
Review Article
Austin J Infect Dis. 2023; 10(3): 1090.
Spectrum of Diseases Affecting the Nervous System Associated with JC - Virus: A Review
Andonova RS1*; Bashchobanov DzhH1,2; Slavcheva BG1; Gadzhovska VP1; Dragusheva ES1; Marinova RV1; Angelova MCh1; Popov GT1
¹Department of Infectious Diseases, Sofiamed Hospital, Sofia, Bulgaria
²Medical Faculty, Medical University of Sofia, Bulgaria
*Corresponding author: Andonova RS Department of Infectious Diseases, Sofiamed Hospital, 10 St Mollov Dimitar, Bulgaria. Tel: +359-878-775-193 Email: radina6@mail.bg
Received: August 19, 2023 Accepted: September 30, 2023 Published: October 07, 2023
Abstract
The JC polyomavirus (JCPyV) belongs to the family Polyomaviridae, genus Betapolyomavirus. These are small, non-enveloped, double-stranded DNA viruses. Approximately 60-80% of the population is carriers of JC polyomavirus. Various ways of spread are being studied. These viruses are associated with a large spectrum of neurological diseases, including progressive multifocal leukoencephalopathy, JC-associated meningitis and encephalitis, JCV granule cell neuronopathy and numerous central nervous system malignancies. In the majority of individuals, the virus remains latent in the kidney tissue. However, in certain subgroups, it leads to the development of severe and frequently disabling or fatal diseases. These subgroups are usually immunosuppressed, which causes the virus to undergo lytic rearrangement. Understanding these diseases and the mechanisms by which they occur is paramount for making accurate diagnoses. Studying these diseases is necessary to develop effective treatments and limit their harmful effects.
Keywords: JC: Polyomavirus; Neurolocigal diseases; PML; JC: Meningitis; JC: Encephalitis
Abbreviations: JCPyV: JC Polyomavirus; PML: Progressive Multifocal Leukoencephalopathy; JCVE, JCVM: JC-Associated Meningitis and Encephalitis; JCV GCN: JCV Granule Cell Neuronopathy; HD: Hodgkin’s Disease; BKV: BK-Virus; SV40: Simian Virus 40; MCPyV: Merkel Cell Polyomavirus; NCCR: Non-Coding Control Region; CNS: Central Nervous System; CSF: Cerebro-Spinal Fluid; LTAg: Large Tumor Antigen; stAg: Small Tumor Antigen
Introduction
In 1963, Zu Rhein & Chou, using an electron microscope and laboratory tests, were able to isolate and prove the presence of the JC virus (JCPyV) from the brain of a person with Hodgkin's Disease (HD) [1]. The virus was associated with the disease - Progressive Multifocal Encephalopathy (PML), first described in 1958 by Å Åströ ööööööööööööööööm et al, in patients with HD and Chronic Lymphocytic Leukemia (CLL) [2]. JCV belongs to the genus polyomavirus, which also includes BK Virus (BKV), SIMIAN VIRUS 40 (SV40), and Merkel Cell Polyomavirus (MCPyV) [3,4]. Polyomaviruses are small, non-enveloped icosahedral capsid viruses containing a circular DNA molecule [3-5]. Their genome is composed of three parts - two coding (early and late) and one noncoding. The early coding region is transcribed before replication and encodes the Large (LTAg) and Small Tumor Antigen (stAg), the Non-Coding Control Region (NCCR) is located between the early and late coding regions and contains enhancers/promoters for their expression. There are two types of JSPyV- Archetype (RR-JCPyV) and Mad-1, which differ in the structure of the NCCR. Archetype is transmitted in humans and is the most common type in the environment. The late control region is transcribed after replication and encodes the structural viral proteins (VP1, VP2, VP3) as well as the small accessory protein (Agno) [5-10]. 60 years after the discovery of JC virus, the aim of our review is to present the associated neurological diseases described in the world literature.
Primary Infection
It is difficult to determine the period of primary JCV infection due to the asymptomatic nature of the infection. Approximately 8-10% of children are seropositive by their first six years [5], this percentage increases to 50% in 10-15 year old children, and in adulthood approximately 80% of individuals are infected [4,5]. Based on genotypic analysis, JCV has been found to be transmitted within the family [5,11]. DNA sequence of JCV is found in tonsils, gastrointestinal tract, it is also possible to be isolated from CSF [5]. This also suggests a possible mechanism of transmission via fecal-oral route and tonsillar tissue [13]. Mazzoni et al. in their study commented on the possibility of mother-to-child transmission [14]. Mopes et al. also present a suggestion of a possible inhalation route of infection [15]. The virus is most commonly isolated in the urine and this is thought to be the main route of spread, but there is as yet no conclusive evidence for this. Primary infection has been suggested to be spread by the haematogenous route [5,12], and the virus may remain latent or persistent in the tonsils, kidneys, spleen, bone marrow, heart, and lungs. Monaco et al. isolated JCPyV DNA from tonsillar tissue and reported that stromal cells were more sensitive than B lymphocytes and CDC34+ cells to JCPyV [15].
