
Special Article - Dengue Fever
Austin J Infect Dis. 2016; 3(1): 1022.
Way Forward for Seasonal Planning of Vector Control of Aedes aegypti and Aedes albopictus in a Highly Dengue Endemic Area in India
Roop K*, Priya S, Sunita P, Mujib M, Kanhekar LJ and Venkatesh S
National Centre for Disease Control, India
*Corresponding author: Kumari Roop, Joint Director, Centre for Medical Entomology & Vector Management, National Centre for Disease Control, Delhi, India
Received: May 09, 2016; Accepted: June 16, 2016; Published: June 17, 2016
Abstract
Aedes aegypti and Aedes albopictus have acquired an international importance in view of recent spurt in zika cases in Brazil as these mosquitoes are the vectors of dengue, chikungunya, yellow fever as well as for Zika fever. Although yellow fever and Zika fever are not reported from India, but both mosquitoes are well prevalent in the country. Ae. aegypti is most prevalent Aedes mosquitoes recorded that preferred to breed in artificial containers in and around the houses and adaptation of Ae. albopictus in man-made containers was also reported from India. Therefore, the objective of present study is to find out season wise key containers for larvae and pupae production of Ae. aegypti and Ae. albopictus in a highly endemic area for dengue in the capital city of India. Delhi has so far witnessed several outbreaks of dengue fever since 1967. Besides larvae survey, pupae survey was also conducted during 2013 and 2014 as pupal production is a better proxy for adult mosquito reproduction than traditional breeding indices and are more appropriate for directing dengue control programs. All pupae were counted and reared for species identification. Total 16168 houses and 48713 containers were checked. Four key containers such as plastic containers, cement tanks, coolers and syntax tanks harboured 89% of Ae. aegypti pupae. Among them Plastic Water Storage Containers (PWSC) are recorded as perennial source of Aedes infestation that contributed 41% of the immature breeding and about half of pupal production. It was observed that Aedes breeding preference has been shifted toward PWSC as compare to previous studies, therefore, public health attention is required to control breeding in such containers. Present study indicates there is aseasonal variation of breeding preference of Ae. aegypti and Ae. albopictus in different containers in Delhi, so it is important for a target and effective planning of seasonwise control measures. The containers that produced the larger proportion of pupae, vector control intervention should be targeted to maximize their intact.
Keywords: Dengue; Aedes aegypti; Aedes albopictus; Key containers; Pupal survey; Breeding; India
Abbreviations
PWSC: Plastic water storage container; HI: House Index; CI: Container Index; BI: Breteau Index; DF: Dengue Fever; DHF: Dengue Haemorrhagic Fever; NCT: National Capital Territory; MCD: Municipal Corporation of Delhi; NDMC: North Delhi Municipal Corporation; ID: Indoor; PD: Peri-Domestic; OD: Outdoor; PPC: Pupae per Container; PI: Pupal Index
Introduction
Dengue Fever (DF) is one of the most rapidly rising mosquito transmitted infections in the world. The maximum burden is contributed by countries of the Asia Pacific Region. Dengue outbreaks are occurring with increasing frequency and intensity. In India, the first outbreak of dengue was reported from Calcutta in 1963 and after that disease has been reported from other States also. So far in India, outbreaks of DF/ Dengue Haemorrhagic Fever (DHF) have been recorded in almost all parts of the country including the NCT of Delhi. The first epidemic of dengue fever was recorded in 1967 [1] after that many outbreaks were recorded from Delhi [2-6]. Among them, the first major outbreak occurred in 1996 with 10252 cases and 423 deaths [7] and during the year 2015, DF/DHF resurged in Delhi and total of 15836 cases and 46 deaths were reported [8]. It is important to study seasonal breeding preference of Aedes aegypti as it is also a vector of yellow fever and Zika fever, although these diseases are not reported from India.
Ae. aegypti is most prevalent dengue vector species recorded that preferred to breed in artificial containers in and around the houses and adaptation of Ae. albopictus in man-made containers was also reported from Delhi [9]. Dengue virus was also detected in both mosquitoes from Delhi [10]. For planning and implementation of control measures for dengue, it is necessary to know the most preferable breeding habitats for larvae and pupae.
Pupal survey is based on the assumption that pupal production is a better proxy for adult mosquito reproduction than traditional indices House Index (HI), Container Index (CI) and Breteau Index (BI) [11]. However, pupal surveys are more appropriate for assessing risk and directing dengue control programs because traditional larval indices correspond poorly with the actual number of pupae per person. Approximately 80% of Ae. aegypti pupae emerge into adult mosquitoes, allowing them to be used as a proxy measure for local adult mosquito abundance [12,13]. The containers that produced the larger proportion of pupae, vector control intervention should be targeted to maximize their intact. This consideration caused us to explore the hypothesis: 1) most pupae of Ae. aegypti are produce in a few types containers, 2) seasonal breeding preference of Aedes mosquitoes in different containers.
Therefore, proposed study was undertaken to detect most productive breeding sites for immature as well as for pupae of Aedes in Delhi and further to identify the key containers for pupal production. It is important to know the seasonal variation for breeding preference of Ae. aegypti and Ae. albopictus in different types of habitats/containers so that season wise planning can be done. In view of this seasonal preference of breeding of Aedes mosquitos in all the five seasons’ of Delhi were studied and presented in the paper. This is useful for season wise planning of vector control measures to be focussed in the key containers.
