
Research Article
Austin J Infect Dis. 2019; 6(1): 1037.
One-Day Old Chicks Vaccinated with Inactivated H5 Antigen are Protected against Highly-Pathogenic Avian Influenza Virus
Seo T1,2,a, Jang Y1,2,a and Seo SH1,2,*
¹Laboratory of Influenza Research, Umuahia Abia State, Republic of Korea
²Institute of Influenza Virus, College of Veterinary Medicine, Chungnam National University, Republic of Korea
aThese authors equally contributed to this work
*Corresponding author: Sang Heui Seo, Laboratory of Influenza Research, College of Veterinary Medicine, Institute of Influenza Virus, Chungnam National University, Korea
Received: September 17, 2019; Accepted: October 17, 2019; Published: October 24, 2019
Abstract
H5-subtype highly pathogenic avian influenza viruses cause considerable economic losses to the poultry industries in many countries, including China, South Korea, and Vietnam. Vaccination is the most important strategy to control highly pathogenic avian influenza viruses in poultry. We studied the efficacy of inactivated H5 antigen in one-day-old chicks. One-day-old chicks were immunized in the pectoral muscles with a single dose (1.0 μg) of inactivated H5 antigen derived from the highly pathogenic H5N8 avian influenza virus. They were then intranasally challenged at 3 weeks and 16 weeks-post-vaccination by the highly pathogenic H5N8 or H5N6 avian influenza viruses. The protective antibody and immunity against homologous H5N8 virus was effective in the vaccinated chickens until 16 weeks-post-vaccination. Vaccinated chickens challenged with H5N8 virus did not show any clinical signs of infection, nor did they shed virus through the trachea or cloaca. Meanwhile, vaccinated chickens challenged with the heterologous H5N6 virus did not survive, and shed lots of viruses from the trachea and cloaca. Vaccination of one-day-old chicks with inactivated H5 antigens is an effective preventive measure to minimize the economic burden of the highly pathogenic H5 avian influenza viruses on of the poultry industry.
Keywords: Highly pathogenic avian influenza virus; Vaccine; H5N8; H5N6
Introduction
Influenza viruses belong to family Orthomyxoviridae, and are divided into four types: A, B, C and D, based on nucleoprotein (N) and matrix (M) genes [1,2]. Influenza A viruses can infect a broad range of hosts, including humans, pigs, horses, dogs, cats, and whales [3-9]. Avian influenza viruses belong to type A, and circulate in water birds [7]. Most avian influenza viruses circulating in water birds exhibit low pathogenicity. Highly-pathogenic (HP) H5 avian influenza viruses which infect poultry in many countries, including China, South Korea, and Vietnam, were originally derived from A/ goose/Guangdong/1/1996 (H5N1)-like HP avian influenza virus [10- 12]. In South Korea, H5N1 HP avian influenza viruses have infected poultry since 2003. After 2014, H5N8 and H5N6 HP avian influenza viruses became prevalent in poultry, and caused huge economic losses to the poultry industry [10,13-16].
Avian influenza viruses possess negative-sense, single-stranded, eight-segmented genomes containing polymerase basic 2 (PB2), polymerase basic 1 (PB1), polymerase acidic (PA), hemagglutinin (HA), and nucleoprotein (NP), neuraminidase (NA), matrix (M), and non-structural (NS) genes that encode 10 proteins: PB2, PB1, PA, HA, NP, NA, M1, M2, NS1, and NS2 [7]. Of these 10 viral proteins, HA is regarded as a major determinant of pathogenicity in poultry, containing polybasic amino acids such arginine (R) and lysine (K) at the cleavage site, which are responsible for systemic infections in poultry [7,14].
Vaccination is one of tools used to control HP avian influenza viruses in poultry. Several vaccine strategies are being studied. Virus-like particle-based vaccine protects mule and pekin duck from infections of homologous H5N8 HP avian influenza virus [17]. The combined H5ND inactivated vaccine including the inactivated reassortant H5N1 avian influenza virus and Newcastle disease virus, can protect chickens from HP H5N1 avian influenza virus and virulent Newcastle disease virus [18]. The recombinant turkey herpesvirus expressing H5 antigen protects chickens from HP H5N8 avian influenza virus [19].