RR-JCPyV is the transmissible form of the virus, it is nonpathogenic. After extensive genomic rearrangement of NCCR, the pathogenic PML-type/Mad-1 form of the virus is produced, which exhibits neurotropicity [13]. When there is an immunosuppression, for example in HIV infection or autoimmune diseases treated with immunomodulatory therapy, the JC virus is reactivated, and can reach the brain via B lymphocytes. However, viral DNA has also been isolated from brains of immunocompetent patients, suggesting that it is an organ where the virus can enter without having undergone genomic rearrangement and remain there in a latent state [16]. Sixty years after JCPyV was first isolated, we now know more about its structure, primary infection, latent state, and the spectrum of diseases associated with it. After reactivation, the virus can affect cerebral oligodendrocytes and astrocytes, leading to progressive multifocal leukoencephalopathy [15-17] In their review, Miskin and Koralnik describe that JCPyV can also affect cerebellar granule cell neurons, cerebral cortical pyramidal neurons, and meningeal and choroid plexi cells, leading to JCV granule cell neuronopathy (JCV GCN), JCV encephalopathy (JCVE), and JCV meningitis (JCVM) [18,19] (Figure 1).
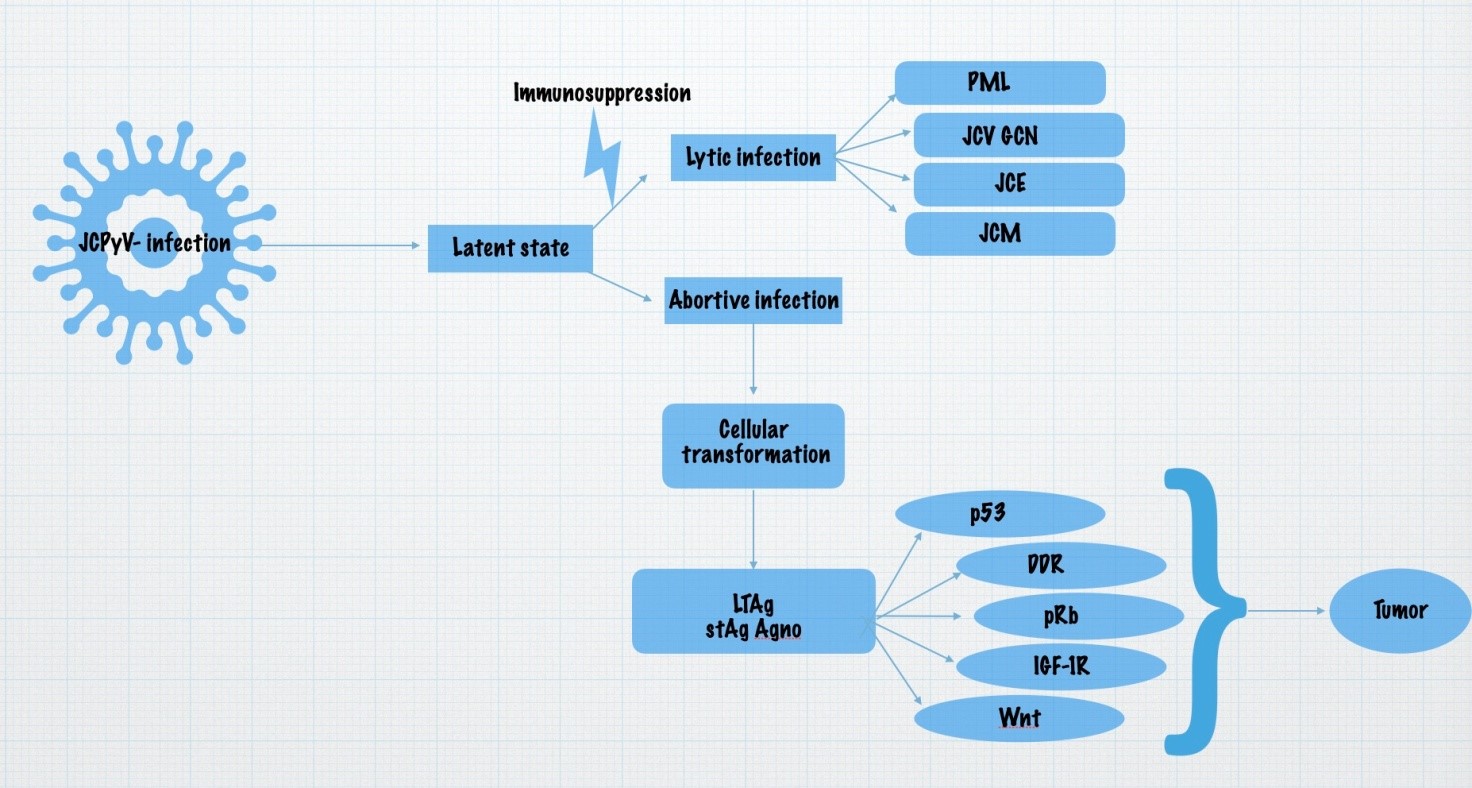
Figure 1: Spectrum of diseases affecting the nervous system associated with JC-virus and the oncogenic potential of this virus.