Materials and Methods
NCT of Delhi is the largest metropolis by area and the secondlargest metropolis by population in India. It is situated at 77o.15' E and 26o.15' N. It occupies 1,485 km2 area of which 900 km2 is classified as urban. Delhi has a population of 16.7 million (2011 Census). The city has three local bodies- New Delhi Municipal Council (NDMC), Delhi Cantonment Board and Municipal Corporation of Delhi (MCD). MCD covers nearly 97% of the total area of the city. The NCT of Delhi receives 611 mm of rainfall on an average annually mainly from July to September. The highest monthly average high temperature is 41oC in May and the lowest monthly average low temperature is 7oC in January. The average annual relative humidity is 49.2% and average monthly relative humidity ranges from 25% in April-May to 73% in August [14]. Delhi has five distinct seasons, viz. summer, rainy, autumn, winter and spring and details are given at (Table 1).
Season
Months
Average
Maximum
Temp.oC
Average
Minimum
Temp oC
Average
Rainfall
mm
Average
Number of Wet Days
Climate
Winter
December to February
24
8
48
7
Cold, dry, Blustery
Summer
April to June
39
26
116
9
Hot-hotter-hottest
Monsoon
July to September
35
25
585
35
Rainy spells
Autumn
October to November
35
13
26
3
Pleasant
Spring
March
29
15
15
3
Sunny and pleasant
Table 1: Season wise information of Delhi.
Entomological surveys
Entomological Surveys for dengue vectors were carried out in different localities in Delhi from 2013 to 2014. Repeated surveys were carried out on monthly basis in sentinel localities, one from each MCD and NDMC zone and other localities were randomly selected for survey. Localities are colonies or urban settlement. All localities were selected on the basis of dengue confirmed cases reported during last three years and also considered different socio-economic factors.
In each survey, about 50 houses in each locality were searched both Indoor (ID) and Peri- Domestic sites (PD) and Outdoor (OD) for breeding of Aedes using single larval techniques [15,16]. Only water holding containers were examined for the presence of mosquito larvae and pupae. A container is considered ‘positive’ for Aedes when one or more larvae or pupae are present. Lawns and campus of the houses were considered as peri-domestic sites and the area outside the compound wall was considered as outdoor. Container/ House with the presence of immatures (larvae & pupae) was marked as positive. Some specific categories of containers (Figure 1) were: Plastic Water Storage Containers (PWSC) which comprised of plastic drums, gallons and cans to store water for domestic purpose; desert coolers are used to cool inside of houses, which have a water tank and a fan; planted pots usually kept indoors or around the houses and solid waste comprises of unused cups, glasses, ceramic pipes, helmets and polythene etc.. Most widely used water tanks in Delhi are sintex water tanks which is lightweight, durable and rustproof with capacity start of 500 litres. It is either keep on ground or on the roof having lids but hardly closed and dried for reuse.

Figure 1: Showing different breeding sites of Aedes mosquitoes in Delhi, India.
The pupae were counted and all immature were reared in laboratory for adult emergence for species identification. Counting the number of pupae in each breeding site (to measure pupal productivity) offers insight not only into the abundance of pupae in the container but also an estimate of how many adult mosquitoes may emerge. Thus, we have assessed the importance of breeding sites to be focused on control operations toward the most productive containers to have the greatest impact on the adult Aedes mosquito populations. To identify the key Aedes breeding sites the percentage contribution of each breeding site to the total count of pupae was calculated. This was done by taking the total number of pupae found in a given container and dividing it by the total number of pupae in all containers in the area being studied of each season. Month wise data has been pooled together as per five seasons to know the variation of seasonal dynamics of vectors and other epidemiological factors.
Statistical analysis
The data were entered in MS Excel 2007 and statistical analysis was done by SPSS software package (version 20). As per standard WHO method HI, CI and BI were calculated [17]. HI was defined as the percentage of houses infested with larvae and/or pupae and CI defined as the percentage of water-holding containers infested with larvae or pupae. BI was calculated as the number of positive containers per 100 houses inspected. Pupal counts were used to calculate the pupal Productivity Indices: PI and Pupae per Container (PPC) according to standard methods [16,17]. Pearson’s correlation, significant differences and Chi-squared analysis was also carried out. The correlation coefficient of two data sets was calculated. Data were analyzed by using SPSS 11.5 software package (SPSS Inc., Chicago, IL, USA).
Results
Breeding preference habitats
During 2013 and 2014, total number of 48713 different types of containers were checked, immature of Ae. aegypti infestation were found maximum in plastic water storage containers (41.86%) followed by coolers, syntax tank, cement tanks, earthen, planted pot, solid waste, tin, tyres and rock/cement-pits (Figure 2a). Unused tyres and other solid waste contributed only 7.28% of Aedes breeding.
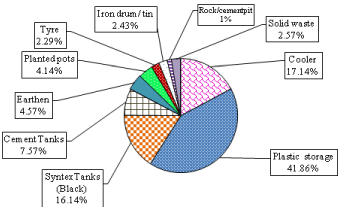
Figure 2a: Breeding Preference of Ae. aegypti (immature) in different
containers in Delhi 2013 & 2014.
(Figure 2b) shows that PWSC contributed maximum (48%) of total pupal production in Delhi followed by coolers, cement tanks and syntax tank. Pupal production was less (0.8%) in solid waste. Unused containers like tyres, tin and polythene sheets contributed only 5.6% of pupal production.
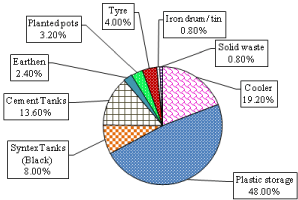
Figure 2b: Preference of Ae. aegypti pupae production in different containers
in Delhi 2013 & 2014.
