
Research Article
Austin J Infect Dis. 2015; 2(1): 1016.
Study of Diversity and Abundance of Anopheline Mosquitoes in Meghalaya, India
Srivastava AK1* and Prasad SB1
1Department of Zoology, North-Eastern Hill University, Shillong-793 022, India
*Corresponding author: Srivastava AK, Department of Zoology, North-Eastern Hill University, Shillong-793022, India
Received: November 03, 2015; Accepted: September 24, 2015; Published: November 07, 2015
Abstract
Malaria is anopheline vector-borne disease of serious worry in Southeast Asia. Understanding the spatial distribution of mosquitoes should contribute to the design of malaria control. The diversity, distribution and relative abundance of Anophelines were surveyed using a sampling method for a period of four years from April 2008 to March 2012 in several biotopes of the varying climatic region of Meghalaya. Meghalaya State is situated in highly malaria endemic North-eastern region of India. The biodiversity of Anopheline was examined and divided into alpha and beta components with the aim of comparing its distribution and abundance in all the seven districts of Meghalaya. A total of 37,026 Anopheline mosquitoes belonging to 33 species were collected. During pre-monsoon, monsoon, and post monsoon 9,345 (25.2%), 23,507 (63.5%) and 4,174 (11.3%) mosquitoes respectively were recorded. The most common species were An. maculatus (21.7%), An. vagus (15.2%), An. annularis (12.91%), An. philippinensis (9.9%), An. nigerimus (9.81%) and An. minimus (8.7%). The result of the study shows significant differences in species richness between districts. Biodiversity indices indicate that species diversity was highest in West Garo Hills and lowest in West Khasi Hills districts. It is suggested that greater variation in the species composition could be due to temperature differences among the different districts of Meghalaya
Keywords: Anopheles; Abundance biodiversity; Meghalaya; Principle component analysis; Survey
Abbreviations
WHO: World Health Organization; NVBDCP: National Vector Borne Disease Control Programme
Introduction
Malaria, a major human health threat, occurs globally in tropical and subtropical regions. It is a worrying disease of Africa, South-east Asia and South America. World Health Organization (WHO) has estimated that there are 106 countries in the world where malaria is endemic and India is one of them. About 36% of the world population (i.e., 2020 million) living in these countries are at risk, with fatal rates being extremely high among young children below 5 year of age [1]. As per WHO report concerning South-east Asian region, out of 1.4 billion people living in 11 countries of South-east Asia, 1.2 billion (about 87%) are exposed to the risk of malaria and most of them live in India [2-4]. The disease primarily affects poor population in tropical and subtropical areas, where the temperature and rainfall are suitable for the development of vectors and parasites [5,6].
Meghalaya (in Sanskrit, Megh = clouds, Alaya = house)” is an important North-eastern State of India famous for the place of highest rainfall in the world. Its geographical territory lies between latitude 25°09’30” N to 26°01’42” N and longitudes 89°51’25 E to 92°50’37 E. The physical features and particular tribal dominance have divided Meghalaya into three zones i.e. Khasi Hills, Jaintia Hills and, Garo Hills. The districts in these zones are East Khasi Hills (EKH), Ri- Bhoi (RB), West Khasi Hills (WKH), Jaintia Hills (JH), East Garo Hills (EGH), West Garo Hills (WGH) and South Garo Hills (SGH) (Roy & Tomar, 2001). In Meghalaya, incidence of malaria has been reported to increase significantly from the year 2001 [7]. The annual average prevalence of malaria in India is 106 per 100,000 populations, whereas in Meghalaya it is 920 per 100,000 populations, which is about 8.6 times more than the national average [7]. The prevalence rate of malaria in Meghalaya is highest in the North-eastern States and second in India [7]. Climatic condition of low land areas of the State is warm and humid, but highland areas are cold. The occurrence of malaria has been reported to be prevalent in foothills and valleys of Meghalaya, but now it has been noted to gradually spread in the highland areas also.
Malaria is a vector-borne disease, which is transmitted by female anopheline mosquitoes. Understanding the spatial distribution of mosquitoes should significantly contribute to the design of malaria control strategies. Earlier studies carried out in Meghalaya started from Shortt (1934) [8] to Prakash et al., (1998) [9] revealed that number of Anopheline species ranged from 8 to 34. These studies were based on the survey mainly done from Ri-Bhoi (RB), East Khasi Hills (EKH), West Khasi Hills (WKH) and Jaintia Hills (JH) districts. However, there are no reports on the survey and distribution of Anopheline mosquitoes in highly malaria-affected areas of East Garo Hills (EGH), West Garo Hills (WGH), and South Garo Hills (SGH) districts of Meghalaya. Further, earlier reports on Anopheline mosquitoes records show the rich mosquito diversity in EKH, WKH, JH and RB, but there is no record about species richness and its composition in these regions.
Therefore, the present study was undertaken to update the status of Anopheline mosquito’s species in all the above-mentioned seven districts of Meghalaya and to determine the species richness, composition, and abundance in view of specific environmental conditions. This is perhaps the first study of its kind in Meghalaya that documents the information on the Anopheline species distribution.
Materials and Methods
Seasonal features of Meghalaya
The Meghalaya plateau lies in the monsoonic region and is directly influenced by the southwest monsoon and the northeastern winter winds. It has four well-defined seasons: spring (March-April), rainy-summer (May - September), autumn (October - November) and winter (December - February). The spring season (March - April) is characterized by moderate temperature, occasional thunderstorms, and high velocity wind. Rainy-summer season is the wettest period of the year and about three-fourth of the annual rainfall is received during this period. The winter season is the coldest period of the year [10].
