
Perspective
Austin J Infect Dis. 2024; 11(1): 1099.
The Dengue Virus
Weledji EP¹*; Taku NJ²; Nsagha ND³
¹Department of Surgery, Faculty of Health Sciences, University of Buea, Cameroon, W/ Africa
²Department of Public Health, Faculty of Health Sciences, University of Buea, Cameroon, W/ Africa
³Department of Public Health, Faculty of Health Sciences, University of Buea, Cameroon, W/ Africa
*Corresponding author: EP Weledji, Livanda Kongo Hill, Lumpsum quarters, Limbe, S.W. Region, Cameroon. Tel: 237699922144 Email: elroypat@yahoo.co.uk
Received: September 12, 2024 Accepted: October 01, 2024 Published: October 08, 2024
Perspective
The Dengue virus falls under the flavivirus group of viruses. There are just over 60 members of the flavivirus group; 29 are mosquito-borne, 15 are tick-borne while the remainder have no known arthropod vector. 26 flaviviruses can cause human disease including Dengue types 1-4, but several of them have produced only laboratory-acquired infections or isolated cases of disease in man (Table 1) [1-3]. Dengue fever is an infectious disease carried by mosquitoes and caused by any of four related dengue viruses. It has been known by health experts for more than 200 years and used to be called "break-bone" fever because it sometimes causes severe joint and muscle pain that feels like bones are breaking. Up to 400 million people get infected with dengue, approximately 100 million people get sick from infection, and 40,000 die from severe dengue. Almost half of the world’s population live in areas with a risk of dengue, and indeed, is often a leading cause of illness in areas with risk. The range of clinical manifestations produced by flaviviruses is similar to those of the alphaviruses- febrile illnesses with or without a rash, or encephalitis. In addition, yellow fever, Kyasanur Forest disease, Omsk haemorrhaggic fever, and the dengue viruses can cause haemorrhagic signs [3]. There are 4 dengue virus serotypes all of which are endemic throughout the tropics particularly in Asia, the Caribbean, the Pacific, and in some areas of West Africa. The various types (dengue-1, dengue-2, dengue-3, and dengue-4) are closely related and no significant biological differences are known between them. Scientists hypothesize that the dengue viruses evolved in nonhuman primates and jumped to humans in Africa or South east Asia between 500 and 1000 years ago which may be corroborated by the four serotypes sharing the same geographic and ecological niche (Tables 2 & 3) [1]. In addition, there is some evidence that monkeys may play a part in virus maintenance but there is no convincing evidence of a vertebrate maintenance host other than man. Like other viruses, the dengue virus is a microscopic structure that can only replicate inside a host organism. The dengue virus has a roughly spherical structure. It is composed of the viral genome and capsid proteins surrounded by an envelope and a shell of proteins (Figure 1). After infecting a host cell, the dengue virus hijacks the host cell's machinery to replicate the viral RNA genome and viral proteins. Newly synthesized dengue viruses can go on to infect other host cells [1,4,5]. Many of the largest epidemics have been caused by dengue-1 as in the case of recent outbreaks in the Carribean. However, in many situations several types co-exist and successive epidemics may be due to different types. Many of the same people may be affected in each outbreak as it has been demonstrated that cross-protection between dengue types in man lasts only a short time. Epidemics of dengue (break-bone fever) have been known since the late eighteenth century and waves of urban epidemics occurred in tropical and subtropical regions during the 19th and early 20th centuries. These epidemics seem to have followed the migration of Aedes aegypti along trade routes from Africa to India and around the coast of Asia to reach Hong Kong and across the Pacific to Hawaii. Originally, dengue was probably a mainly rural infection in tropical Asia transmitted by indigenous Stegomyia. As no virological diagnosis was possible, ‘dengue’ has been a symptom complex which includes a large number of tropical febrile illnesses [1]. Dengue is endemic in tropical areas where Stegomyia species are constantly active, the boundaries are the winter isotherms for 17.8°C. Large epidemics occur outside these areas from time to time, e.g. Brisbane (1906), Durban (1927), and Athens (1928). Aedes aegypti is the most important vector particularly in urban areas, but other Stegomyia species play a role in rural areas of Asia and the Pacific Islands. These include Ae. Albopictus, Ae. Polynesiensis, and Ae. Scutellaris. The ‘classical’ form of dengue fever usually affects adults and older children. Following an infective mosquito bite there is an incubation period of five to eight days followed by the sudden onset of fever, which often becomes biphasic, severe headache, pain behind the eyes, backache, chilliness, and generalised pains in the muscles and joints. A maculopapular rash generally appedars on the trunk between the 3rd and 5th day of illness and spreads later to the face and extremities. Lymphadenopathy, anorexia, constipation, and altered taste sensation are common. Ocassionally, petechiae are seen in the dorsum of the feet, legs, hands, axillae and palate late in the