It seems from the result that number of containers with immatures was significantly more as compared to number of containers with pupae. During 2013 and 2014, immatures of Ae. aegypti was found in 700 containers, whereas, pupal production was recorded only in 125 containers (17.85%). Larvae were recorded in rock and cement pits but pupal stages were not found. Some of the key containers like plastic storage, coolers, cement tanks and sintex tanks contributed 89% of pupal production (Figure 2b). However, these containers also contributed 83% of total breeding of immatures (including larvae and pupae) but pupal production has been decreased in some containers like earthen, planted pots, iron/tin and other solid wastes.
In Delhi, during 2013 & 2014, PWSC was the most favourable containers for larvae and pupae growth of Aedes aegypti. However, our earlier studies during 2008-2009 revealed thatmost preferable breeding sites of these mosquitoes were cement tanks and coolers. Thus, in recent year’s percent proportion of Aedes breeding in coolers has been decreased; and almost no breeding was recorded where Temephos granules were used. It was also observed that coolers were found as main target by MCD for control interventions. However, infestation of Ae. aegypti has been increased in PWSC from 12% (2008-09) to 48% (2013-14) as use of different type plastic containers has also increased by the residents of Delhi to store water due to irregular supply.
However, in some abundant containers like earthen, planted pots, iron/tin and other solid wastes where number of immatures were found more but number of pupae were less, it seems most of the immatures did not reach up to pupal stages. Thus, unused containers with temporary water collection had low pupal production.
During 2013 and 2014, total of 2407 Aedes adult mosquitoes merged out from all immatures collected from the Delhi, 2194 were Aedes aegypti (91.15%), 157 Ae. albopictus (6.52%) and 56 Aedes vittatus (2.3%). Percent contribution of breeding preference of Ae. albopictus in different containersis shown in (Figure 2c), which indicates that this species prefer to breed in plastic water storage container 26.23%, followed tanks, planted pots, cooler, tyre, earthen (bird feeding pots) and rock-pits, respectively. In urban area of Delhi, Ae. albopictus has adapted for man-made containers like Aedes aegypti.
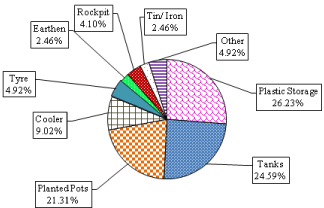
Figure 2c: Breeding Preference of Ae. albopictus in different containers in
Delhi during 2013 & 2014.
During the study period, Ae. aegypti was most predominant Aedes mosquito, found through-out the year. However, Ae. albopictus breeding was recorded during summer and rainy season (Figure 3). However, only two adult Ae. albopictus emerged out during winter season. Aedes Vittatus breeding was found only during rainy season. Aedes aegypti breeding was found maximum during the summer and rainy season followed by autumn, spring and winter, respectively.
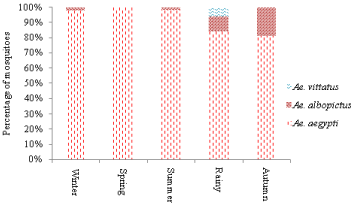
Figure 3: Season wise distribution of different species of Aedes mosquitoes
in Delhi during 2013 & 2014.
Season-wise sex and species-wise distribution shows 52.37% male and 47.63% female Ae. aegypti were reared from wild collected immatures. However, percentage of female of Ae. albopictus was 52.86% and Aedes vittatus was 91.08%. Except winter season the proportion of male Ae. aegypti was higher than female mosquito. There is no significant distribution in male and female Ae.albopictus. However, the number of female Aedes vittatus was significantly more during the rainy season (Table 2).
Season
Species
No. of Male
No. of female
Total Mosquitoes
%
Winter
Ae. aegypti
45
53
98
98
Ae. albopictus
0
2
2
2
Total
45
55
100
Spring
Ae. aegypti
86
75
161
100
Summer
Ae. aegypti
500
448
948
98.44
Ae. albopictus
2
13
15
1.56
Total
502
461
963
Rainy
Ae. aegypti
403
367
770
84.06
Ae. albopictus
47
43
90
9.83
Ae. vittatus
5
51
56
6.11
Total
455
461
916
Autumn
Ae. aegypti
115
102
217
81.27
Ae. albopictus
25
25
50
18.73
Total
140
127
267
Table 2: Season and Sex-wise distribution of Ae. aegypti, Ae. albopictus and Aedes vittatus in Delhi in 2013 & 2014.
Seasonal variation of Aedes breeding habitats
Season-wise variation in breeding indices and preference of particular containers are given below:
Winter season: During winter months, low breeding indices were recorded and PPC was also low (Table 3). During 2013, 1899 houses and 4547 containers were searched in winter season, house index was 0.90 and container index was 0.42. However in 2014, total of 1653 houses and 3657 container searched breeding was recorded in 0.67% houses and 0.41% containers. BI was 0.95 and 0.91 in 2013 and 2014 respectively. Similarly PI was 0.58 in 2013 and 0.18 in 2014 (Table 3).
Season
House
Searched
House
House
Container
Container
Container
Breteau
No. of
Pupal
Pupae per
positive
Index
Searched
positive
Index
Index
Pupae
Index
Container
Winter
3552
28
0.79
8204
33
0.43
0.99
14
0.39
0.17
Spring
1314
23
1.75
3590
27
0.67
1.83
43
3.27
1.2
Summer
5629
226
4.01
16697
252
1.55
4.6
212
3.77
1.27
Rainy
5205
247
4.75
14454
331
2.29
6.36
224
4.3
1.55
Autumn
2468
60
2.43
5768
55
1.23
2.88
47
1.9
0.81
Total
18168
584
3.21
48713
699
1.40*
3.76*
537
2.96*
1.1
Table 3: Details of Aedes breeding survey report in Delhi during 2013 & 2014.