For the purpose of survey in present study, the year was divided into three phases, i.e. pre-monsoon (February - May), monsoon (June - October) and post-monsoon (November - January). In each village, one thermometer and relative humidity data loggers (Onset Computer Corporation, Bourne, MA, USA) were placed and one person was appointed to record temperature and humidity daily. Rainfall data were collected from the Meteorological department of India, Shillong.
Mosquitoes collection and identification
The mosquitoes were collected from the 35 sampling sites comprising five sites per district in Meghalaya from April 2008 to March 2012. These sampling sites are as shown in (Figure 1).
Figure 1: A schematic map of Meghalaya showing the sampling sites (board
pins) for Anopheline mosquito in different districts. The mosquito samples
were obtained from 28 selected sampling sites. Source: Google Earth. https://
www.google.com/earth/download/ge/agree.html
All catches were conducted by standard techniques of WHO [11]. All possible habitats of mosquitoes situated within 5 Km radius were searched, for obtaining the maximum number of specimen from every district. Sampling of mosquitoes in each selected area was done at least once approximately at the mid of a phase during a year. In each village, 10 houses were normally examined and the worst ventilated room was selected for sampling as these surroundings usually contain a large number of breeding grounds for mosquitoes. Special attention was paid to the sleeping areas and bathrooms. The collection was done during early morning at about 6 to 8 am. Immature forms of mosquitoes were collected by standard dipping technique as described by Reuben [12] and Service [13]. All collected larvae and pupae were kept in a rearing tray for the emergence of adults. The emerged and collected adults were preserved in glass and plastic vials. Adult and larval forms of mosquitoes were morphologically identified using catalogues of Christopher’s [14] Gillie’s and Coetzee [15], Das et al., [16] and Nag pal & Sharma [17].
Data analysis
Mosquito community structure was analyzed using following ecological parameters like population abundance, species richness, species evenness (Pileou’s index), diversity of species (Simpson index, Shannon - Wiener index), wealth of mosquito (Margalef index) and species dominance (Barger - Parker index) in each district. Population abundance at each district was defined as the sum of individuals of a particular species, counted at each site during study [18]. The number of species found at each study site during the study period expresses species richness. The indices of diversity were calculated using the software PAST 2.16 [19]. The similarity between habitats based on number of species was estimated by Jaccard and Whittaker index. Cluster analysis and Principal Component Analysis (PCA) were used to group the sampling sites by similarity of Anopheline abundance. The Jolliffe cut-off var-covar was also calculated for PCA to know the degree of reliability of the classification system used. Nonparametric abundance estimates were used to verify sampling sufficiency to assess the richness directly related to the number of rare species in the samples. All results are presented as mean ±SE.
Results
Environmental setting of study sites
The average season wise data on different environmental parameters such as rainfall, humidity and temperature, water temperature and pH are shown in Table 1. Analysis of data revealed the presence of higher temperature in East, West and South Garo hills during the month of May and June and maximum rainfall occurred in EKH while minimum rainfall was recorded in Ri-Bhoi district (Table 1). It was noted that during July and August there was more rain fall in study areas. It was also noted that the water pH in the sampling sites was acidic (Table 1).
Districts
Parameters
2010-11
2011-12
Pre-Monsoon
Monsoon
Post-Monsoon
Pre-Monsoon
Monsoon
Post-Monsoon
East Khasi Hills
Temperature
11.5±0.6
22.5±2.8
13.5±2.1
19.2±2.8
21.2 ±0.9
9.6±2.5
Rain fall
33.7±35.7
265.4 ±116.4
7.9 ±0.8
20.0 ±18.3
230.8 ±137.2
32.7±19.4
pH
6.3±0.2
6.6 ±0.2
6.5±0.2
6.5 ±0.4
6.6 ±0.2
6.6±0.3
Humidity
50 ±3.4
90±4.3
70±5.3
57±5.5
89±6.7
60±.5
West Khasi Hills
Temperature
12.7±1.6
20.5 ±1.8
30.5±0.1
19.2 ±2.8
21.2±0.9
4.6 ±0.5
Rain fall
133.7±35.7
274.5 ±66.4
12.5 ±0.8
50.0 ±18.3
254.8 ±137.2
32.7±11.2
pH
6.4±0.2
6.3 ±0.2
6.6±0.2
6.3±0.4
6.7±0.7
6.6±0.5
Humidity
66±3.4
90±4.3
60-70±5.3
50-70±5.5
70-90±6.7
60-70±.5
Jaintia Hills
Water Temp.