Here, we studied the vaccine efficacy in one-day-old chicks immunized with a vaccine consisting of inactivated H5 antigen of HP H5N8 avian influenza virus, and tested how long the protective immunity lasted.
Material and Methods
Viruses and animals
Highly Pathogenic (HP) avian influenza viruses, A/Waterfowl/ S005/Korea/2014 (H5N8) (clade 2.3.4.4) and A/Waterfowl/Korea/ S57/2016 (clade 2.3.4.4.) were amplified by inoculation into 10-dayold fertilized eggs. This work with HP H5 viruses were performed in an animal BSL-3 facility approved by the Korean government.
Chicks (White Leghorn) were hatched from fertilized eggs purchased from the local farm in Korea. One-day-old chicks were used to inoculate H5 inactivated antigen.
Animal rights statement: Chungnam National University (CNU) Internal Animal Use Committee approved the protocol for the chicken vaccine study and collection of clinical chicken samples.
The authors declare that there is no conflict of interests.
Preparation of H5 vaccine antigen
Vaccine virus, H5N8-RG-CNUK4-2014 (H5RG), which was constructed using an A/PR/8/34 (H1N1) backbone and H5 of A/ Waterfowl/S005/Korea/2014 (H5N8) using reverse genetics in our laboratory [20] was used to prepare the vaccine antigen.
Allantoic fluid was harvested from 10-day-old embryonated eggs which were inoculated with H5RG virus. The egg fluid was concentrated to 1/10 volume by an Amicon concentrator. The concentrated fluid was purified by a 20% to 75% continuous sucrose gradient by centrifugation at 26,000 rpm for 2 h. The purified viruses were inactivated by treatment with 0.01% formalin at 4 oC for over 14 h. The protein concentration of the purified H5RG vaccine was measured by the Bradford method (Bio-Rad) with a bovine serum albumin (BSA) standard curve.
Vaccination and infection of one-day-old chicks
One-day old chicks (n=10 per group) were injected into pectoral muscles with 300 μl of 1.0 μg of inactivated H5RG antigens with oil adjuvant (70% oil and 30% antigens in PBS) (Montanide IMS 1313 NPR, SEPPIC Co., France). Immunized chickens were intranasally infected with 100 μl of 105EID50/ml of A/Waterfowl/Korea/S57/2016 (clade 2.3.4.4) or A/Waterfowl/S005/Korea/2014 (H5N8) (clade 2.3.4.4) at 3 weeks and 16 weeks post-vaccination.
Measurement of antibody titers
Hem Agglutination-Inhibition (HI) assay was performed to determine antibody titers in chickens. The collected sera from the vaccinated chickens were treated with Receptor-Destroying Enzyme (RDE) (DENKA SEIKEN, Tokyo, Japan) before the sera were serially 2-fold diluted in PBS (pH 7.4) in 96-well plates (V bottom). Twenty five microliters (eight HA units) of A/Waterfowl/Korea/S57/2016 (clade, 2.3.4.4) or A/Waterfowl/S005/Korea/2014 (H5N8) (clade, 2.3.4.4) virus was added to the diluted sera, and was incubated for 15 min at room temperature. Then, 50 μl of 0.5% turkey red blood cells in PBS (pH 7.4) was added and was incubated at room temperature for 40 min. HI titer was read as reciprocal dilutions which inhibit hem agglutination.
Determination of virus titer
Swabs were performed of trachea and cloacae in the surviving chickens infected with HP H5N6 or H5N8 avian influenza virus. Tracheal and cloacal swab samples were suspended in 0.5 ml and 1 ml of PBS (pH 7.4) supplemented with Antibiotic-Antimitotic solution (SIGMA, St. Louis, MO, USA), respectively, and were serially diluted (10 fold) in PBS (pH 7.4). Each sample was inoculated into four 10-day-old fertilized eggs. Virus presence in the inoculated eggs was determined by hem agglutination assay using 0.5% turkey red blood cells in PBS (pH 7.4). Viral titers were expressed by log10 egg infectious dose 50/ml (log10EID50/ml). The detection limit was 1.0 log10EID50/ml.