PML is a demyelinating disease of the brain, primarily affecting elderly individuals, but cases in which children are also affected have been described in the world literature [7,9,20]. Although initially associated with malignant hematologic diseases, the incidence of the disease increased along the HIV pandemic, and cases have been described in which treatment with immunomodulatory therapy for various autoimmune diseases also led to the development of PML. The disease can also develop in rheumatoid patients, transplant patients and people with idiopathic immunodeficiency [7,9,21]. Depending on the pathogenesis of the development of PML, we distinguish several types:
Classical PML
Clinical Presentation
Clinical presentation of the classical type of PML (cPML) varies. The disease usually affects oligodendrocytes to a greaterextent and astrocytes to a less extent [15-19]. This also explains the clinical picture of the disease depending on the localization of the lesions in the brain. Hemiparesis and sensory loss may be the most frequently observed features in patients with PML, as well as aphasia and cognitive impairment [22]. Some of the patients with PML (18%) also suffer from seizures, which are explained by the close localization of some lesions with the cerebral cortex [23]. Although lesions are generally not seen in the optic nerve, complaints of decreased vision may also occur if the occipital lobe is affected, speech disorders if the parietal lobe is affected, and dysmetria and ataxia if the cerebellum is affected [24]. Subacute onset of the disease is typical, lasting up to several months. Very rarely, lesions in the spinal cord can also be described [25].
Brain Imaging
Although lesions in PML can also be emphasized on CT, MRI of the brain remains a method of diagnosing PML. The lesions seen are localized in the cerebral white matter and are not associated with characteristic vascular areas. They can be either unifocal or multifocal, localized in the subcortical white matter or cerebellar peduncles, although those in the basal ganglia or thalamus, which are structures of the cerebral gray matter, have also been described. On T1 -weighted MRI, PML lesions are hypotensive and well defined, even if small in size. On T2 -weighted and Diffusion-Weighted (DWI) and/or fluid-attenuated inversion recovery (Flair) images, lesions are hyperintense with sharp borders to the gray matter and indistinct ones to the white matter. Typically, cPML is not characterized by edema and mass effect, and there is no gadolinium enhancement in MRI very often [7,26]. Lesions in PML appear before the onset of clinical symptoms, so MRI is an appropriate method for monitoring patients who are at risk for developing PML. The results of proton magnetic resonance spectroscopy show that in PML lesions the levels of N-acetyl-aspartate are reduced at the expense of those of choline, which may be related on the one hand to neuronal damage and on the other hand to the process of demyelination. Lactate and lipids are markers that increase and indicate a necrotic process. Unfortunately, these results are not specific and cannot be used for the diagnosis of the disease [27].
Histology
Demyelination is the main histological finding, with the initial foci growing and merging into larger areas; with advanced process, necrosis or, in rare cases, even hemorrhages may be seen in these areas [28]. Lytically infected oligodendrocytes are seen along the active edges of the lesions, which are swollen with enlarged, basophilic nuclei [29]. Astrocytes can also be infected by the JC virus, they also appear enlarged and multilayered hyperchromatic nuclei resembling neoplastic cells can be observed in them. These astrocytes are called " bizarre astrocytes” [9,30].
Diagnosis
For a definitive diagnosis of PML it is necessary to isolate JCPyV DNA from CSF, by PCR, despite its high sensitivity, at low viral load this PCR can give a false negative result, another way is the detection of viral DNA or proteins, by in situ hybridization or immunohistochemistry of brain biopsy.
A diagnosis of "possible" PML can also be made in the case of typical imaging findings and corresponding neurological symptoms, having previously excluded other possible diseases and tumors [7,31].
PML Associated with Monoclonal Antibodies
Treatment of autoimmune diseases such as Crohn's disease, multiple sclerosis and psoriasis (which are not usually associated with PML) with monoclonal antibodies is a risk factor for the development of PML [7]. Back in 2011 Keene et al. in their study reported 182 cases in the global literature: adalimumab -1 case, alemtuzumab-14, bevacizumab -3, cetuximab -1, efalizumab - 8, ibritumomab tiuxetan-5, infliximab-4, natalizumab-32, and rituximab-114 [32]. We will focus on PML cases in the two largest cohorts- rituximab and natalizumab.
Rituximab is a chimeric IgG1 monoclonal antibody that targets CD20+ B lymphocytes for lysis and depletion from the peripheral circulation. It is used in CD20+ non-Hodgkin lymphoma, rheumatoid arthritis, multiple sclerosis, and lupus erythematosus [7]. Carson et al. described 57 cases of PML in HIV-negative patients treated with rituximab, the mortality rate described was 90% due to the fact that patients receiving rituximab have underlying lymphoproliferative disease [33], it is difficult to clarify the role of the monoclonal antibody in the development of PML.
The pathogenesis of PML with rituximab may be due to a decrease in the level of B-lymphocytes in the brain perivascular spaces, leading to a decreased presentation of antigens to T-lymphocytes and therefore also to an altered cellular immune response [34].
Natalizumab is a humanized IgG4 class monoclonal antibody that binds to the alpha 4 subunit of the very late antigen-4 integrin present on leukocytes, preventing these cells from escaping outside the circulation. Indications for treatment include Multiple Sclerosis (MS) and Crohn's disease [7].