20.7±1.65
29.3±4.9
16.2 ±1.83
26.67 ±2.48
29.4±0.93
15.57±1.42
Rain fall
44.7±27.1
291.7 ±138.2
9.47 ±0.65
45.0 ±9.82
324.4±62.9
13.47±2.17
pH
7.7±.25
6.92 ±1.3
6.9 ±0.51
6.8 ±0.70
6.7±0.39
6.2±.21
Humidity
50 ±2.6
80±3.1
60±3.3
55±3.6
82±3.1
64±2.4
Ri-Bhoi
Temperature
23.27 ±1.65
32.3 ±4.9
19.2 ±1.83
26.67±2.48
31.4±0.93
17.5±1.42
Rain fall
49.27±37.1
251.5 ±138.2
11.47±0.78
25.0 ±10.86
244.4 ±67.9
9.47 ±2.97
pH
7.67±.25
6.97±0.3
7.2 ±0.21
7.8 ±.70
7.3 ±0.40
7.2 ±.26
Humidity
64±3.2
88±4.5
68±3.1
60±2.8
85±5.4
66±3.9
East Garo Hills
Temperature
27.3±5.7
30.8±1.9
19.1±26.6
26.7±4.32
33.8±3.1
16.0±3.7
Rain fall
170.0±17.51
209.5±33.6
21.9±3.2
173.0 ±17.5
6.6±0.57
22.0±2.9
pH
72.0±5.8
73.3±4.1
65,3±12.2
70±5.8
76.0±4.1
66.0±12.2
Humidity
50±4.6
80±5.1
62±2.8
68±2.6
80±3.7
66±3.7
West Garo Hills
Temperature
26.8±5.14
32.8±3.5
19.4±6.2
27.3±5.7
33.8±3.0
20.1±5.6
Rain fall
31.0 ±22.7
356.1±122.8
73.4±82.7
170.0 ±170.51
315.4±99.9
15.1±24.3
pH
7.1 ±0.15
6.7±0.2
6.9 ±0.17
6.9 ±0.17
6.8 ±0.05
6.9 ±0.2
Humidity
62±2.8
88±2.6
70±3.7
64±3.3
84±4.4
70±4.1
South Garo Hills
Temperature
29.3 ±1.8
30.1 ±2.4
17.9 ±3.5
26.7±2.0
32.7±2.9
18.7 ±2.1
Rain fall
92.0 ±143.2
278.0 ±129.1
12.4 ±3.5
96.1±108.1
301.4 ±139.6
1.3 ±1.1
pH
7.6 ±0.3
7.2 ±0.2
7.3 ±0.1
7.0 ±0.1
7.1 ±0.3
7.3 ±0.20
Humidity
50±2.9
84±5.4
67±4.8
55±3.4
82±5.4
67±3.4
Table 1: Environmental analysis of sampling in different districts of Meghalaya. The results are shown as Mean±SD. Sampling districts were surveyed 3 times.
Sample based rarefaction curve
Rarefaction curve provides measures of species diversity involving the expected number of sampling against the number of individuals (Figure 2). The rise in the curve denotes quick increase in the number of species during sampling, while the flattening of the graph at the later stages may denote the repetition of similar species. This may infer that a reasonable number of individual samples have been taken. More intensive sampling may yield only few additional species.
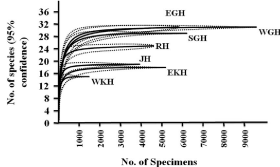
Figure 2: Sample based individual rare fraction curve showing number of
individuals against number of species recorded in different districts. Solid
lines show rare fraction curves of Anopheline mosquito communities and
dotted lines show 95% confidence limits of related solid lines. EGH and WGH
rare fraction curve are overlaps. The curve steepness is a function of the
community taxon evenness, while its height indicates the taxon richness.
EKH: East Khasi Hills district; WKH: West Khasi Hills district; RB: Ri-Bhoi
district; JH: Jaintia Hills district; EGH: East Garo Hills districts; WGH: West
Garo Hills District and SGH: South Garo Hills district
Species composition and relative abundance
A total of 37,026 Anopheline mosquitoes (larvae: 23,614 and adults: 13,412) were collected during the survey from 35 sampling sites of all seven districts of Meghalaya. Analysis of data revealed the presence of 33 species of two subgenera as: Anopheles (An. aitkenii, An. ahomi, An. barbirostris, An. gigas, An. lindesayiand An. maculates) and Cellia (An. aconitus, An. annularis, An. balabacensis, An. culicifacies, An. crawfordi, An. fluviatilis An. dirus, An. jamesii, An. jeyporiensis, An. karwari, An. kochi, An. maculates, An. minimus, , An. maculates, An. majidi, An. nivipes, An. philippinensis, An. peditaeniatus, An. pallidus, An. pseudojamesi, An. splendidus, An. subpictus, An. stephensi, An. tessellates, An. vagus An. varuna and An. willmorei,) (Table 2).
Subgenera
Species
Adult
Larvae
Total
RA (%)
Anopheles
An. nigerimus Giles, 1900
1269
2357
3626
9.79
An. lindesayi Giles, 1900
304
573
877
2.37
An. ahomi Chowdhury, 1929
53
95
148
0.40
An. gigas Giles, 1901
33
85
118
0.32
An. barbirostris Van der Wulp, 1884
32
78
110
0.30
An. gigas var baileyiedwards 1929
26
81
107
0.29
An. aitkenii James 1903
21
51
72
0.19
Total (Anopheles)
1738
3320
5058
13.67
Cellia
An. maculatus Theobald, 1901
2712
5316
8028
21.68
An. vagus Donitz, 1902
2089
3535
5624
15.19
An. annularis Vander wulp, 1884
1688
3087
4775
12.90
An. philippinensis Ludlow,1902*
1444
2207
3651
9.86
An. minimus Theobald, 1901*
1083
2136
3219
8.69
An. varuna Iyengar, 1924
451
937
1388
3.75
An. balabacensis Baisas, 1936
381
710
1091
2.95
An. subpictus Grani,1899*
345
664
1009
2.73
An. culicifacies Giles, 1901
300
403
703
1.90
An. aconitus Donitz, 1902
146
261
407
1.10
An. kochi Doenitz, 1901
186
218
404
1.09
An. stephensi Liston, 1901*
132
211
343
0.93
An. crawfordi Reid, 1953
173
98
271
0.73
An. jamesii Theobald, 1901
119
140
259
0.70
An. jeyporiensis James, 1902
104
71
175
0.47
An. pseudojamesii Strickland and Chowdhury, 1927
36
78
114
0.31
An. tessellatus Theobald, 1901
40
69
109
0.29
An. karwari James, 1903
74
0
74
0.20
An. Pallidus
0
74
74
0.20
An. splendidus Koidzumi, 1902
0
55
55
0.15
An. fluviatilis James, 1902
16
21
37
0.10
An. willmorei James, 1903
22
13
35
0.09
An. majidi Young and Majid, 1928
12
21
33
0.09
An. dirus Peyton and Harrison, 1979*
0
32
32
0.09
An. nivipes Theobald, 1903
20
11
31
0.08
An. peditaeniatus Leicester, 1908
27
0
27
0.07
Total (Cellia)
11674
20294
31978
86.36
Grand Total (Anopheles and Cellia)
13338
23688
37026
100
RA: Relative Abundance
* = potential malaria vector species
Table 2: Anopheline species collected from different districts of Meghalaya. Species are listed in descending order of the total number of each species collected.