(Table 2) shows that during winter, Aedes breeding was limited to three types of permanent containers (syntax tank, cement tank and large PWSC). Maximum immature collection of Aedes mosquitoes was found in syntax tank (39.39%), followed by PWSC and cement tanks. However, pupae could be collected from two types of containers, from plastic storage containers and cement tanks (Figure 4). Number of pupae was found maximum in cement tanks that contributed 50% of total pupae collected during winter. Total of 33 containers with immatures, pupae was present only in 18.18% of containers.
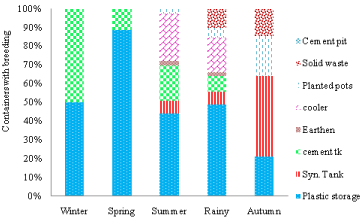
Figure 4: Season-wise Aedes breeding Preference (Pupae) in different
containers in Delhi during 2013 & 2014.
During winter, due to extreme low temperature, slow development takes place. Breeding is limited to few permanent principal key containers such as Cement tank, Syntax tanks and PWSC used to store potable water in and around houses which ensured year round availability of water, acted as mother foci during winter. Larvae and pupae stay there for long period of time due to slow development due to extreme low temperature. Therefore, vector control may be required to focus in these containers during winter season.
Spring season: During this season, 1314 houses were checked, of which 23 houses (1.75%) were found positive whereas, total of 3590 containers checked and 0.67% of containers infested with Aedes breeding in 2013 & 2014 (Table 3). PI was recorded as 3.27%. Number of positive containers was present more in peri-domestic area. A Pupa per Container (PPC) was found highest during this season. Aedes breeding was recorded in 331 containers but pupae production was recorded only in 17.82% of these containers (Table 4).
Infestation of Ae. aegypti was significantly higher (p<0.001) in plastic containers (85.19%) followed by syntax tank and cement tank. Breeding was limited in three types of containers like winter months (Table 4 & Figure 5). Total of 27 containers positive for immatures, pupae were present only in 9 containers. Pupae could be collected only from plastic containers and cement tanks (Figure 3). Thus, during the spring also, larvae and pupae were found mainly limited to three types of containers which need special attention for planning of vector control measures.
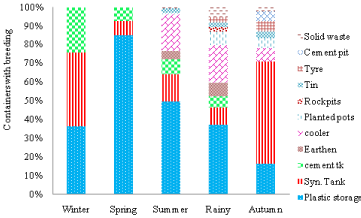
Figure 5: Season wise Aedes breeding preference (immature) in different
containers in Delhi during 2013 & 2014.
Winter
Plastic storage
12
3
7
50
Sintex Tank
13
0
0
0
Cement tank
8
3
7
50
Total
33
6
14
2.59
Spring
Plastic storage
23
8
35
81.4
Sintex Tank
2
0
0
0
Cement Tank
2
1
8
18.6
Total
27
9
43
7.96
Summer
Plastic storage
125
19
75
35.38
Sintex Tank
37
3
14
6.6
Cement Tank
20
8
63
29.72
Cooler
50
11
52
2.83
Earthen
11
1
6
24.53
Planted pots
3
1
2
0.94
Solid waste
7
0
0
0
Total
252
43
212
39.26
Rainy
Plastic storage
123
29
111
49.55
Sintex Tank
31
4
6
2.68
Cement Tank
20
5
33
14.73
Cooler
66
11
39
1.34
Earthen
24
1
3
17.41
Planted pots
25
3
8
3.57
Solid waste
42
6
24
10.71
Total
331
59
224
41.48
Autumn
Plastic storage
9
3
10
21.28
Sintex Tank
30
6
18
38.3
Cement Tank
3
0
0
0
Cooler
4
3
5
0
Planted pots
3
0
0
10.64
Solid waste
6
2
14
29.79
Total
55
14
47
8.7
Table 4: Showing the details of positive containers with immatures and pupae along with percent pupal production for the Year 2013 & 2014.
Summer season: (Table 3) shows that during summer, 1.55% of containers were infested with immature, whereas, pupal production recorded only in 43 containers (17.1%). Pupae per container were 1.27. During the survey the number of containers with pupae was found less number as compared to containers with immature, hence development till pupae stages could be reached in less number of containers. It might be due to hot weather when temperature reaches above 40oC, mortality would be more.
During the study period, Ae. aegypti preferred to breed significantly more in PWSC (49.60%) followed by cooler and syntax tank (Table 4). These containers are mostly kept in peri-domestic areas to store water for domestic purpose, due to irregular supply of water in the colonies during hot climate. PWSC was also recorded as most productive habitat for pupae followed by cement tanks and coolers. In colonies with low socio economic status, in Delhi, PWSC are most abundant, cheap and easily available containers such as gallons, cans, even bottle, empty containers cut into making wide mouth (Figure 1) using to store water where breeding extents. There is also maximum use of desert coolers in the season to protect from dry hot weather, which are favourable sites for Aedes breeding with humid environment.
Rainy season: During rainy season in 2013 and 2014, 5205 houses were checked, of which 4.75% were found positive whereas 2.29% containers were found positive breeding (Table 3). Average pupal index was recorded as 4.40% in 2013 and 4.04% in 2014. Total number of pupae collected was found maximum during this season as compared to other season. Highest number of positive containers was present in peri-domestic area. Number of containers positive were more in outdoor as compared to other seasons.
During rainy season, total of 331 containers found positive with Ae. aegypti breeding, maximum breeding was recorded in plastic containers 123 (37.16%) followed by coolers (9.94%). About 12.69% breeding was recorded in solid waste lying in peri-domestic and outdoor areas. Total 331 containers infested with immature stages of Aedes, pupae could be collected only from 17.82% of positive containers.