PML is usually not considered in the differential diagnostic plan in patients with autoimmune diseases, which delays the diagnosis on the one hand strangely, and on the other hand the disease itself may present with a different clinical picture, making it even more difficult to differentiate from MS. T
he initial symptoms of patients treated with rituximab were mental confusion (54.4%), hemiparesis (33.3%), loss of motor coordination (24.6%), speech difficulties (21.1%), and visual changes (17.5%) [33]. PML-associated natalizumab administration in patients with Crohn's disease begins with mental confusion without focal neurological deficit, in patients with MS it is difficult to distinguish symptoms of the two diseases, cases have been described including: initial mental confusion, mild myoclonic jerking of the arm, difficulties with hand-eye coordination and speech, and the last with attention deficits [35-38]. A patient also presented with seizure as the initial diagnosis [37].
MRI results are similar to those of cPML. Due to the prolonged modulation of the immune system, cavitating lesions may be emphasized. Another feature that may serve to differentiate the two types of PML is gadolinium enhancement due to the prolonged inflammatory response of the host to JCPyV particles when receiving a monoclonal antibody, in contrast to cPML [39].
Diagnosis is complex, with consideration given to the history of autoimmune disease treated with monoclonal antibody, imaging findings, and detection of JC DNA in CSF, although the latter may be negative due to the host's preserved immune response. This may necessitate brain biopsy, taking care not to misdiagnose astrocytoma due to atypical astrocytes [7].
IRIS-PML
Progressive Multifocal Leukoencephalopathy Immune Reconstitution Inflammatory Syndrome (PML-IRIS) is an overactive immune response that manifests as an inflammatory reaction to a clinically manifest or sublinear pathogen. It is associated with the recovery of immunocompetence in HIV-negative patients undergoing immunosuppressive therapy, for example, in people with autoimmune diseases treated with monoclonal antibodies who are on immunosuppressive therapy or in HIV-positive patients initiating Combined Antiretroviral Therapy (cART) [7,9]. Approximately 20% of patients with HIV-PML and most patients with PML associated with natalizumab treated with immunosuppressive medicines develop IRIS [40]. Studies have shown that HIV-positive patients started on cART between 1 and 104 weeks may develop IRIS-PML [41]. IRIS-PML is a potentially debilitating or fatal condition characterized by excessive cytotoxic CD8-positive T-cell infiltration in the brain. In HIV-positive patients, an increase in CD4+ T-cell counts and a decrease in plasma HIV RNA levels from baseline are observed [41].
In IRSI-PML, it is possible to observe contrast enhancement of lysis on MRI due to the acute inflammatory response and blood-brain barrier disruption [42], mass effect, edema and swelling, which in severe cases can lead to brain herniation and death[43]. However, this uctal enhancement may be transient. Similar to PML associated with monoclonal antibody administration, IRIS-PML may not isolate viral DNA due to the activated immune system in CSF [43].
JCV Granule Cell Neuronopathy (JCV GCN)
In PML, lesions affecting the cerebellar peduncles, cerebellum, but also the pons have been described. In contrast to PML, in JCV Granule Cell Neuropathy (JCV GCN) the glial cells are not affected, but the granule cells in the cerebellum are affected [44]. JCV GCN can present as a stand-alone disease or in association with PML [44]. It was originally described by Pasquier et al., who reported a case of an HIV-positive patient with typical PML lesions in the cerebral hemispheres, but these were not described in the cerebellum. In parallel, they described cerebellar atrophy. Histologically, productive JCPyV infection of cerebellar granule cells was demonstrated [45]. Subsequently, such an infection, combined or not with PML, was demonstrated in both HIV-positive and HIV-negative patients, and cases have been described in a patient with sarcoidosis [46-48].
The incidence of JCV GCN is likely to be higher because cerebellar biopsy is not commonly performed [7]. The tropism of the virus to this cell type is thought to be associated with a particular mutation in the VP1 gene, which encodes the VP1 capsid protein [49].
When granule cells are infected with the JC virus, they are destroyed, leading to isolated (if not associated with PML) cerebellar dysfunction, which may include dysarthria, ataxia and discordance. On MRI in the early stages, no typical findings are seen, however in the later stages cerebellar atrophy is observed [7].
JC Meningitis (JCVM)
To date, only a few cases of proven JC virus-associated meningitis have been described in the world literature [7]. In their study, Behzad-Behbahani et al. demonstrated the presence of JC viral DNA in the CSF of two patients out of 89 studied (19 HIV-positive and 70 HIV-negative), both cases belonging to the cohort of HIV-negative patients [50]. JC virus meningitis presents with typical meningeal symptoms. In contrast to PML, in JCM we have no white matter involvement and no typical lesions are found. The only imaging finding that can be seen is enlarged ventricles [7]. To prove the disease it is necessary to isolate JC virus DNA from CSF.
JC Encephalitis (JCVE)
JC viral encephalitis is a newly described manifestation of JC virus infection affecting the CNS. It was first described by Wuthrich, who present the case of a 74-year-old woman with non-small cell lung cancer treated with lobectomy, radiation, carboplatin and taxol chemotherapy, which had been completed five months prior to her neurological symptoms. Two weeks after her neurological presentation she was treated with a 7- week Prednisone therapy. She presented with global cognitive deficits and aphasia, the lesions in her case were limited to the hemispheric grey matter of the brain, and in the final stages had begun to affect the white matter of the brain. In her case, there was no evidence of cerebellar atrophy and despite white matter involvement, the typical PML lesions were absent and the diagnosis of JCV GCN was rejected, although JCPyV infection of cerebellar neurons was histologically proven, they were not localized to the granule cell layer. The presence of virus DNA in CSF was detected by PCR [51].