The average density of Anopheline was about 5289 ±931.15 per district. Out of the total 37,026 mosquitoes, the most common species were An. maculatus (21.7%), An. vagus (15.2%), An. annularis (12.91%), An. philippinensis (9.9%), An. nigerimus (9.81%) and An. minimus (8.7%). These form nearly 78.2% (p =0.01) of the total mosquitoes caught. The relative abundance of subgenera Cellia (86.4%) was higher than subgenera Anopheles (13.6%). The number of specimens of potential malaria vector species, i.e. An. minimus Theobald, 1901, An. philippinensis Ludlow, 1902, An. annularis Vander wulp, 1884 and An. culicifacies Giles, 1901 was 33.4% of total individual caught. A total 71.4% (p = 0.01) of Anopheles were collected from lowland areas of Meghalaya, out of which 25.9% (p = 0.01) were collected from WGH, 19.7% (p = 0.01) from EGH and 16.8% (p = 0.01) from SGH districts (Table 3).
Species
Districts
EKH
WKH
RB
JH
EGH
WGH
SGH
An. aitkenii
0
9
0
16
11
22
14
An. lindesayi
436
56
195
98
32
41
19
An. gigas Giles
0
31
0
49
16
22
0
An. gigas var
0
18
0
29
19
41
0
An. nigerimus
613
149
548
300
555
915
546
An. ahomi
28
0
55
25
2
33
5
An. barbirostris
0
0
55
0
26
29
0
An. balabacensis
357
104
133
0
41
285
171
An. aconitus
56
0
61
0
60
123
107
An. tessellatus
39
0
25
0
13
28
4
An. fluviatilis
16
20
1
0
0
0
0
An. minimus
263
0
686
420
418
864
568
An. varuna
95
0
146
236
227
383
301
An. culicifacies
0
73
161
153
71
141
104
An. subpictus
86
0
104
200
105
458
56
An. vagus
822
164
498
662
849
1706
923
An. annularis
699
216
396
414
1040
1180
830
An. jamesii
23
6
49
60
41
54
26
An. maculatus
1309
595
850
710
1344
1810
1410
An. philippinensis
352
0
379
476
690
1038
716
An. splendidus
0
0
12
0
0
17
26
An. stephensi
0
45
77
54
43
66
58
An. jeyporiensis
10
6
21
14
32
49
43
An. karwari
0
0
0
0
21
19
34
An. willmorei
3
0
10
0
11
2
9
An. majidi
9
0
0
0
3
5
16
An. nivipes
0
0
5
0
1
11
14
An. dirus
0
23
0
0
5
0
4
An. peditaeniatus
0
0
0
0
7
11
9
An. kochi
0
0
112
0
118
70
104
An. crawfordi
0
0
34
35
43
106
53
An. pallidus
0
0
0
0
15
33
26
An. pseudojamesii
0
0
15
3
9
53
34
Total number of Individual
5216
1515
4628
3954
5868
9615
6230
EKH: East Khasi Hills, WKH: West Khasi Hills, RB: Ri-Bhoi, JH: Jaintia Hills, EGH: East Garo Hills, WGH: West Garo Hills and SGH: South Garo Hills.
Table 3: Number of Anopheles species collected from different districts of Meghalaya from April, 2010 to March, 2012.
The district wise species abundance and species richness relationship shows that species abundance was highest in WGH and lowest in WKH (Figure 3).
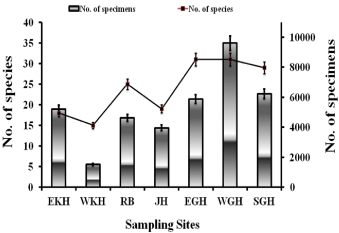
Figure 3: Number of Anopheles species collected from different districts of
Meghalaya from April, 2010 to March, 2012.
Seasonal distribution
During pre-monsoon, monsoon, and post-monsoon 9,345 (25.2%), 23,507 (63.5%) and 4,174 (11.3%) mosquitoes respectively were recorded (Figure 4). It was noted that Anopheline species abundance was associated with temperature and rainfalls because the abundance of Anopheline peaked in month of July to October (20840, 56.2%) and fell progressively from November to January (4,174, 11.3%). Gradual increase in the density of An. annularis, An. philippinensis, An. minimus and An. subpictusstarted during premonsoon, peaked in post-monsoon and declined during winter. An. peditaeniatus and An. pallidus was found only in border areas of EGH and WGH in the month of July and August. The abundance of all species declined gradually at different rates in winter season (Figure 4).