It was observed that (Figure 6) that during rainy season, Aedes breeding proliferated in different kind of temporary water collections particularly e.g. stagnant water on the roof surface and in unused containers, discarded tyres, discarded unused small plastic/glasses/ tin containers and other solid waste etc. in outdoor and peri domestic areas. Therefore, intensive planning for elimination of breeding and source reduction are required to plan.
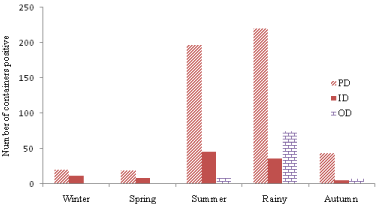
Figure 6: Season wise preference of breeding of Aedes mosquitoes in
Indoor, Outdoor and Peri-domestic areas in Delhi during 2013 & 2014.
Autumn season: During this season, there was a declining trend of average breeding indices as compared to rainy season (Table 3). Pupal Index was recorded as 1.9 while PPC was 0.81. During this season in both years number of positive containers presents more in peri-domestic area. Larval and pupal production was found maximum in syntax tank (Table 4). However, total of 55 positive containers with immatures, pupae were present only in 14 containers. Pupae production was higher in sintex tanks, limited to Percent proportion of pupal production was also found higher as compared to other season.
Number of containers with immature and pupae were significantly more during summer and rainy months. However, percent proportion of containers with pupae productivity was found high during spring and autumn seasons (Table 4).
Seasonal variation of breeding in Indoor (ID), Peri Domestic (PD) and Outdoor (OD)
During the year 2013 & 2014, total of 700 positive containers with Aedes breeding, 504 numbers of containers (72%) were in peridomestic area followed by 15% in Indoor and 13% outdoor area.
(Figure 6) shows that maximum number of containers (331) was found with Aedes breeding during the rainy season followed by summer (252), autumn (57), winter and spring, respectively. In all five season dengue vector preferred to breed in peri-domestic area with peaks during summer and monsoon. Breeding in outdoor containers increased during rainy season. However, breeding in indoor containers was found maximum during summer; it might be due to hot climatic condition of Delhi. During winter and spring season not a single container was found positive in outdoor area except one positive container recorded in late winter it might be due to low outdoor temperature.
PWSC, coolers, planted pots and earthen pots contributed more than 80% of breeding in peri-domestic area. Therefore, it needs to pay attention and control breeding. Adult mosquito emerged from these breeding sites may bite indoor and in peri-domestic sites as mostly human activities are concentrated in this area. Breeding in ID and PD areas was found throughout the year in all seasons and maximum percentage positivity for indoor containers was recorded in the month of July and for peri-domestic area.
Discussion
Present study revealed that Ae. aegypti was found the most predominant Aedes mosquitoes (91.15%) followed by Ae. albopictus (6.52%) and Aedes vittatus (2.3%) in Delhi. However, during 2011- 12, the proportion of Ae. aegypti was 86.89% and Ae. albopictus was 11.5% in Delhi [10]. During 2013-14, PWSC, cement tanks, coolers and sintex tanks contributed 83% of total Aedes breeding of immatures and these containers also contributed 89% of total pupal production. These containers were found to be perennial and key containers for production of larvae as well as pupae. Identification of key containers breeding of Aedes is important to plan for priorities for implementation of control interventions in those containers.
Our studies show that Aedes breeding in various plastic water storage containers have been significantly increased from 27% (2008- 09) to 50% (2013-14), thus, preference of breeding has been shifted in PWSC, while in earlier studies, breeding was more in cement tanks and desert coolers. PWSC are most common containers used by the residents of Delhi to store water for domestic purpose due to shortage of water supply in the colonies. Pipe water supply does not fulfil the requirement of influx huge population growth in the city, unplanned development activities and extension of colonies. Plastic containers such as cans, buckets and drums are found most abundant containers, cheaper and easily available to store water, most of them don’t have lids and residents pour water without emptying completely, these containers constituted the major breeding sources of Ae. aegypti and Ae. albopictus in Delhi. Similarly, it was reported earlier from Tirunelveli, Tamil Nadu [18] that the most common storage containers used by the residents were plastic drums and other plastic containers and aluminium utensils that were found major breeding resources. Singh et al., 2013 [19] reported that in all the localities surveyed the plastic containers were maximum followed by tin containers, earthen pots, desert coolers and cement tanks. The plastic drums and plastic containers were identified a key sites (50.7%) for breeding of Ae. aegypti in Philippines also [20]. Seng et al.[21] reported that 15.2% containers were infested with Aedes immatures stages and the majority of these were water storage jars (64.2%) followed by concrete tanks, small pots and tyre in Cambodia and Aedes pupae were also collected maximum from water storage jar (76.1%). However, pupae production in solid waste was found less in Delhi and recorded only during autumn and rainy season (Table 4). The relative importance of container categories, plastic items and cement tanks were the categories contributing the most to the risk of dengue transmission in the capital.
During all seasons in Delhi, Aedes breeding was predominantly recorded in different water storage containers particularly those were kept in and around houses (ID/OD) for domestic use. These are rarely cleaned and dried, thus resulting in high Aedes breeding, thus water storage habits were found as one of the most important factor in determining the breeding of Aedes. Similar observation was recorded by Balakrishnan et al. [22] from Tamil Nadu. Rajesh et al. [23] also reported that rapid spread of Aedes specie was due to the storage of water in plastic containers and cement tank. It seems from the published reports from India [18], 2014; from Thailand [24], Etiopia [25], in Philippines [20] as well as from our studies that water storage practices are favourable to give rise breeding of Ae. aegypti and these storage containers were found closely associated to the spread of dengue infection. Therefore, there is a need to create awareness in the community to cover store water containers with proper lid and storage containers should be cleaned, empty and dried regularly on weekly basis. Proper surveillance followed by health education is important for effectively checking rise in Aedes breeding vis a vis dengue transmission [19].