illness. In young children, upper respiratory tract symptoms predominate and dengue is rarely suspected. The illness generally lasts for about ten days after which recovery is usually complete although convalescence may be protracted. Laboratory findings show leucopenia, a mild thrombocytopenia, and a relative lymphocytosis. Epidemics continue to occur in Thailand, and only indigenous populations are involved with neither ethnic origin nor socio-economic conditions apparently having any effect on the incidence of the disease. Outbreaks of classical dengue are uncommon during haemorrhagic disease epidemics but immigrants from non-endemic areas often suffer from classical dengue while haemorrhagic disease occurs in the indigenous population. Dengue haemorrhagic fever syndrome is almost entirely confined to indigenous children, usually orientals, often as young as six months [1]. In a proportion of children, the initial phase is followed by an abrupt collapse with hypotension, peripheral vascular congestion, petechiae, and sometimes a rash. There are varying degrees of shock. The child is often restless, sweating and has cold, clammy extremities and a hot feverish trunk. The fourth and fifth days are critical and purpura, ecchymoses, epistaxis, haematemesis, melaena, coma, convulsions, and severe shock indicate a poor prognosis. Should the patient survive this period, recovery is generally complete [6,7]. Laboratory studies show thrombocytopenia, a prolonged bleeding time, an elevated prothrombin time, a raised haematocrit, hypoproteinaemia, a positive tourniquet test. The liver is often enlarged, soft and tender. It has been suggested that the acute onset of shock and the rapid and often dramatic clinical recovery when the shock is treated properly, together with the absence of inflammatory vascular lesions, suggest short-term vascular lesions. The central role of complement activation with the formation of immune complexes has been demonstrated. C3a and C3b anaphylatoxins, which are products of complement activation are thought to be the cause of plasma leakage. Their rapid inactivation and elimination from the circulation are consistent with the short duration of shock. There is increased fibrinogen consumption which indicates DIC. Both leucopenia and leucocytosis have been reported. There may be maturation arrests of megakaryocytes in the bone marrow and phagocytic activity of reticulum cells. Immunoelectrophoretic studies of serum proteins has shown the disappearance of the βIC line at the onset of shock suggesting an immunological phenomenon- a massive antigen-antibody reaction. There is a very rapid rise in flavivirus antibody in the early stages of the disease which suggests that patients may have been previously sensitized to the infecting virus by earlier infection with a closely related virus, probably another type of dengue virus. Post-mortem studies show that vascular changes predominate with vasodilatation, congestion, oedema, and haemorrhages. Pleural and peritoneal effusions are seen with haemorrhages in the stomach and intestines; there are widespread petechial haemorrhages. There is no specific treatment for dengue fever. The focus is on treating pain symptoms and acetaminophen (paracetamol) is often used. Maintenance of the patient’s body fluid volume is critical to severe dengue care. The management of dengue haemorrhagic fever is entirely symptomatic with the basic principle being directed towards correction of plasma leakage. Immediate replacement of plasma loss with isotonic salt solution and plasma or plasma expanders in cases of profound shock; further plasma leakage is continually replaced to maintain the circulation volume for another 12-24 hours to allow extravasated plasma to be reabsorbed; correct the electrolyte and acid-base disturbance; give transfusions of fresh blood in cases of massive bleeding. The use of the microhaematocrit has been claimed to be invaluable as a guideline. Corticosteroids have not been found to be of great benefit, and heparin is not generally indicated in the management of dengue shock syndrome even when there is evidence of disseminated intravascular coagulation [1,6-8]. For severe dengue, medical care by physicians and nurses experienced with the effects and progression of the disease can decrease mortality rates from more than 20% to less than 15% (Table 3) [4]. The control of dengue depends principally on control of the vector, particularly Ae. Aegypti. A great deal can be done by eliminating the periodomestic breeding places such as flower pots, old jars, and tin cans around houses, using insecticides carefully and by wearing loose-fitting, long-sleeved shirts and pants. Rural breeding sites are much more difficult to control. Several live attenuated strains are under development but Dengvaxia is the only dengue vaccine currently available and three doses are required for full protection [1,9-11].
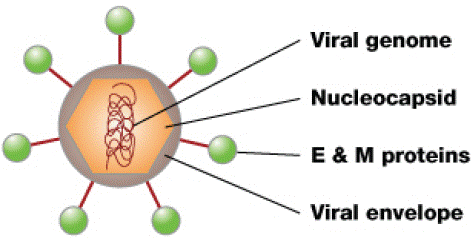
Figure 1: The dengue virus. Inside is the nucleocapsid made of the viral genome and C proteins. The viral envelope is a lipid bilayer membrane taken from the host that surrounds the nucleocapsid. Embedded in the viral envelope are E and M Proteins that span through the lipid bilayer forming a protective outer layer that controls the entry of the virus into human cells [5].