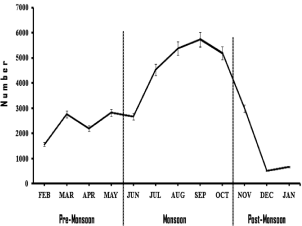
Figure 4: Seasonal distribution of Anopheline mosquitoes.
Species richness and diversity
Alpha and beta biodiversity indices (mentioned above) were calculated district wise for the entire study area (Table 4). The average Anopheline species richness was about 24 ±2.5 species per district and ranged from 15 (WKH) to 31 (WGH and SGH). The Welch F test showed that species richness varied significantly among districts (F96.47 =3.092, p =0.001). The comparison of alpha diversity using Simpson and Shannon indices showed that RB district is the most diverse (H’= 2.56, λ = 0.90,) and E ranged from 0.33 to 0.58. According to the Margalef richness index, East Garo Hills district was the most diverse (Dmg = 3.46) and the West Khasi Hills districts showed least diversity (Dmg = 1.91, λ = 0.79, H’= 2.00).The order of the districts on the basis of higher to lower diversity is noted to be as EGH>WGH>SGH>RB>JH>EKH>WKH.
Alpha Diversity
WKH
EKH
RB
JH
EGH
WGH
SGH
Species Richness
15
18
25
19
31
31
29
Individuals
5216
1515
4628
3954
5868
9615
6230
Simpson (?)
0.79
0.86
0.90
0.89
0.86
0.88
0.88
Shannon (H')
2.00
2.21
2.56
2.40
2.33
2.45
2.40
Evenness (E")
0.49
0.50
0.52
0.58
0.33
0.37
0.38
Margalef (Dmg)
1.91
1.99
2.84
2.17
3.46
3.27
3.21
Barger-Parker
0.39
0.25
0.18
0.18
0.23
0.19
0.23
EKH: East Khasi Hills, WKH: West Khasi Hills, RB: Ri-Bhoi, JH: Jaintia Hills, EGH: East Garo Hills, WGH: West Garo Hills and SGH: South Garo Hills.
Table 4: comparision of various community indices among the study sites in seven districts of Meghalaya.
The β biodiversity analysis showed that EGH and SGH are the closest districts with reference to Anopheles diversity (IJ= 0.94) with the specific alternate among them being very low (βw = 0.03). There is low similarity of 0.38, 0.38, 0.39, 0.38, and 0.44 found when WKH was compared to the EKH, RB, EGH, WGH and SGH districts (Table 5).
Comparison
Jaccard (Ij)
Whittaker (βw)
EKH-WKH
0.38
0.45
EKH- RB
0.65
0.20
EKH-JH
0.48
0.35
EKH-EGH
0.53
0.31
EKH-WGH
0.53
0.31
EKH-SGO
0.57
0.27
WKH-RB
0.38
0.45
WKH-JH
0.55
0.29
WKH-EGH
0.44
0.39
WKH-WGH
0.39
0.43
WKH-SGH
0.38
0.45
RB-JH
0.57
0.27
RB-EGH
0.70
0.18
RB-WGH
0.75
0.14
RB-SGH
0.74
0.15
JH-EGH
0.61
0.24
JH-WGH
0.61
0.24
JH-SGH
0.55
0.29
EGH-WGH
0.94
0.03
EGH-SGH
0.88
0.06
WGH-SGH
0.88
0.07
- The Whittaker average for comparisons of the districts is 0.375.
- EKH: East Khasi Hills; WKH: West Khasi Hills; RB: Ri-Bhoi; JH: Jaintia Hills; EGH: East Garo Hills; WGH: West Garo Hills; SGH: South Garo Hills.
Table 5: Pair wise Whittaker and Jaccard β comparisons for each district.
Cluster analysis and principal component analysis
The districts were also classified on the basis of species similarity in richness and abundance as depicted in the form of Bray-Curtis similarity cluster analysis. Cluster analysis produced 3 separate groups, out of which the major group (B) consisted of four districts followed by group A with two districts and group C with one district (Figure 5). Group B is the largest one which is further subdivided into small sub-units. Group C (West Khasi hills district) separated first at a major distance with only 39% similarity with other group, followed by West Garo hills districts (72.5% similarity). Anopheline mosquitoes in WKH were the poorest in terms of richness and abundance where mainly An. maculatus dominated with many rare species (relative abundance is less than 1%).The high value of Bray- Curtis cophenitic correlation (rc= 0.9361) indicates a high correlation between ecological distance observed in our study.
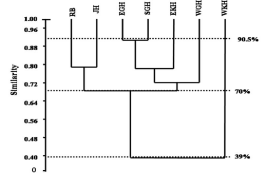
Figure 5: Pair wise Whittaker and Jaccard β comparisons for each district.
The Principal Component Analysis (PCA) was used to analyze the correlation of all seven districts. The high value of the Jolliffe cutoff (1.035E) indicates a high correlation as noted after cluster analysis. The results of PCA also showed similar result with cluster analysis (Figure 6).
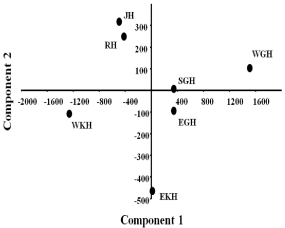
Figure 6: Principal component analysis of different districts based on the
Anopheles species richness. The two axes explained 59% of the variation.
