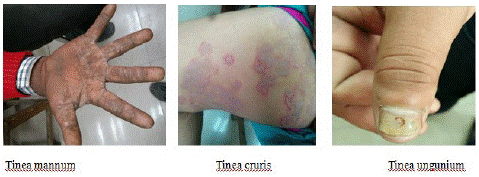
Figure 4:
Dermatophytes can survive solely on outer cornified layers of the skin [11,12]. The ability of certain fungi to adhere to particular host arises from numerous mechanisms and host factors, including the ability to adapt to the human body [11]. Natural infection is acquired by the deposition of viable arthrospores or hyphae on the surface of the susceptible individual [13]. After the inoculation in the host skin, suitable conditions favor the infection to progress through the following stages [14].
Adherence
The kinetics of adherence to the skin or nail surface was investigated in several Trichophyton and Microsporum species, using different experimental models and microscopy techniques. These studies showed a time-dependent increase in the number of adhering spores, followed by germination and invasion of the stratum corneum by hyphae growing in multiple directions. Zurita and Hay [14] observed that maximum adherence of Trichophyton spp. arthroconidia to keratinocytes in suspension occurred within 3–4h. Aljabre et al. [15,16] used stripped sheets of stratum corneum or separate keratinocytes to demonstrate that adherence of Trichophyton mentagrophytes arthroconidia is maximum by 6h and that germination of these spores begins by 4h. In a nail plate model, adherence and germination of T. mentagrophytes arthrospores were observed at 6h and side branches at 16h [17]. The early stages of T. mentagrophytes infection were investigated using skin explants of full epidermis thickness [18]. Adherence was maximum at 12h, germination had started by 24h, and penetration of the stratum corneum occurred after 3 days.
Little is known about the factors that mediate adherence of dermatophytes. The ability of T. rubrum to adhere to epithelial cells has been attributed to carbohydrate-specifi c adhesins, expressed on the surface of microconidia [19]. From a morphological point of view, fi brillar projections have been observed in T. mentagrophytes during the adherence phase [20,21]. At the skin surface, long and sparse fi brils connect fungal arthroconidia to keratinocytes and to each other, while in the inner skin layers, newly formed arthroconidia show thin and short appendices covering their entire surface; the latter begin to vanish as a large contact area is established between conidia and skin tissue [21]. Based on the fi ndings made in the yeast Candida albicans, where secreted aspartic proteases (Saps) have been shown to play a fundamental role in fungal adherence to epithelia [22,23], so that is dermatophytic-secreted proteases could facilitate or even be necessary for effi cient adherence. We have cheched the expression patteren of Exoprotease and Endoprotease genes with their non-protease genes in real time PCR from that data we hypothsised that the Endoprotease have majorly expressed in dermatophytic patient during infection.
Penetration
Dermatophytes are provided with an arsenal of proteases aimed at the digestion of the keratin network into assimilable oligopeptides or amino acids [24]. Once established, the spores must germinate and penetrate the stratum corneum at a rate faster than desquamation. Penetration is accompanied by dermatophytes secreting multiple serine-subtilisins and metallo-endoproteases (fungalysins) formerly called keratinases that are found almost exclusively in the dermatophytes [24,25]. A direct relationship between keratinases and pathogenicity was established by Viani et al.The protease production in T. rubrum is highly host specific showing reduced physiological activity when growing on their preferred host [26,27].
Immunity Behind Dermatophytic Infection
Development of Host Response
Fungal metabolic products diffuse through the malphigian layer to cause erythema, vesicle or even pustule formation along with pruritus. There in vivo activity is restricted to the zone of differentiation, newly differentiated keratin and Adamson’s fringe within the hair shaft [28]. Acute dermatophytosis is associated with a DTH skin response against them, while persistent disease corresponds to IH responses, to high levels of IgE and IgG4 antibodies, and to the production of Th2 cytokines by mononuclear leukocytes [29].
Acquired Resistance
The efficient and protective response against dermatophytosis is a cell-mediated response of the DTH, characterized namely by the action of macrophages as effector cells, interferon-a secretion from type 1 T-helper lymphocytes and by some key cytokines like interferon-? (IFN-?). Immune detection and chemotaxis occur via low-molecular weight chemotactic factors or alternative complement pathway activation. However, the immune response that is raised, and especially the degree of inflammation, varies according to the dermatophyte species, the host species and the pathophysiological status of the host. In general, the zoophilic species cause more inflammatory infections, which may heal spontaneously and result in relative resistance to re-infection. The anthropophilic species usually cause more chronic, less circumscribed infections, which result in less resistance to re-infection [30]. Primary infection produces negative trichophytin test and minimal inflammation (mild erythema and scaling) due to increased keratinocyte turnover.