Treatment and Prognosis
Despite the development of pharmacology in the last decades, it is still not possible to find a suitable drug for the management of JCPyV-related diseases affecting the CNS. In recent years, different strategies of action have been developed, two main avenues are being worked on- direct antiviral therapy and indirect strategies to restore cellular immunity [9,52].
Direct antiviral therapy relies on several approaches- attachment inhibition, entry inhibitors, transport inhibitors, TAg helicase inhibitors, replication inhibitors, and extracellular vesicle inhibitors [7,9,52]. These studies have not yielded good results for several reasons, the difficulty of PML development in animal models, the difficulty of viral replication in culture, the fact that PML is a rare disease with nearly 4000 cases reported annually in the US and Europe [53], the severity of the disease and ethical principles in medicine also prevent such studies in patients. It is the worsening of the patients' disease and the lack of therapeutic response that stops most of the studies [7]. However, some of the molecules investigated produce good results in vitro but do not show a reasonably good therapeutic response in vivo, a common reason being the presence of the haemato-encephalic barrier [7,52].
Restoring the immune response has proven to be an effective treatment, particularly in HIV-positive patients who can receive cART. In HIV-negative patients, limiting immunosuppressive therapy is preferred. However, this is not always achievable, particularly in transplanted patients [7]. This treatment also poses a risk of developing PML-IRIS. HIV-negative patients are often treated with corticosteroids, as there is evidence of their beneficial effects [7]. In HIV-positive patients, corticosteroids are not the preferred treatment due to their immunosuppressive nature, which increases the risk of HIV mutations. For patients who are already diagnosed with HIV, temporary cessation of cART is preferred, but this also poses a risk of HIV resistance [7,41]. Monoclonal antibodies are often used to treat PML, but their use curtails the use of corticosteroids [7]. While the immune system recovers following the withdrawal of monoclonal antibodies, drugs like natalizumab have residual activity and may induce leukocytosis and alter the CD4/CD8 ratio in the CSF, which increases the risk of developing PML-IRIS [54].
PML is a rare but severe disease that can be fatal or severely disabling. In a study including HIV-positive and HIV-negative individuals, we observed similar 1-year survival in PML-IRIS (54%) compared with PML without IRIS (49%) [55]. According to other studies, survival in HIV-associated PML is increased if timely treatment with cART is initiated [56]. However, nearly 70% of individuals with long-term survival have residual neurological deficits, with 25-50% having moderate to severe [57] and over 40% having seizures [58].
There are no studies of PML recurrence, although such cases are reported. Furthermore, studies have demonstrated persistence of JC DNA in CSF up to 3 years after PML diagnosis. This means that the JC virus, despite restored immune competence, is difficult to clear from the brain and remains dormant [7].
The Role of JCPyV in the Development of CNS Malignancies
Many studies have demonstrated the presence of JCPyV in human malignancies [5]. Some of the proteins entering the structure of the viral particles may interact in a particular way with the products of genes involved in the regulation of the cell life cycle or with components of regulatory pathways in the cell associated with cell proliferation. For example, LTAg can interact with Retinoblastoma protein (pRb) [59,60] and the tumor suppressor p53 [61]. When LTAg interacts with pRb, on the one hand, cell elongating factors are activated, which promotes cell cycle progression, on the other hand, this interaction leads to the activation of the E2F tanscription factor, which in turn leads to cell proliferation [5,60]. LTAg also blocks the action of p53, which blocks apoptosis [5,61]. Interactions of LTAg with β-catenin, Insulin Receptor Substrate-1 (IRS-1), and survivin have also been described.
β-catenin is a component of the Wnt-signaling pathway. LTAg stabilizes β-catenin by protecting it from degradation, which leads to cell proliferation [60,62]. Similar phenomena have been described in malignancies associated with JC infection- medulloblastoma, colon cancer, and esophageal cancer [5,10].
IRS-1 is the downstream docking molecule of the Insulin Growth Factor 1 receptor (IGF-1R) pathway. Upon binding to LTAg, this stabilizes it, leading to nuclear translocation of IGF-1R, this in turn strangely leads to inactivation of the enzyme Rad51. Inactivated Rad51 prevents Homologous Recombination (HR), forcing the cell to repair Double-Strand Breaks (DSBs) via Non-Homologous End Joining (NHEJ), which is the more primitive process and leads to more errors [63].
In addition to inactivating Rad51, the interaction between IRS-1 and LTAg, results in increased levels of survivin, which is a potent antiapoptotic protein. LTAg on the other odd can activate the survivin promoter and thus increase its expression [5].
stag is another protein of the JCPyV structure that has oncogenic potential [64], it can interact with pRb and with Protein Phosphatase A2 (PPA2) [5,65]. Expression of the late Agno protein also affects DNA Damage Repair (DDR). Infection of glial cells with JC virus per se results in DNA damage to host cells [66].
There are many suspected JCPyV-related CNS neoplasms, including gangliocytoma, choroid plexus papilloma, pilocytotic astrocytoma, subependymoma, pleomorphic xanthoastrocytoma, oligodendroglioma, all subtypes of astrocytomas, ependymomas, oligoastrocytomas, glioblastomas, glioblastoma multiforme, medulloblastoma, pineoblastoma, gliosarcoma, and primitive neuroectodernal tumours [5,10] (Figure 1).