Chicken lung tissues (1g) from vaccinated 16-week-old chickens challenged with HP H5N6 or H5N8 avian influenza virus on day 3 p.c. were homogenized and suspended in PBS (pH 7.4). Virus titer was measured by log10EID50/ml.
Histopathology of chicken lung
Lung tissues were collected from vaccinated 16-week-old chickens (n=3 per group) challenged with HP H5N6 or H5N8 avian influenza virus on 3 days p.c., were fixed in 10% neutral-buffered formalin, and were embedded in paraffin. Thin sections (5 μm) were made and were stained with H&E. The stained tissues were observed under an Olympus DP70 microscope (Olympus Corporation, Tokyo, Japan).
Statistical analysis
The statistical significance was analyzed by the Mann-Whitney U test. A P value of less than 0.05 (P‹0.05) was considered significant.
Results
Antibody response
We vaccinated one-day-old chicks (n=10 per group) with the inactivated H5 antigen derived from HP H5N8 avian influenza virus to determine the possibility on whether early-age vaccination could protect chicks from HP avian influenza virus. One-day-old chicks were immunized with one-dose (1.0 μg) of inactivated H5 antigen with oil adjuvant. Sera were collected from 1 to 17 weeks postvaccination (p.v.). Antibody titers against homologous H5N8 and heterologous H5N6 avian influenza viruses were measured by HI assays (Figure 1). Specific antibody titers against homologous H5N8 avian influenza virus were detected from 3 weeks p.v. and lasted until 16 weeks p.v., while no specific antibody titers were detected against heterologous H5N6 avian influenza virus. The mean HI titers against homologous H5N8 avian influenza virus at 3 weeks and 16 weeks p.v. were 61 and 80, respectively.
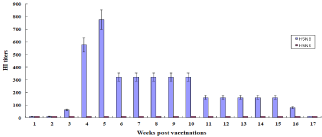
Figure 1: Antibody responses in one-day-old chicks. One-day old chicks (n=10 per group) were immunized with 300 μl of one-dose 1.0 μg of inactivated H5RG
antigens with oil adjuvant. The immunized chickens were bred in one-week intervals until 17 weeks p.v. Antibody titers in sera were measured by HI assay against
A/Waterfowl/Korea/S57/2016 (H5N6) or A/Waterfowl/S005/Korea/2014 (H5N8). Data are the mean HI titers ± standard deviations.
Protective efficacy in 3-week-old chicks
We intranasal challenged the immunized chicks (n=10 per group) ay 3 weeks p.v. using homologous HP H5N8 or heterologous H5N6 avian influenza viruses (Figure 2). All vaccinated chicks challenged with homologous HP H5N8 avian influenza virus survived without apparent clinical signs, but all vaccinated chicks challenged with heterologous HP H5N6 avian influenza virus died until day 6 postchallenge (p.c.). All unvaccinated chicks challenged with either homologous HP H5N8 or heterologous HP H5N6 avian influenza virus died until day 4 p.c.
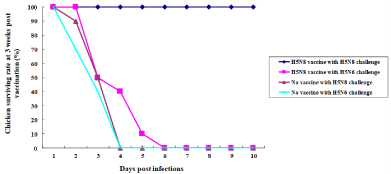
Figure 2: Efficacy of inactivated H5 antigens in 3-week-old chickens. The immunized chickens (n=10 per group) were i.n. infected with 100 μl of 105EID50/ml of A/
Waterfowl/Korea/S57/2016 (H5N6) or A/Waterfowl/S005/Korea/2014 (H5N8) on 3 weeks p.v. The surviving rates were recorded.
Protective efficacy in 16-week-old chickens
We were interested in finding out the protective duration provided by the vaccination against HP avian influenza viruses in chicks by one dose at one day of age (Figure 3). One-day-old chicks (n=10 per group) were immunized with 1.0 μg of inactivated H5 antigen with oil adjuvant, and at 16 weeks p.v., chickens were challenged with homologous HP H5N8 or heterologous HP H5N6 avian influenza virus. All vaccinated chickens challenged with homologous HP H5N8 avian influenza virus survived, while all vaccinated chickens challenged with heterologous HP H5N6 avian influenza virus died until day 6 p.c. All unvaccinated chickens challenged with either homologous HP H5N8 or heterologous H5N6 avian influenza virus died until day 4 p.c.