Antibodies
Antibody formation does not seem to be protective [31]. The dermatophyte antigen is thought to be processed by epidermal Langerhans cells and presented in local lymph nodes to T lymphocytes which proliferate, migrate to the infected site, and produce inflammation. The epidermal barrier becomes permeable to transferring and migrating cells leading to spontaneous resolution of lesions. Trichophytin skin test is now positive and clearing of second infection will be more rapid [32]. Rivalier showed that a dermatophytic infection in humans results in a relative resistance to subsequent infection [33] mainly by the inflammatory forms (kerion), caused by zoophilic species, but not always follow the more chronic anthropophilic infections [18,34]. Barlow and Chattaway [34] pointed out that fungi which do not invade the hair follicle do not seem to give rise to an equivalent immunity when growing in the horny layer of the smooth skin. It demonstrate such acquired immunity in experimental T. rubrum infection of smooth skin [19,35].
Hypersensitivity ("Trichophytin" Reaction)
Dermatophytid reactions (4–5% of patients) are inflammatory eczematous allergic skin reactions at sites distant from primary fungal infection [36]. Being KOH and culture negative, it is associated with a DTH response to trichophytin test and may involve a local DTH response to systemically absorbed fungal antigen [36,37].
Treatment
The treatment is chosen based on the infection site, etiological agent and penetration ability of the drug. The penetration ability and retention in the site of infection of the agent determines its efficacy and frequency of utility. Since the dermatophytes reside in the stratum corneum especially within the keratinocytes, the antifungal agents should have a good penetrating ability. The duration of treatment mainly depends on the type of infection and symptom. Generally a two-three week treatment is required for skin lesions whereas four-six week for feet inflammation [38].
The year 1970 saw the release of Miconazole, the first in the line of azoles group. Since then many more were subsequently synthesized and added to this list during the same period. These antimycotic drugs belonged to the Azoles class of antifungal drugs. The major target of the azoles unlike the other antifungal agents is the cytochrome P450 enzyme [39] (Figure 5).
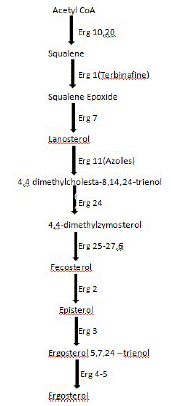
Figure 5: Ergosterol Biosynthesis Pathway.
Based on the number of nitrogen atoms the azoles derivatives are classified into 2 groups as imidazoles and triazoles [40]. In general the imidazoles exhibit side effects such as anorexia, constipation, headache, hepatitis, pruritis, exanthema and inhibition of synthesis of steroid hormone [41]. Triazoles include fluconazole, voriconazole, itraconazole (1980), posaconazole, teraconazole and ravuconazole. In comparison to the imidazoles, the triazoles exhibit lesser degree of side effects which includes nausea, dizziness and gastrointestinal upset [42]. Allylamines and benzyl amines were synthesized in the 1980s’. Allylamines include naftifine and terbinafine. Naftifine, terbinafine and benzylamine obtained FDA approval in United States in the year 1988, 1992 and 2001, respectively. The mode of action of these drugs is inhibition of the key enzyme squalene epoxidase, an essential enzyme involved in the synthesis of squalene epoxide from squalene [43].
Griseofulvin is a narrow spectrum antimycotic drug with fungistatic activity. It is very effective against all the dermatomycoses. The side effects include headache, nausea, bad taste, skin rash, Systemic Lupus Erythematosus (SLE), porphyria and arthralgia. With all its side effects, griseofulvin still remains to be the gold-standard for treating dermatophytic infections [44]. Treatment of cutaneous infection using natural sources is the ongoing research work of many research groups across the globe.
A Novel Approach to Solve the Problem
More recently the scientific community has turned its attention to secondary metabolites from actinobacteria and its exploitation for various purposes which include therapeutic, environmental and industrial applications. With developing microbial resistance and need for safe and cost-effective antidermatophytic drugs, screening of action-bacteria for potential bioactive secondary metabolites becomes indispensible [45]. The anti-dermatophytic activity of these three strains is anticipated to be due to high salt concentration of the environment. Under stress conditions microorganisms inhabiting the particular environment is said to produce complex chemicals that can be exploited medicinally.