Our hypothesis proofed that pupal development takes place only in few key containers. Four key containers (PWSC, cement tanks, sintex tanks and desert coolers) harboured 86% of Aedes pupae in Delhi, except desert coolers, all were the perennial source of breeding. Coolers contributed only during summer and rainy season for pupae growth. Similarly, Seng et al.reported that two container types (jar and concrete tanks) harbour 89.3% of the Aedes pupae recovered during the survey and an intervention successfully targeting both containers would be of considerably effective in reducing dengue outbreak. Observations from several areas also suggested most pupae of Ae. aegypti and Ae. albopictus reproduce in only few types of containers [12,20]. Results also show that PWSC contributed maximum (48%) for the pupal production in Delhi. Edillo et al., 2012 [20] also reported that plastic containers as key site for Ae. aegypti pupae (50.7%) in Philippines. However, in Kerala, plastic sheets and buckets were found the key containers for pupae [26]. However, present study indicated that pupae developed in specific containers in different seasons, which is useful for planning of targeted control measures. Since the pupae of dengue vectors emerge to become adults, controlling the key breeding sites that produce the most pupae could have the greatest impact on the adult population [27]. Therefore, it needs to target these pupal habitats for prevention and control of Dengue.
It was recorded that number of pupae production was also found maximum during rainy season followed by summer, autumn, spring and winter. Average pupal index ranged from 0.18 to 4.91 in all five seasons of Delhi. Pupal index is important to know the intensity of transmission and were considered the better and alternate indicator for adult mosquito abundance, than traditional larval surveillance [28]. In Delhi, there is variation in pupal production in different containers in all five seasons and it was found varied in different containers according to the dry and wet seasons in Kerala [26].
The results indicate that seasonal variation of Aedes immatures as well as pupal breeding in Delhi. During rainy season, number of containers with pupae and total number of pupae collected, both were found higher in number as compared to other seasons, it might be due to most favourable condition like high humidity and temperature for development of pupae. Similarly other studies also showed that Aedes breeding was high in monsoon and post-monsoon and in semiarid areas of India, Ae. aegypti populations fluctuate with rainfall and other water storage practices [29].
During rainy season, maximum pupae productivity and relative importance of particular container was recorded in PWSC, cement tankand coolers, so best measures for prevention of breeding to empty and dry the containers (Table 4). We have promoted to cover tanks and plastic water containers with clean clothes, if lids are not available. NCDC also develop a mosquito free desert cooler.
However, Aedes breeding spread in small water collection in solid waste in outdoor and on top of the roof and good proportion of pupae production (14.3%). Source reduction and cleaning of garbage are recommended to eliminate such type of breeding habitats. During this season, control measures are required to be implemented in war foot manner. Awareness in the community through health education is required to be developed for cleaning and drying the containers, at least once in a week. Top up all depressions to prevent hatching out of eggs. Change water in vases completely and remove water in saucers underneath potted plants every week. Source reduction at least once a week is the best solution for prevention and control of Aedes breeding in and around the houses and on roof top by involving community.
However, Aedes breeding persist during autumn season so all measures of rainy season should be planned for autumn also. During Summer, breeding extents in other storage containers due to demand of more water in hot weather and irregular water supply and in desert coolers which needs to be targeted vector control measures in IN & OD areas only. In Delhi, during winter season and spring season, Aedes immatures infestation was limited in three types of breeding foci namely PWSC, cement tanks and sintex tanks, which ensured year round availability of water, acted as mother foci during winter. Larvae and pupae stay there for long period of time due to slow development due to extreme cold temperature and their eggs can withstand at edges and sides of the containers for long period of desiccation, and will hatch when the temperature becomes suitable. Aedes mosquitoes infected with dengue virus can pass the virus from generation to generation through their eggs (Kumari et al., 2011). Therefore, it is important to target these permanent containers for vector control during winter season, so as to prevent their proliferation. It needs to involve the community to empty and dry such containers/tanks with proper cleaning and scrubbing edges and sides with household detergent to remove possible deposited Aedes eggs of previous season to eliminate mosquito eggs so as to prevent their proliferation.
Result of pupal survey shows that during rainy season 7 different types of containers produced pupae, followed by summer when 6 types of containers produced pupae while in autumn, pupae production was recorded in 4 types of containers. However, during spring and winter season pupae production was limited in to 2 types of containers (PWSC & tanks), that acted as mother foci and showing most conducive environment for survival of pupae during extreme cold condition. However, as per earlier reports, cement tanks, clay jars and overhead tanks acted as mother foci of Aedes [30,6] during pre-monsoon season. Pupal productivity in key containers identified in all seasons in Delhi provided valuable information to target for implementation of control measures. The containers that produced the larger proportion of pupae, vector control intervention should be targeted to maximize their intact.
Thus, in Delhi population of Ae. aegypti and Ae. albopictus fluctuates with rainfall, water storage practices, temperature. During the post monsoon season and vector density increases and virus load also increases in vectors [9,10] that lead to transmission of dengue at the end of rainy season followed in autumn, and it declined on the onset of winter. Similarly, Pandya et al. [31] reported that dengue in Delhi encounter during or after rainfall as an outcome of rise in vector. Rajesh et al. [23] also reported that the population of Ae. aegypti fluctuates with temperature rainfall and humidity.