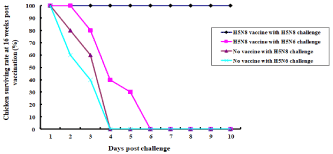
Figure 3: Efficacy of inactivated H5 antigens in 16-week-old chickens. The immunized chickens (n=10 per group) were i.n. infected with 100 μl of 105EID50/ml of A/
Waterfowl/Korea/S57/2016 (H5N6) or A/Waterfowl/S005/Korea/2014 (H5N8) on 16 weeks p.v. Surviving rates were recorded.
Virus titers in 16-week-old chickens
Chickens challenged at 16 weeks p.v. with homologous HP H5N8 or H5N6 avian virus were swabbed in the trachea and cloaca until day 10 p.c. Virus titers in the swabbed samples were measured by log10EID50/ml (Figure 4). In tracheas, no viruses were detected in vaccinated chickens challenged with homologous HP H5N8 avian influenza virus. In contrast, the range of detected virus titers in vaccinated chickens challenged with heterologous HP H5N6 avian virus was from 2.0 to 5.0 EID50/ml (Figure 4A). The range of virus titers detected in the unvaccinated chickens challenged with either homologous HP H5N8 or heterologous H5N6 avian influenza virus was 3.0 to 5.5 EID50/ml (Figure 4A). In cloacae, the virus was not detected in the vaccinated chickens challenged with homologous HP H5N8 avian influenza virus, while the range of virus titers in the vaccinated chickens with heterologous HP H5N6 avian influenza virus was 1.5 to 4.0 EID50/ml (Figure 4B). The range of virus titers in the unvaccinated chickens challenged with either homologous HP H5N8 or heterologous H5N6 avian influenza virus was 1.5 to 5.5 EID50/ml (Figure 4B).
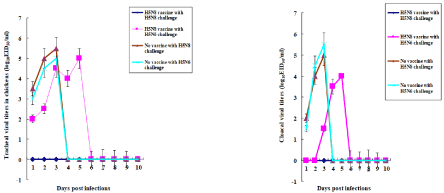
Figure 4: Viral titers in 16-week-old infected chickens. Swabs were performed in tracheas and cloacae in PBS in the surviving chickens infected with HP H5N6 or
H5N8 viruses in the experiment in Figure. 3. Viral titers were measured by log10EID50/ml. Data are the mean viral titers ± standard deviations. A, Tracheal swabs;
B, Cloacal swabs.
Lung histopathology
Lung tissues were collected from 16-week-old chickens challenged with either homologous HP H5N8 or heterologous H5N6 avian influenza virus on day 3 p.c., and these were stained with hematoxylin and eosin (H&E) (Figure 5). The lung tissue of the vaccinated chicken which was challenged with homologous HP H5N8 avian influenza virus did not have pneumonia (Figure 5B-1), while that of vaccinated chickens challenged with heterologous HP H5N6 avian influenza virus showed severe interstitial pneumonia with accumulation of inflammatory cells (Figure 5C-1). The lung tissues of unvaccinated chickens challenged with either homologous HP H5N8 or H5N6 avian influenza virus showed the severe interstitial pneumonia (Figure 5B, 5C). Lung tissue of uninfected chicken did not show any sign of pneumonia (Figure 5A).
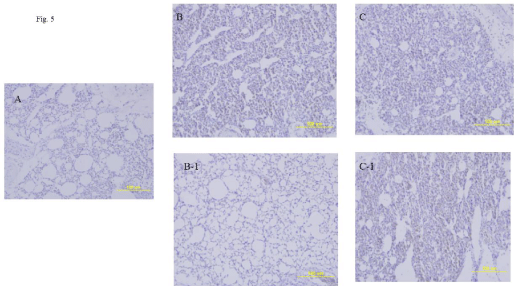
Figure 5: Lung histopathology in the infected 16-week-old chickens. The immunized chickens were i.n. infected with 100 μl of 105EID50/ml of A/Waterfowl/Korea/
S57/2016 (H5N6) or A/Waterfowl/S005/Korea/2014 (H5N8) on 16 weeks p.v. Chickens were euthanized on 3 days after infections prior to the collections of lung
tissues. Lung tissues were stained with H&E.