Author Statements
Conflict of Interest
The authors report no conflicts of interest.
References
- Emmons CW, Bindford CH, Utz JP, Kwon-Chung KL. Dermatophytoses. Medical mycology. 3rd ed. Philadelphia: Lea & Febiger. 1977; 117-67.
- Gurgel LA, Sidrim JJ, Martins DT, Cechinel Filho V, Rao VS. In vitro antifungal activity of dragon’s blood from Croton urucurana against dermatophytes. J Ethnopharmacol. 2005; 97: 409-12.
- Bristow IR, Spruce MC. Fungal foot infection, cellulitis and diabetes: a review. Diabet Med. 2009; 26: 548-51.
- Jagdish C. Dermatophytoses. Med Mycol, 1st Etdition. 1995; 106-7.
- Chen BK, Friedlander SF. Tinea capitis update: a continuing conflict with an old adversary. Curr Opin Pediatr. 2001; 13: 331-5.
- Ciavaglia MD, de Carvalho TU, de Souza W. Interaction of Trypanosoma cruzi with cells with altered glycosylation patterns. Biochem Biophys Res Commun. 1993; 193: 718-21.
- Zurita J, Hay RJ. Adherence of dermatophyte microconidia and arthroconidia to human keratinocytes in vitro. J Invest Dermatol. 1987; 89: 529-34.
- Aljabre SH, Richardson MD, Scott EM, Shankland GS. Germination of Trichophyton mentagrophytes on human stratum corneum in vitro. J Med Vet Mycol. 1992; 30: 145-52.
- Aljabre SH, Richardson MD, Scott EM, Rashid A, Shankland GS. Adherence of arthroconidia and germlings of anthropophilic and zoophilic varieties of Trichophyton mentagrophytes to human corneocytes as an early event in the pathogenesis of dermatophytosis. Clin Exp Dermatol. 1993; 18: 231-5.
- Rashid A, Scott E, Richardson MD. Early events in the invasion of the human nail plate by Trichophyton mentagrophytes. Br J Dermatol. 1995; 133: 932-40.
- Duek L, Kaufman G, Ulman Y, Berdicevsky I. The pathogenesis of dermatophyte infections in human skin sections. J Infect. 2004; 48: 175-80.
- Tabart J, Baldo A, Vermout S, Nusgens B, Lapiere C, et al. Reconstructed interfollicular feline epidermis as a model for Microsporum canis dermatophytosis. J Med Microbiol. 2007; 56: 971-5.
- Esquenazi D, Alviano CS, de Souza W, Rozental S. The infl uence of surface carbohydrates during in vitro infection of mammalian cells by the dermatophyte Trichophyton rubrum. Res Microbiol. 2004; 155: 144-53.
- Kaufman G, Horwitz BA, Duek L, Ullman Y, Berdicevsky I. Infection stages of the dermatophyte pathogen Trichophyton: microscopic characterization and proteolytic enzymes. Med Mycol. 2007; 45: 149-55.
- Ollert MW, Söhnchen R, Korting HC, Ollert U, Bräutigam S, et al. Mechanisms of adherence of Candida albicans to cultured human epidermal keratinocytes. Infect Immun. 1993; 61: 4560-8.
- Monod M, Borg-von ZM. Secreted aspartic proteases as virulence factors of Candida species. Biol Chem. 2002; 383: 1087-93.
- De Bernardis F, Liu H, O’Mahony R, La Valle R, Bartollino S, et al. Human domain antibodies against virulence traits of Candida albicans inhibit fungus adherence to vaginal epithelium and protect against experimental vaginal candidiasis. J Infect Dis. 2007; 195: 149-57.
- Coloe SV, Baird RW. Dermatophyte infections in Melbourne: trends from 1961/64 to 1995/6. Pathology. 1999; 31: 395-7.
- Marques SA, Robles AM, Tortorano AM, Tuculet MA, Negroni R, Mendes RP. Mycoses associated with AIDS in the Third World. Med Mycol. 2000; 38: 269-79.
- White TC, Oliver BG, Gräser Y, Henn MR. Generating and testing molecular hypotheses in the dermatophytes. Eukaryot Cell. 2008; 7: 1238-45.