Geographical distribution of viruses
Other features
Australia encephalitis
mosquito
Australia, New Guinea S. and E. Africa
one case only
Banzi
mosquito
Bussuquara
mosquito
Brazil, Colombia, Panama
one case only
Dengue types 1-4
mosquito
S., S.E. Asia, Pacific Is., New Guinea, Carribean area, Venezuela, Colombia, W. Africa
tropics and subtropics wherever the virus and a Stegomyia vector exist
Ilheus
mosquito
Central America, Trinidad, Colombia, Brazil, Argentina
mosquito
Japanese encephalitis
ixodid tick
E., S.E., and S. Asia, W. Pacific
Laboratory case only
ixodid tick
Kunjin
Australia, Sarawak
Kyasanur Forest
ixodid tick
Mysore, India
Langat
ixodid tick
Malaysia
Louping ill
ixodid tick
N. and W. British Isles
Only experimental cases proven
Omsk haemorrhagic fever
ixodid tick
Central former USSR
Powassan
ixodid tick
Canada, USA
Laboratory cases more severe epidemics of encephalitis
Rio Bravo
? bat saliva
USA, Mexico
Rocio
Poss. mosquito
Brazil
St Louis encephalitis
mosquito
N. America, Panama, Jamaica, Trinidad, Brazil, Argentina New Guinea E., W., and S. Africa
One case only
Sepik
mosquito
Spondweni
mosquito
Tick-borne encephalitis
ixodid tick
Central Europe from Scandinavia to Balkans and from Germany to W. former USSR E. USSR and sometimes in W. USSR and Czechoslovakia E., W., and S. Africa, Thailand E., W., and S. Africa, S and S.E. Asia, Mediterranean area W. and Central Africa, S. and Central America
Disease recognized mainly in Israel and S. France Periodical epidemics in neighbouring areas, e.g. Ethiopia
(Central European)
(Far Eastern)
Wesselsbron
mosquito
West Nile
mosquito
Yellow fever
mosquito
Zika
mosquito
E. and W. Africa, Malaysia, Philippines
One case in Uganda
Table 1: Flaviviruses known to cause human disease [1].
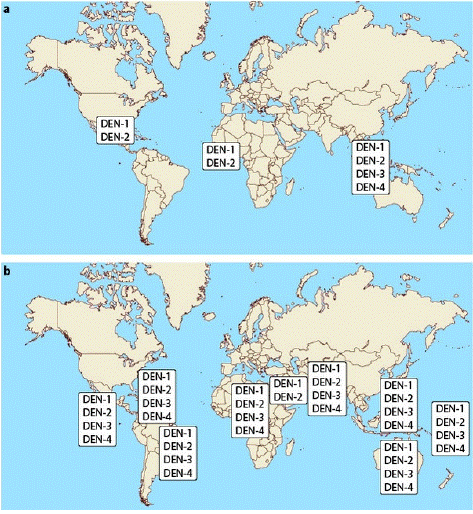
Table 2: The change in distribution of Dengue serotypes (a) 1970, (b) 2004 [1].
Year
Countries
Cases
Deaths
2017
Burkino Faso
Cote d’Ivoire9029
62318
22016
Burkina Faso
1.266
15
2015
Egypt
28
-
2009
Cape Verde
16,744
-
Table 3: Recent outbreaks in Africa [4].
References
- Beasley DWC, Barrett ADT. The infectious agent. In Dengue: tropical Medicine: Science and Practice, vol5 eds G. Pasvol & SL. Hoffman (London: Imperial College Press. 2008; 9: 29-74.
- Halstead SB. Observations related to pathogenesis of dengue haemorrhagic fever. VI. Hypotheses and discussion. Yale J Biol Med. 1970; 42: 350-62.
- Hotta S. Dengue and related tropical viruses. Susuma Hotta, Kube, Japan. 1978.
- Africa Centres for Disease Control (Africa CDC) and Prevention. Dengue fever. Epidemiology. 2024.
- Kuhn RJ, Zhang W, Rossmann MG, Pletnev SV, Corver J, Lenches E, et al. Structure of dengue virus: Implications for flavivirus organization, maturation, and fusion. Cell. 2002; 108: 717-725.
- Guzman MG, Halstead SB, Artsob H, Buchy P, Farrar J, Gubler DJ, et al. Dengue: A continuing global threat. Nature Reviews Microbiology. 2010; 8: S7-S16.
- Lupi O. Mosquito-borne haemorrhagic fevers. Dermatologic clinics. 2011; 29: 33-38.
- Weledji EP, Simo AW. Disseminated Intravascular coagulation (DIC): What the physician should know. Thromb Haemost Res. 2024; 8: 1094.
- World Health Organization. Dengue. Guidelines for Diagnosis, treatment and Prevention and Control. Geneva: World health Organization and the special Programme for Research and training in Tropical Diseases. 2009.
- Whitehead SS, Blaney JE, Durbin AP, Murphy BR. Prospects for a dengue virus vaccine. Nature Reviews Microbiology. 2007; 5: 518-528.
- Centre for Disease Control and Prevention (CDC, USA) 2024: Data and statistics on Dengue in the USA. Current year data. 2024.