A, Uninfected lung tissue of chicken; B, Lung tissue of unvaccinated infected chicken with H5N8 virus; B-1, Lung tissue of vaccinated infected chicken with H5N8
virus; C, Lung tissue of unvaccinated infected chicken with H5N6 virus; C-1, Lung tissue of vaccinated infected chicken with H5N6 virus.
Virus titers in lung tissues in vaccinated 16-week-old chickens
Lung tissues from vaccinated chickens (n=3 per group) challenged with either homologous HP H5N8 or Heterologous HP H5N6 avian influenza virus on 16 weeks p.v. were collected on day 3 p.c., and were homogenized in phosphate-buffered saline (PBS) (pH 7.4). Virus titers in the homogenized lung tissues were measured by log10EID50/ ml (Figure 6). Virus was not detected in lung tissues of the vaccinated chickens challenged with homologous HP H5N8 avian influenza virus, while the virus titer in the lung tissues of vaccinated chickens challenged with heterologous HP H5N6 avian influenza virus was 5.0 EID50/g. The mean virus titer in the lung tissues of unvaccinated chickens challenged with homologous HP H5N8 or Heterologous H5N6 avian influenza virus was 5.0 and 5.5 EID50/g, respectively.
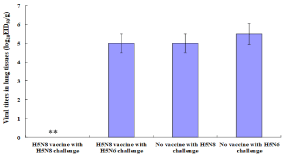
Figure 6: Viral titers of lung tissues in the infected 16-week-old chickens. Viral titers in the lung tissues from Figure. 5 were measured by log10EID50/g. Data were
the mean viral titers (n=3 per group) ± standard deviations. Statistical analysis was performed between the mean viral titer in the lungs of vaccinated chickens
challenged with homologous HP H5N8 avian influenza virus and that in the lungs of vaccinated chickens challenged with heterologous HP H5N6 avian influenza
virus.
**P‹0.001
Discussion
HP avian influenza viruses cause tremendous economic losses to the poultry industry around the world. Vaccination is the most important means to protect poultry from infections of HP avian influenza viruses. We studied the efficacy of a one-dose vaccine consisting of inactivated H5 antigen in one-day-old chicks against HP H5N8 and H5N6 avian influenza viruses. Protective immunity was conferred against homologous HP H5N8 avian influenza virus.
Our study showed that the protective antibody titers and protective immunity lasted until 16 weeks p.v. in one-day-old chicks immunized with one-dose (1.0 μg) of inactivated H5 antigen. Previous studies showed that the protective antibody lasted only 12 weeks p.v. in six-week-old chickens immunized with one-dose (5.0 ug) of inactivated H5N1 antigen [21]. In a study of short-and longterm protective efficacy against H5N2 HP avian influenza virus, Santos et al [22] showed that two-day-old turkeys primed with the recombinant a-replicon-expressing HA gene of A/Gyrfalcon/ Washington/41088-6/2014 (H5N2) and boosted with oil-adjuvanted inactivated H5N2 antigen of A/Gyrfalcon/Washington/41088-6/2014 (H5N2), were protected against HP H5N2 avian influenza virus on 16 weeks p.v.
Protective immunity was mainly conferred against homologous HP H5N8 avian influenza virus in one-day-old chicks immunized with inactivated H5 antigen with oil adjuvant. In the study with Herpesvirus turkey (HVT) vector-based live recombinant expressing the H5 gene of a clade 2.2 HP H5N1 avian influenza virus [HVT-AI (H5)], when one-day-old broilers and layers were immunized with HVT-AI (H5) and challenged with heterologous 2017 Hungarian HP H5N8 avian influenza virus, they were protected and exhibited reduced shedding of viruses [19]. The lack of protective immunity against heterologous HP avian influenza virus in our study may be due to the lack of cellular immunity inductions by inactivated H5 antigen.