- Woodfolk JA. Allergy and dermatophytes. Clin Microbiol Rev. 2005; 18: 30-43.
- Rippon JW. Medical Mycology: the pathogenic Fungi and the Pathogenic Actinomycetes. 3rd ed. Philadelphia: Saunders; 1988; 140-275.
- Samdani AJ. Dermatophyte growth and degradation of human stratum corneum in vitro (pathogenesis of dermatophytosis). J Ayub Med Coll Abbottabad. 2005; 17: 19-21.
- Hay RJ, Moore MK. Mycology. In: textbook of dermatology Burns T, Breathnach S, Cox N, Griffiths C, editors. 7th ed. Vol. 2. Blackwell Publishing Oxford; 2004.
- Shannon V, Heffernan M. In: superficial fungal infections chapter 188 of Dermatology in general medicine Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. 7th ed. New York: McGraw-Hill; 2008; 2.
- Vermout S, Tabart J, Baldo A, Mathy A, Losson B, et al. Pathogenesis of dermatophytosis. Mycopathologia. 2008; 166: 267-75.
- Aljabre SH, Richardson MD, Scott EM, Rashid A, Shankland GS. Adherence of arthroconidia and germlines of antropophilic and zoophilic varieties of T. mentagrophytes to human corneocytes as an early event in the pathogenesis of dermatophytosis. Clin Exp Dermatol. 1993; 18: 231-5.
- Dahl MV. Dermatophytosis and the immune response. J Am Acad Dermatol. 1994; 31: S34-41.
- Rippon JW. Medical Mycology, the Pathogenic fungi and Pathogenic Actionmycetes. 3rd ed. Philadelphia: W B Saunders; 1988.
- Rippon JW, McGinnis MR. The changing epidemiology and emerging patterns of dermatophyte species. Curr Top Med Mycol. 1995; 209-34.
- Chander J. Dermatophytoses, textbook of medical mycology. 1995; 1: 91-112.
- Grappel SF, Bishop CT, Blank F. Immunology of dermatophytes and dermatophytosis. Bacteriol Rev. 1974; 38: 222-50.
- Hay RJ, Reid S, Talwat E, Macnamara K. Immune responses of patients with tinea imbricate. Br J Dermatol. 1983; 108: 581-6.
- Rivalier E. Recherches experimentales sur l’allergie et l’immunite trichophytiques. Ann Dermatol Syphiligr. 1929; 10: 618-40.
- Barlow AJ, Chattaway FW. The parasitism of ringworm group of fungi. AMA Arch Derm. 1958; 77: 399-405.
- Desai SC, Bhat ML, Modi PJ. Biology of T. rubrum infections. Indian J Med Res. 1963; 51: 233-43.
- Grappel SF, Bishop CT, Blank F. Immunology of dermatophytes and dermatophytosis. Bacteriol Rev. 1974; 38: 222-50.
- Kaaman T, Torssander J. Dermatophytid - A misdiagnosed entity? Acta Derm Venereol. 1983; 63: 404-8.
- Elewski BE, Hazen PG. The superficial mycoses and the dermatophytes. J Am Acad Dermatol. 1989; 21: 655-73.
- Elewski BE. Mechanisms of action of systemic antifungal agents. J Am Acad Dermatol. 1993; 28: S28-34.
- Jagdish C. Dermatophytoses. Med Mycol, 1st Etdition. 1996: 108-9.
- Katsambas A, Antoniou CH, Frangouli E, Avgerinou G, Michailidis D, Stratigos J. A doubleblind trial of treatment of seborrhoic dermatitis with 2% ketaconazole cream compared with 1% hydrocortisone cream. Br J Dermatol. 1989; 12: 353-7.
- Grant SM, Clissold SP. Itraconazole: a review of its pharmacodynamic and pharmacokinetic properties and therapeutic use in superficial and systemic mycosis. Drugs. 1989; 37: 310-44.
- Wolverton SE. Systemic antifungal drugs. Comprehensive dermatologic drug therapy. 2nd ed. Saunders; 2001.
- Gentles JC. Experimental ringworm of guinea pigs: oral treatment with griseofulvin. Nature. 1958; 182: 476-7.
- Lakshmipat DT, Kannabiran K. A morphological, biochemical and biological studies of halophilic Streptomyces sp isolated from saltpan environment. Am J Infect Dis. 2009; 5: 207-13.