Dots are showing study sites.
EKH: East Khasi Hills district; WKH: West Khasi Hills district; RB: Ri-Bhoi
district; JH: Jaintia Hills district; EGH: East Garo Hills districts; WGH: West
Garo Hills District and SGH: South Garo Hills district
Discussion
Meghalaya has a unique landscape of North-eastern India, supporting a varied flora and fauna because of highly humid, tropical, and subtropical climate [20]. The study areas have highly dissected and irregular topography, which includes warmer foothill areas and subalpine, alpine central plateau areas (Figure 1). Thus, it may be expected to find the presence of Anopheline species from two broad bio-geographical zones with unique possibilities for studies on the impact of climate and habitat on mosquito’s species richness and biodiversity. Similar correlation has also been proposed in many other highlands [21,22]. Climate variability is widely considered to be a major driver of inter-annual variability of Anopheles species richness and abundance. The relationship between environmental variability and Anopheles abundance was assessed in this study. Climate suitability for Anopheles was defined as the coincidence of precipitation accumulation greater than 100 mm, mean temperature between 18°C and 32°C, and relative humidity greater than 60 percent [23-25]. Considering these climate suitability, present findings revealed that the environmental conditions of all the districts should be suitable for Anopheles mosquito survival except East Khasi Hills and West Khasi hills districts (Table 1).
The obtained rare faction curves (Figure 2) showed that the mosquitoes in an environment are randomly distributed. The sample size is sufficiently large and taxonomically similar and all the samples have similar pattern of distribution in each district. The curve is flattened to right (for each district) which suggests very high beta diversity. Rarefaction only works well when no species is extremely rare or common, or when beta diversity is very high. Rarefaction assumes that the number of occurrences of a species reflects the sampling intensity, but if one species is especially common or rare, the number of occurrences will be related to the extremity of the number of individuals of that species, not to the intensity of sampling [26]. In EGH and WGH, the curve has tendency to stabilize with 31 species. According to Magurran [27] geometric models for the abundance curves, more flattened distributions correspond to diverse sample, which is consistent with our present study (Figure 2).
During the present study, 33 species and 2 sub-genera of Anopheles were recorded. The number of Anopheline species recorded were more as compared to neighboring States i.e. Nagaland, 22 [28], Assam, 30 [29,30], Manipur, 17 [31], Mizoram 16 [32] and Tripura, 22 [29,33]. EGH (31), WGH (31), SGH (29) and RB (25) are warmer in spring- summer season recording maximum temperature range of 29°C – 32°C and wettest districts with humidity ranging from 60% - 80%. These factors may help to increase the larval habitat density and related biodiversity. Most parts of WKH (15) and EKH (18) are situated on central plateau area, thus, only a few species were capable of adapting to this adverse situation [21,34]. Overall species abundance and richness revealed that subgenera Cellia (86.4%) were the most dominant in all the districts. Earlier also An. Maculatus has been reported as the most dominant species of Meghalaya [35]. Further, our findings reveal that there is abundance of different species in different districts i.e. EKH is dominated by An. lindesayi (n =1309), WKH is dominated by An. annularis (n = 595) and WGH is dominated by An. vagus (n = 1810) although they are found in all seven surveyed districts (Table 2). Some species of Anopheles (An. willmorei, An. majidi, An. dirus, An. nivipes and An. paditaeniatus) were found in very low number during the study. The results of this study also support the report of NVBDCP, 2012 regarding the increase of malaria cases in Meghalaya compared to other part of India [36,7]. This could be due to high density of vector Anopheline mosquitoes.
Anopheles mosquitoes’ survival and development has been suggested to significantly depend on ambient temperature [37-40]. The present findings on the occurrence of more diverse and abundant Anopheline species in low land and mid range areas of Garo Hills, Khasi Hills and Jaintia Hills, may also suggest the presence of suitable environmental conditions such as high temperature, humidity and acidic pH of aquatic habitat (Table1). Another possible cause of Anopheles abundance and richness in central plateau area of Khasi Hills could be anthropogenic land use changes. Normally at high altitude, mosquitoes population is controlled by low temperature and availability of less breeding place. Several studies have suggested that land use changes in any area may increase the Anopheline density and diversity [41-44].
Total nine malaria vector species were known in India and all were encountered and captured at varying density at study sites. Similar results have been reported from Sonitpur district of Assam [45,46] from Nagaland [28] and from Mizoram [32].
Alpha and beta biodiversity study is one of the easiest ways to measure the diversity and similarity in pair of study sites. Alpha diversity index like Shannon-Wiener, Simpson, Pileou’s, Margalef and Barger-Parker are the most useful tool to measure the species diversity, species evenness, wealth of mosquito and species dominance [18]. The analysis of similarity in Shannon diversity index, Simpson’s evenness and Barger–Parker dominance of each district indicated that uneven biodiversity prevailed in the whole study area. The Anopheles communities in the districts of Garo Hills zone showed higher species richness and diversity, and lower dominance index compared to the districts in Khasi Hills and Jaintia Hills zone. The decline in species richness and diversity from foothills to mountain habitat could be due to unfavorable environmental low temperature. Several hypotheses and studies suggested that Land Use and Land Cover (LULC) changes have influenced malaria vector, larval habitat availability, productivity, density and distribution in the world [24,39,47,48].
The observed increase in Anopheline mosquitoes in EKH having mostly plateau areas may be attributed due to human land manipulation and disturbance through urbanization involving LULC. Similar type of observation and suggestion has been given for study areas in Kenya and Africa [40].