Conclusion
After six decades since the discovery of JCPyV, significant knowledge has been accumulated regarding its structure, spread, and mode of entry into the host cell. It is understood that the virus is carried by over half the population and can be transmitted to other individuals. There is an increasing understanding about the range of illnesses that the virus can cause, such as PML, JCVE, JCVM, JCV GCN, and how it is the cause of many malignancies. Having a thorough understanding of this information is extremely important for the accurate diagnosis and effective treatment, particularly when considering patient comorbidity.
In literature, there are descriptions of JCPyV-related diseases among patients receiving immunosuppressive therapy, patients with HIV, transplant patients, people with malignant and hematologic diseases, patients with idiopathic immunodeficiency, and cases of PML among people with liver disease, type 2 diabetes mellitus and dementia. As such, JCPyV-related disease should be considered as part of the differential diagnostic plan.
The study of the virus's structure, its impact on the macrovirus, its activation, dormancy, and cellular entry mechanism are essential for effective treatment.
References
- Zurhein G, Chou SM. Particles resembling Papova viruses in human cerebral demyelinating disease. Science. 1965; 148: 1477-9.
- Åström KE, Mancall EL, Richardson EP, Jr. Progressive multifocal leuko-encephalopathy; a hitherto unrecognized complication of chronic lymphatic leukaemia and Hodgkin’s disease. Brain. 1958; 81: 93-111.
- Frisque RJ, Bream GL, Cannella MT. Human polyomavirus JC virus genome. J Virol. 1984; 51: 458-69.
- Pinto M. Dobson S. BK and JC virus: a review. J Infectol. 2014; 68: S2-8.
- Ahye N, Bellizzi A, May D, Wollebo HS. The role of the JC virus in central nervous system tumorigenesis. Int J Mol Sci. 2020; 21: 6236.
- Corbridge SM, Rice RC, Bean LA, Wüthrich C, Dang X, Nicholson DA, et al. JC virus infection of meningeal and choroid plexus cells in patients with progressive multifocal leukoencephalopathy. J Neurovirol. 2019; 25: 520-4.
- Tan CS, Koralnik IJ. Progressive multifocal leukoencephalopathy and other disorders caused by JC virus: clinical features and pathogenesis. Lancet Neurol. 2010; 9: 425-37.
- Neu U, Maginnis MS, Palma AS, Ströh LJ, Nelson CD, Feizi T, et al. Structure-function analysis of the human JC polyomavirus establishes the LSTc pentasaccharide as a functional receptor motif. Cell Host Microbe. 2010; 8: 309-19.
- Cortese I, Reich DS, Nath A. Progressive multifocal leukoencephalopathy and the spectrum of JC virus-related disease. Nat Rev Neurol. 2021; 17: 37-51.
- Delbue S, Comar M, Ferrante P. Review on the role of the human Polyomavirus JC in the development of tumors. Infect Agent Cancer. 2017; 3: 10.
- Kitamura T, Kunitake T, Guo J, Tominaga T, Kawabe K, Yogo Y. Transmission of the human polyomavirus JC virus occurs both within the family and outside the family. J Clin Microbiol. 1994; 32: 2359-63.
- Ciappi S, Azzi A, De Santis R, Leoncini F, Sterrantino G, Mazzotta F, et al. Archetypal and rearranged sequences of human polyomavirus JC transcription control region in peripheral blood leukocytes and in cerebrospinal fluid. J Gen Virol. 1999; 80: 1017-23.
- Atkinson AL, Atwood WJ. Fifty years of JC polyomavirus: A brief overview and remaining questions. Viruses. 2020; 12: 969.
- Mazzoni E, Pellegrinelli E, Mazziotta C, Lanzillotti C, Rotondo JC, Bononi I, et al. Mother-to-child transmission of oncogenic polyomaviruses BKPyV, JCPyV and SV40. J Infect. 2020; 80: 563-70.
- Monaco MC, Atwood WJ, Gravell M, Tornatore CS, Major EO. JC virus infection of hematopoietic progenitor cells, primary B lymphocytes, and tonsillar stromal cells: implications for viral latency. J Virol. 1996; 70: 7004-12.
- Chapagain ML, Nerurkar VR. Human polyomavirus JC (JCV) infection of human B lymphocytes: a possible mechanism for JCV transmigration across the blood-brain barrier. J Infect Dis. 2010; 202: 184-91.
- Bag AK, Curé JK, Chapman PR, Roberson GH, Shah R. JC virus infection of the brain. AJNR Am J Neuroradiol. 2010; 31: 1564-76.
- Miskin DP, Koralnik IJ. Novel syndromes associated with JC virus infection of neurons and meningeal cells: no longer a gray area. Curr Opin Neurol. 2015; 28: 288-94.
- Kartau M, Sipilä JO, Auvinen E, Palomäki M, Verkkoniemi-Ahola A. Progressive multifocal leukoencephalopathy: current insights. Degener Neurol Neuromuscul Dis. 2019; 9: 109-21.
- Zheng HY, Kitamura T, Takasaka T, Chen Q, Yogo Y. Unambiguous identification of JC polyomavirus strains transmitted from parents to children. Arch Virol. 2004; 149: 261-73.