Prevalence of Ae. albopictus occurred during monsoon and post monsoon season in Delhi while Ae. aegypti reported throughout the year. However, its population increases during rainy season and maintain until autumn. It has been evidenced from studies that Ae. albopictus is now well adopted for artificial containers in urban areas of Delhi and harbouring dengue virus [10]. Therefore, I needs to study bionomic of Ae.albopictus, since the report is available, it is slowly displacing Ae. aegypti from its habitat in USA [32]. In SEAR, Ae. aegypti has been incriminated as the principal epidemic vector, while Ae. albopictus has been incriminated as the principal epidemic vector, while Ae. albopictus has been given the status of secondary vector, responsible for maintenance of the virus [33].
Four key perennial containers, identified as the most favourable sites for Aedes larvae as well as pupae. Among them, PWSCs contributed maximum (48%) for the pupal production in Delhi, which need public health attention. Pupal survey was also found the most productive breeding sites for Ae. aegypti and Ae.albopictus in order to reduce dengue mosquito populations in Philippines. This was relevant for both the National Dengue Prevention and Control Program and special program and the special program for Research and Training in Tropical Diseases of the WHO [34]. This might be used as a model for dengue prevention in the Philippines and in other countries as well.
Pupal surveys of Ae. aegypti are based on the assumption that pupal mosquito production is a better proxy for adult mosquito reproduction than traditional indices or larval counts [34] and is more appropriate for directing dengue control programs because traditional larval indices correspond poorly with the actual number of pupae per person [12]. Barrera et al., 2006 [35] emphasizes that pupal survey technique generates an estimate of pupal density of Ae. aegypti in containers as a proxy for the number of adults. The pupal survey may be feasible approach for vector control programs because the preliminary observations in several urban areas suggested that most pupae of Ae. aegypti were produced in a few types of containers [12]. Thus vector control efforts on be concentrated on eliminating or treating the most productive types of containers to reduce mosquito density below a target threshold. However, the threshold levels of vector infestation that constitute a trigger dengue transmission are influenced by many factors, including mosquito longevity and immunological status of the human population. There are instances (in Singapore), where dengue transmission occurred even when the HI was <2% [36].
Conclusion
It is an important finding that four key containers (PWSC, cement tanks, sintex tanks and desert coolers) harboured 86% of Aedes pupae in Delhi, except desert coolers, all were the perennial source of breeding. Therefore, an intervention successfully targeting these containers would be of considerably effective in reducing dengue outbreak. Among them, PWSCs are recorded as main source for production of pupae of Aedes aegypti and Ae. albopictus and identified as most abundant and perennialkey breeding habitat during all five season in Delhi. The containers that produced the larger proportion of pupae, vector control intervention should be targeted to maximize their intact. Therefore, public health attention is required to create awareness to cover them properly and dry these containers at least once in a week. The source reduction of Aedes breeding habitats in and around living and working areas (ID & PD) needs be taken into consideration, since the presence of water in storage containers is probably the most important factor in determining the breeding of dengue vectors.
During rainy season 7 different types of containers produced pupae, while first time it was recorded that during winter and spring seasons, Aedes breeding was limited to plastic storage containers and tanks that acted as mother foci and it needs focussed intervention. Identification of season wise key containers for production of pupae of Aedes mosquitoes, are important for season wise planning of effective control interventions.
Acknowledgement
The authors gratefully acknowledge all the co-operation extended by the Municipal Corporation of Delhi. Technical help of all staffs of Centre for Medical Entomology & Vector management, National Centre for disease Control is gratefully acknowledged.
References
- Balaya S, Paul SD, D'Lima LV, Pavri KM. Investigations on an outbreak of dengue in Delhi in 1967. Indian J Med Res. 1969; 57: 767-774.
- Dinesh P, Pattanayak S, Singha P, Arora DD, Mathur PS, Ghosh TK. An outbreak of dengue fever in Delhi. J of Commun Dis. 1972; 4: 13-18.
- Acharya SK, Buch P, Irshad M, Gandhi BM, Joshi YK, Tandon BN. Outbreak of Dengue fever in Delhi. Lancet. 1988; 2: 1485-1486.
- Baroor DS, Sengupta S, Xess I, Seth P. The first Major outbreak of dengue haemorrhagic fever in Delhi, India. Emer Infect Dis. 1999; 5: 589-590.
- Kabra SK, Verma IC, Arora NK, Jain Y, Kalra V. Dengue haemorrhagic fever in children in Delhi. Bull World Health Organ. 1992; 70: 105-108.
- Sharma RS, Kaul SM, Sokhay J. Seasonal fluctuations of dengue fever vector, Aedes aegypti (Diptera: Culicidae) in Delhi, India. Southeast Asian J Trop Med Public Health. 2005; 36: 186-190.
- Kaul SM, Sharma RS, Sharma SN, Panigrahi N, Phukan PK, Lal S. Preventing dengue/dengue haemorrhagic fever outbreaks in the National Capital Territory of Delhi--the role of entomological surveillance. J of Commun Dis. 1998; 30: 187-192.
- National Vector Borne Disease Control Programme Directorate General of health services. Ministry of Health & Family Welfare.
- Roop K, Kumar K, Chauhan LS. First time Dengue virus detected in Aedes albopictus and its breeding ecology from urban localities of National Capital Territory of Delhi, India. Trop Med Int Health. 2011; 16: 949-954.
- Roop K, Sharma RS, Kumar K, Singh P, Krishnan S, Dashet AP, et al. Mapping of dengue vectors and dengue virus activity in Delhi during 2011-2012. Dengue Bull. 2013; 37: 87-100.
- Daniel P, Lenhart A, Manrique- Suade P, Siqueria JB, da Rocha WT, Kroeger A. Is routine dengue vector surveillance in central Brazil able to accurately monitor the Aedes aegypti population? Results from a pupal productivity survey. Trop Med Int Health. 2011; 16: 1365-3156.