Meri & Peydro [34] and Confalonieri &Costa-Neto [49] grouped Anopheline mosquitoes habitats on the basis of population. In present study, the same method was used to construct the dendrogram to group Anopheline mosquito habitat. This cluster analysis showed 90.5% similarity between EGH and SGH which is probably due to the similar environmental conditions. However, the similarity between EKH and Garo hills zone is suggested to be due to land use disturbances. The WKH had very low similarity with another groups (39%), may be due to the low temperature and less land use changes.
The results of the present studies show that biodiversity indices are very useful tool for environmental changes assessments. The similarity in Shannon diversity index, Simpson’s evenness and Barger–Parker dominance of each district showed that uneven biodiversity prevail in the whole study area. Thus, based on the present study it may be suggested that the local environment plays the significant role in explaining Anopheline mosquitoes species distributions and indicates that community composition can be sustained under changing land use.
Acknowledgement
We duly acknowledge the contribution of (late) Prof. B. Kharbuli in planning and carrying out the present work and the paper is dedicated to him. The research fellowship was provided from DSASAP, UGC and UGC Non NET to Atin K. Srivastava. We are very grateful to the people of the study area who kindly allowed us to use their houses to sample mosquitoes, as well as to the members of National rural health mission, Ms. Eseena Sangma (Chellang), Ms. Tinku Sangma (Garobada) who daily recorded temperature and humidity throughout the study period. We also thank Defiance and Medical Entomology department of USA for providing the identification keys and Mr. Mani of Regional Health Centre, Govt. of India, Shillong for helping in identifying adult and larval mosquitoes.
References
- WHO: World Malaria Report. 2012.
- Narain JP. Malaria in South - East region: Myth and the reality. Indian Journal of Medical Research. 2008; 128: 1-3.
- Bhattacharjee A, Bhattacharjee DA. Bayesian approach to compare the state wise malaria death counts in India. Healthline. 2011; 2: 54-58.
- Sinka ME, Bangs MJ, Manguin S, Chareonviriyaphap T, Patil AP, Temperley W, et al. The dominant Anopheles vector of human malaria in the Asia-Pacific region: occurrence data, distribution map and bionomic précis. Parasite and Vectors. 2011; 4: 1-78.
- Greenwood BM, Fidock DA, Kyle DE, Kappe SH, Alonso PL, Collins FH, et al. Malaria: progress, perils, and prospects for eradication. The Journal of clinical investigation. 2008; 118: 1266-1276.
- Dash AP. "Estimation of true malaria burden in India: 12. A profile of National Institute of Malaria Research". 2009.
- Srivastava AK, Kharbuli B, Shira DS, Sood A. Effect of land use and land cover modification on distribution of Anopheline larval habitat in Meghalaya, India. Journal of Vector Borne Diseases. 2013; 50: 121-126.
- Shortt H E. The occurrence of malaria in hill station. Indian Journal of Medical Research 11. 1925; 767-771.
- Prakash A, Bhattacharyya DR, Mohapatra PK, Mahanta J. Anopheline fauna of the north-eastern states of India with notes on vectors of Malaria. Proceeding of the Indian National Science Academy. 1998; 68: 217-229.
- Marak CP. State profile of community forestry: Meghalaya, Ne India. Community forestry international (CFI), USA. 2007; 1-13.
- WHO: Manual on practical entomology in malaria vector bionomics and organization of antimalaria activities, Part I and Part II. Offset Publication No. 13. World Health Organization, Geneva. 1975.
- Reuben R. A report on mosquitoes collected in the Krishna-Godavari delta, Andhra Pradesh. Indian Journal of Medical Research. 1978; 68: 603-609.
- Service MN. Mosquito ecology: Field Sampling Methods. Edition II. London, Kluwer Academic Publishers. 1993; 889.
- Christophers SR. The fauna of British India, including Ceylon and Burma. Diptera. Family Culicidae, Tribe Anopheline Taylor & Franci, London. 1933; 1-371.
- Gillies MT, Coetzee M. A supplement to the Anophelinae of Africa South of the Sahara (Afro-tropical region). Johannesburg: South Africa institute of Medical Research. 1987; 55: 1-143.
- Das BP, Rajagopal R, Akiyama J. Pictorial keys to the species of Indian Anophelines mosquitoes. Zoology: Journal of Pure and Applied Zoology. 1990; 2: 131-162.
- Nagpal BN, Sharma VP. Indian Anophelines. New Delhi, India, Oxford and IBH Publishing Co Pvt Ltd. 1995; 416.
- Montes J. [Culicidae fauna of Serra da Cantareira, Sao Paulo, Brazil]. Rev Saude Publica. 2005; 39: 578-584.
- Hammer DAT, Harper, Ryan PD. Past: paleontological statistics software package for education and data analysis. Paleontologica Electronica. 2001; 4: 1- 9.
- Roy PS, Tomar S. Landscape cover dynamics pattern in Meghalaya. International Journal of remote sensing. 2001; 22: 3813-3825.
- Eisen D, Bolling BG, Blair CD, Beaty BJ, Moore CG. Mosquito species richness, composition, and abundance along habitat-climate-elevation gradients in the Northern Colorado Front range. Journal of Medical Entomology. 2008; 45: 800-811.
- Pinault LL, Hunter FF. New highland distribution records of multiple Anopheles species in the Ecuadorian Andes. Malaria Journal. 2011; 10: 1-11.