- Moens U, Krumbholz A, Ehlers B, Zell R, Johne R, Calvignac-Spencer S et al. Biology, evolution, and medical importance of polyomaviruses: an update. Infect Genet Evol. 2017; 54: 18-38.
- Weber T. Progressive multifocal leukoencephalopathy. Neurol Clin. 2008; 26: 833-54.
- Lima MA, Drislane FW, Koralnik IJ. Seizures and their outcome in progressive multifocal leukoencephalopathy. Neurology. 2006; 66: 262-64.
- Bartsch T, Rempe T, Leypoldt F, Riedel C, Jansen O, Berg D, et al. The spectrum of progressive multifocal leukoencephalopathy: a practical approach. Eur J Neurol. 2019; 26: 566-e41.
- Bernal-Cano F, Joseph JT, Koralnik IJ. Spinal cord lesions of progressive multifocal leukoencephalopathy in an acquired immunodeficiency syndrome patient. J Neurovirol. 2007; 13: 474-76.
- Whiteman ML, Post MJ, Berger JR, Tate LG, Bell MD, Limonte LP. Progressive multifocal leukoencephalopathy in 47 HIV-seropositive patients: neuroimaging with clinical and pathologic correlation. Radiology. 1993; 187: 233-40.
- Chang L, Ernst T, Tornatore C, Aronow H, Melchor R, Walot I, et al. Metabolite abnormalities in progressive multifocal leukoencephalopathy by proton magnetic resonance spectroscopy. Neurology. 1997; 48: 836-45.
- Brooks BR, Walker DL. Progressive multifocal leukoencephalopathy. Neurol Clin. 1984; 2: 299-313.
- Ono D, Shishido-Hara Y, Mizutani S, Mori Y, Ichinose K, Watanabe M, et al. Development of demyelinating lesions in progressive multifocal leukoencephalopathy (PML): comparison of magnetic resonance images and neuropathology of post-mortem brain. Neuropathology. 2019; 39: 294-306.
- Berger JR, Aksamit AJ, Clifford DB, Davis L, Koralnik IJ, Sejvar JJ, et al. PML diagnostic criteria: consensus statement from the AAN Neuroinfectious Disease Section. Neurology. 2013; 80: 1430-8.
- Cinque P, Koralnik IJ, Clifford DB. The evolving face of human immunodeficiency virus-related progressive multifocal leukoencephalopathy: defining a consensus terminology. J Neurovirol. 2003; 9: 88-92.
- Keene DL, Legare C, Taylor E, Gallivan J, Cawthorn GM, Vu D. Monoclonal antibodies and progressive multifocal leukoencephalopathy. Can J Neurol Sci. 2011; 38: 565-71.
- Carson KR, Evens AM, Richey EA, Habermann TM, Focosi D, Seymour JF, et al. Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood. 2009; 113: 4834-40.
- Martin P, Cravens PD, Winger R, Kieseier BC, Cepok S, Eagar TN, et al. Depletion of B lymphocytes from cerebral perivascular spaces by rituximab. Arch Neurol. 2009; 66: 1016-20.
- Langer-Gould A, Atlas SW, Green AJ, Bollen AW, Pelletier D. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N Engl J Med. 2005; 353: 375-81.
- Kleinschmidt-DeMasters BK, Tyler KL. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N Engl J Med. 2005; 353: 369-74.
- Lindå H, von Heijne A, Major EO, Ryschkewitsch C, Berg J, Olsson T, et al. Progressive multifocal leukoencephalopathy after natalizumab monotherapy. N Engl J Med. 2009; 361: 1081-7.
- Wenning W, Haghikia A, Laubenberger J, Clifford DB, Behrens PF, Chan A, et al. Treatment of progressive multifocal leukoencephalopathy associated with natalizumab. N Engl J Med. 2009; 361: 1075-80.
- Clifford DB, De Luca A, Simpson DM, Arendt G, Giovannoni G, Nath A. Natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: lessons from 28 cases. Lancet Neurol. 2010; 9: 438-46.
- Cinque P, Koralnik IJ, Gerevini S, Miro JM, Price RW. Progressive multifocal leukoencephalopathy in HIV-1 infection. Lancet Infect Dis. 2009; 9: 625-36.
- Tan K, Roda R, Ostrow L, McArthur J, Nath A. PML-IRIS in patients with HIV infection: clinical manifestations and treatment with steroids. Neurology. 2009; 72: 1458-64.
- Du Pasquier RA, Koralnik IJ. Inflammatory reaction in progressive multifocal leukoencephalopathy: harmful or beneficial? J Neurovirol. 2003; 9: 25-31.
- Vendrely A, Bienvenu B, Gasnault J, Thiebault JB, Salmon D, Gray F. Fulminant inflammatory leukoencephalopathy associated with HAART-induced immune restoration in AIDS-related progressive multifocal leukoencephalopathy. Acta Neuropathol. 2005; 109: 449-55.
- Richardson EP, Jr., Webster HD. Progressive multifocal leukoencephalopathy: its pathological features. Prog Clin Biol Res. 1983; 105: 191-203.