- Focks DA and Chadee DD. Pupal survey: an epidemiologically significant surveillance method for Aedes aegypti: an example using data from Trinidad. Am J Trop Med Hyg. 1997; 56: 159-167.
- Focks DA, Alexander N, Villegas E, Barrera R,Claudia ME, Romero-Vivas, et al . Multi-country study of Aedes aegypti pupal productivity survey methodology: findings and recommendations. World Health Organization, Special Programme for Research and Training in Tropical Diseases. TDR/IRM/Den/06.1, 2006.
- Maps of India.
- Sheppard PM, Macdonald WW, Tonn RJ. A new method of measuring the relative prevalence of Aedes aegypti. Bull World Health Organ. 1969; 40: 467-468.
- World Health Organization. Operational guide for assessing the productivity of Aedes aegypti breeding sites. World Health Organization on behalf of the Special Programmefor Research and Training in Tropical Diseases. 2011.
- World Health Organization. Dengue guidelines for diagnosis, treatment, prevention and control. World Health Organization, Geneva, Switzerland. 2009; 147.
- Bhat Mohd. Ahmad, Krishnamoorthy. Entomological investigation and distribution of Aedes mosquitoes in Tiruneveli, Tamil Nadu, India. Int J Curr Microbiol Appl Sc. 2014; 3: 253-260.
- Singh Sunder, Vandna, Abdul Rahman. Contribution of Aedes aegypti breeding by different income group communities of Dehradun city, Uttrakhand, India. Biol forum- An Int J. 2013; 5: 96-99.
- Edillo FE, Roble ND, Otero ND. The key breeding sites by pupal survey for dengue mosquito vectors, Aedes aegypti (Linnaeus) and Aedes albopictus (Skuse), in Guba, Cuba City, Phillipines. Southeast Asian J Trop Med Public Health. 2012; 43: 1365-1374.
- Chang SM, Setha T, Nealon J, Socheat D. Pupal sampling for Aedes aegypti (L.) surveillance and potential stratification of dengue high risk areas in Cambodia. Trop Med Int Health. 2009; 14: 1233-1240.
- Balakrishnan N, Venkatesh S, Lal S. An entomological study on the dengue vectors during outbreak of dengue in Tiruppur town and its surroundings, Tamil Nadu, India. J Commun Dis. 2006; 38: 164-168.
- Rajesh K, Dhansekaran D, Tyagi B.K. Survey of container breeding mosquito larvae (Dengue vector) in Tiruchirapalli district, Tamil Nadu, India. J Entomol and Zool Stud. 2013; 1: 88-91.
- Swaddiwudhipong W, Chaovakiratipong C, Nguntra P, Koonchote S, Khumklam P, Lerdlukanavonge P. Effect of health education on community participation in control of dengue hemorrhagic fever in an urban area of Thailand. J Trop Med Public Health. 1992; 23: 200-206.
- Dejene G, Habte T, Gebre-Michael, Teshome, Meshesha B, Mesfin, et al. Breeding Sites of Aedes aegypti: Potential Dengue Vectors in Dire Dawa, East Ethiopia. Interdisciplinary Perspectives of Infect Dis. 2015.
- Balasubramanian R, Anukumar B, Nikhil T. Stegomyia indices of Aedes mosquito infestation and container productivity in Alappuzha district Kerala. Int J Mosq Res. 2015; 2: 14-18.
- Lenhart AE, Castillo CE, Oviedo M, Villegas E. Use of pupal/ demographic survey technique to identify the epidemiologically important types of containers producing Aedes aegypti (L.) in a dengue-epidemic area of Venezuala. Ann Trop Med Parasitol. 2006; 100: S53-S59.
- Wai K, Arunachalam N, Tana S, Espino F, Kittayapong P, Abeywickreme W. Estimating dengue vector abundance in the wet and dry season: implications for targeted vector control in urban and peri-urban Asia. Pathogen Global Health. 2012; 106: 436-445.
- Vijaykumar K, TK Sudheesh Kumar, Nujum ZT, Umarul F, Kuriakose A. A study on container breeding mosquitoes with special reference to Aedes aegypti (Stegomyia) and Aedes albopictus in Thiruvananthpuram District, India. J Vector Borne Dis. 2014; 51: 27-32.
- Katyal R, Singh K, Kaushal K. Seasonal variations in Aedes aegypti population in Delhi, India. Dengue Bull. 1996; 20: 78-81.
- Pandya G. Prevalence of dengue infection in India. Defence Sci. J1982; 32: 359-370.
- O'Meara GF, Evans LF Jr, Gettman AD, Cuda JP. Spread of Aedes albopictus and decline of Ae.aegypti (Diptera: Culicidae) in Florida. J Med Entomol. 1995; 32: 554-562.
- Reinert JF, Harbach RE, Kitching IJ. Phylogeny/ and classification of Aedini (Diptera: Culcuidae) based on morphological characters of all life stages. Zoological J LinneanSoc. 2004; 142: 289-368.
- Focks DA. A review of entomological sampling methods and indicators for Dengue vectors. 2003.
- Barrera R, Amador M, Clark GG. Use of the pupal survey technique for measuring Aedes aegypti (Diptera: Culicidae) productivity in Puerto Rico. Am J Trop Med Hyg. 2006; 74: 290-302.
- Chan YC, Ho BC, Chan K.L. Aedes aegypti (L) and Aedes albopictus (Skuse) in Singapore City: 5. Observation in relation to dengue haemorrhagic fever. Bull World Health Organization. 1971; 44: 651-657.