- Koenraadt CJ, Paaijmans KP, Githeko A, Knols B, Takken W. Egg hatching, larval movement and larval survival of the malaria vector Anopheles gambiae in desiccating habitats. Malaria Journals. 2003.
- Munga S, Minakawa N, Zhou G, Mushinzimana E, Barrack OJ, Githeko AK, et al. Association between land cover and habitat productivity of malaria vectors in western Kenyan Highlands. American Journal of Tropical Medicine and Hygiene. 2006; 74: 69 -75.
- Afrane YA, Little TJ, Lawson BW, Githko A, Yan G. Deforestation and vectoral capacity of Anopheles gambiae Giles mosquitoes in malaria transmission, Kenya. Emerging Infectious Diseases. 2008; 14: 1533-1538.
- Bush AM, Markey MJ, Marshall CR. Removing bias from diversity curves: the effects of spatially organized biodiversity on sampling-standardization. Paleobiology. 2004; 30: 666-686.
- Magurran AE. Measuring Biological Diversity. Malden, USA, Blackwell Publishing Company. 2004; 215.
- Dutta P, Khan SA, Khan AM, Sharma CK, Mahanta J. Survey of mosquito species in Nagaland, a hilly state of north east region of India. Journal of Environmental Biology. 2010; 31: 781-785.
- Malhotra PR, Mahanta HC. Check- list of mosquitoes of Northeast India (Diptera: Culicidae). Oriental Insects. 1994; 28: 125-149.
- Dutta P, Prakash A, Bhattacharyya DR, Khan SA, Gogoi PR, Sharma CK, et al. Mosquito biodiversity of Dibru-Saikhowa biosphere reserve in Assam, India. J Environ Biol. 2010; 31: 695-699.
- Dutta P, Khan SA, Khan AM, Sharma CK, Mahanta J. Biodiversity of mosquitoes in Manipur State and their medical significance. J Environ Biol. 2005; 26: 531-538.
- Vanramliana, Lalramnghinglova H. Anopheline diversity in Undivided Aizawl district of Mizoram, India. Science vision. 2013; 13: 35-39.
- Nagpal BN, Sharma VP. Survey of mosquito fauna of northeastern region of India. Indian J Malariol. 1987; 24: 143-149.
- Marí RB, Jiménez-Peydró R. Differences in mosquito (Diptera: Culicidae) biodiversity across varying climates and land-use categories in Eastern Spain. Entomologica Fennica. 2011; 22: 190-198.
- Das SC, Das NG, Baruah I. Mosquito survey in Meghalaya. Indian J Public Health. 1984; 28: 147-151.
- Delhi India: National Vector Borne Disease Control Programme Ministry of Health and Family Welfare.
- Leeson HS. Longevity of Anopheles maculipennis race atroparvus, Van Thiel, at controlled temperature and humidity after one blood meal. Bulletin of Entomological Research. 1939; 30: 103-301.
- DeMeillon, B. Observations on Anopheles funestus and Anopheles gambiae in the Transvaal. South African Institute of Medical Research. 1934; 6: 199-248.
- Mouchet J, Manguin S, Sircoulon J, Laventure S, Faye O, Onapa AW, et al. Evolution of malaria in Africa for the past 40 years: impact of climatic and human factors. J Am Mosq Control Assoc. 1998; 14: 121-130.
- Afrane YA, Githeko AK, Yan G. The ecology of Anopheles mosquitoes under climate change: case studies from the effects of deforestation in East African highlands. Ann N Y Acad Sci. 2012; 1249: 204-210.
- GARNHAM PC. The incidence of malaria at high altitudes. J Natl Malar Soc. 1948; 7: 275-284.
- Lindblade KA, Walker ED, Onapa AW, Katungu J, Wilson ML. Land use change alters malaria transmission parameters by modifying temperature in a highland area of Uganda. Tropical Medicine and International Health. 2005; 5: 263-274.
- Munga S, Minakawa N, Zhou G, Githeko AK, Yan G. Survivorship of immature stages of Anopheles gambiae s.l. (Diptera: Culicidae) in natural habitats in western Kenya highlands. J Med Entomol. 2007; 44: 758-764.
- Phasomkusolsil S, Lerdthusnee K, Khuntirat B, Kongtak W, Pantuwatana K, Murphy JR. Effect of Temperature on laboratory reared Anopheles dirus Peyton and Harrison and Anopheles sawadwongporni Rattanarithikul and Green. Southeast Asian Journal of Tropical Medicine and Public Health. 2011; 42: 63-70.
- Baruah I, Das NG, Kalita J. Seasonal prevalence of malaria vectors in Sonitpur district of Assam, India. J Vector Borne Dis. 2007; 44: 149-153.
- Adhikari ML, Kalita S, Deka TC. Diversity and abundance of malaria vectors in Dhekiajuli Subdivision of Sonitpur District, Assam. Asian Journal of Experimental Biological Sciences. 2013; 14: 103-111.
- Lindsay SW, Martens WJ. Malaria in the African highlands: past, present and future. Bull World Health Organ. 1998; 76: 33-45.
- Githeko AK, Lindsay SW, Confalonieri UE, Patz JA. Climate change and vector-borne diseases: a regional analysis. Bull World Health Organ. 2000; 78: 1136-1147.
- Confalonieri UE, Neto CC. Diversity of mosquito vectors (Diptera: culicidae) in caxiuan&aTilde;, pará, Brazil. Interdiscip Perspect Infect Dis. 2012; 2012: 741273.