- Du Pasquier RA, Corey S, Margolin DH, Williams K, Pfister LA, De Girolami U, et al. Productive infection of cerebellar granule cell neurons by JC virus in an HIV+ individual. Neurology. 2003; 61: 775-82.
- Otis CN, Moral LA. Images in pathology: granule cell loss in AIDS-associated progressive multifocal leukoencephalopathy. Int J Surg Pathol. 2005; 13: 360.
- Hecht JH, Glenn OA, Wara DW, Wu YW. JC virus granule cell neuronopathy in a child with CD40 ligand deficiency. Pediatr Neurol. 2007; 36: 186-89.
- Granot R, Lawrence R, Barnett M, Masters L, Rodriguez M, Theocharous C, et al. What lies beneath the tent? JC-virus cerebellar granule cell neuronopathy complicating sarcoidosis. J Clin Neurosci. 2009; 16: 1091-92.
- Dang X, Koralnik IJ. A granule cell neuron-associated JC virus variant has a unique deletion in the VP1 gene. J Gen Virol. 2006; 87: 2533-37.
- Behzad-Behbahani A, Klapper PE, Vallely PJ, Cleator GM, Bonington A. BKV-DNA and JCV-DNA in CSF of patients with suspected meningitis or encephalitis. Infection. 2003; 31: 374-78.
- Wüthrich C, Dang X, Westmoreland S, McKay J, Maheshwari A, Anderson MP, et al. Fulminant JC virus encephalopathy with productive infection of cortical pyramidal neurons. Ann Neurol. 2009; 65: 742-48.
- Kaiserman J, O’Hara BA, Haley SA, Atwood WJ. An elusive target: inhibitors of JC polyomavirus infection and their development as therapeutics for the treatment of progressive multifocal leukoencephalopathy. Int J Mol Sci. 2023; 24: 8580.
- Kartau M, Verkkoniemi-Ahola A, Paetau A, Palomäki M, Janes R, Ristola M et al. The incidence and predisposing factors of john Cunningham virus-induced progressive multifocal leukoencephalopathy in Southern Finland: A population-based study. Open Forum Infect Dis. 2019; 6: ofz024.
- Stüve O, Marra CM, Bar-Or A, Niino M, Cravens PD, Cepok S, et al. Altered CD4+/CD8+ T-cell ratios in cerebrospinal fluid of natalizumab-treated patients with multiple sclerosis. Arch Neurol. 2006; 63: 1383-87.
- Marzocchetti A, Tompkins T, Clifford DB, Gandhi RT, Kesari S, Berger JR, et al. Determinants of survival in progressive multifocal leukoencephalopathy. Neurology. 2009; 73: 1551-58.
- Engsig FN, Hansen AB, Omland LH, Kronborg G, Gerstoft J, Laursen AL, et al. Incidence, clinical presentation, and outcome of progressive multifocal leukoencephalopathy in HIV-infected patients during the highly active antiretroviral therapy era: a nationwide cohort study. J Infect Dis. 2009; 199: 77-83.
- Lima MA, Bernal-Cano F, Clifford DB, Gandhi RT, Koralnik IJ. Clinical outcome of long-term survivors of progressive multifocal leukoencephalopathy. J Neurol Neurosurg Psychiatry. 2010; 81: 1288-91.
- Miskin DP, Herman ST, Ngo LH, Koralnik IJ. Predictors and characteristics of seizures in survivors of progressive multifocal leukoencephalopathy. J Neurovirol. 2016; 22: 464-71.
- Gan DD, Khalili K. Interaction between JCV large T-antigen and beta-catenin. Oncogene. 2004; 23: 483-90.
- Caracciolo V, Reiss K, Crozier-Fitzgerald C, De Pascali F, Macaluso M, Khalili K, et al. Interplay between the retinoblastoma related pRb2/p130 and E2F-4 and -5 in relation to JCV-TAg. J Cell Physiol. 2007; 212: 96-104.
- Reich NC, Levine AJ. Specific interaction of the SV40 T antigen-cellular p53 protein complex with SV40 DNA. Virology. 1982; 117: 286-90.
- Ripple MJ, Parker Struckhoff A, Trillo-Tinoco J, Li L, Margolin DA, McGoey R et al. Activation of c-Myc and cyclin D1 by JCV T-antigen and β-catenin in colon cancer. PLOS ONE. 2014; 9: e106257.
- Trojanek J, Croul S, Ho T, Wang JY, Darbinyan A, Nowicki M, et al. T-antigen of the human polyomavirus JC attenuates faithful DNA repair by forcing nuclear interaction between IRS-1 and Rad51. J Cell Physiol. 2006; 206: 35-46.
- Verhaegen ME, Mangelberger D, Harms PW, Vozheiko TD, Weick JW, Wilbert DM, et al. Merkel cell polyomavirus small T antigen is oncogenic in transgenic mice. J Invest Dermatol. 2015; 135: 1415-24.
- Safak M, Barrucco R, Darbinyan A, Okada Y, Nagashima K, Khalili K. Interaction of JC virus agno protein with T antigen modulates transcription and replication of the viral genome in glial cells. J Virol. 2001; 75: 1476-86.
- Darbinyan A, Siddiqui KM, Slonina D, Darbinian N, Amini S, White MK et al. Role of JC virus agnoprotein in DNA repair. J Virol. 2004; 78: 8593-600.