We showed that inactivated H5 antigen could provide the protective immunity to chickens against HP H5 avian influenza virus. Previous studies also showed that the inactivated vaccine could be efficient at protecting chickens from lethal infections of HP avian influenza viruses. Peng et al. [23] showed that inactivated PR8-H7/H5 vaccine containing HA proteins of A/chicken/Guangdong/RZ/2017 (H7N9) and A/chicken/Fujian/5/2016 (H5N6) considerably reduced viral shedding in the infected chickens, and protected chickens from the lethal infections of H5 and H7N9 avian influenza viruses. The study with oil-emulsion-inactivated H5 avian influenza vaccine protected broiler chickens and ducks from HP H5N1 avian influenza viruses [24].
Conclusion
Our results suggest that vaccination of one-day-old chicks with the inactivated H5 antigens should be applied to prevent chickens from the infections of HP avian influenza viruses.
Acknowledgments
This work was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry (IPET) through Livestock Disease Preparedness Program funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (Grant number, 118092031SB010).The manuscript was edited by a staff of Editage Co.
References
- Bouvier NM, Palese P. The biology of influenza viruses. Vaccine 26 Suppl. 2008; 4: 49-53.
- Thielen P, Nolting JM, Nelson SW, Mehoke TS, Howser C, Bowman AS. Complete Genome Sequence of an Influenza D Virus Strain Identified in a Pig with Subclinical Infection in the United States. Microbiol Resour Announc. 2019; 8: 1462-1418.
- Baudon E, Peyre M, Peiris M, Cowling BJ. Epidemiological features of influenza circulation in swine populations: A systematic review and metaanalysis. PLoS One. 2017; 12: e0179044.
- Hatta M, Zhong G, Gao Y, Nakajima N, Fan S, Chiba S, et al. Characterization of a Feline Influenza A(H7N2) Virus. Emerg Infect Dis. 2018; 24: 75-86.
- Lvov DK, Zdanov VM, Sazonov AA, Braude NA, Vladimirtceva EA, Agafonova LV, et al. Comparison of influenza viruses isolated from man and from whales. Bull World Health Organ. 1987; 56: 923-930.
- Miño S, Mojsiejczuk L, Guo W, Zhang H, Qi T, Du C, et al. Equine influenza virus in Asia: phylogeographic pattern and molecular features revealed the circulation of an autochthonous lineage. 2019. J Virol pii: JVI.00116-00119.
- Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol Rev. 1992; 56: 152-179.
- Wiman Å, Enkirch T, Carnahan A, Böttiger B, Hagey TS, Hagstam P, et al. Novel influenza A(H1N2) seasonal reassortant identified in a patient sample, Sweden, January Euro Surveill . 2019; 24.
- Zhou L, Sun H, Song S, Liu J, Xia Z, Sun Y, Lyu Y, H3N2 canine influenza virus and Enterococcus faecalis coinfection in dogs in China. BMC Vet Res. 2019; 15: 113.
- Lee IH, Jin SY, Seo SH. Genetic and pathogenic analysis of a novel reassortant H5N6 influenza virus isolated from waterfowl in South Korea in 2016. Arch Virol. 2017; 162: 3507-3510.
- Smith GJ, Naipospos TS, Nguyen TD, de Jong MD, Vijaykrishna D, Usman TB, et al. Evolution and adaptation of H5N1 influenza virus in avian and human hosts in Indonesia and Vietnam. Virology. 2006; 350: 258-268.
- Xu X, Subbarao K, Cox NJ, Guo Y. Genetic characterization of the pathogenic influenza A/Goose/Guangdong/1/96 (H5N1) virus: similarity of its hemagglutinin gene to those of H5N1 viruses from the 1997 outbreaks in Hong Kong. Virology. 1999; 261: 15-19.
- Kim HR, Park CK, Lee YJ, Woo GH, Lee KK, Oem JK, et al. An outbreak of highly pathogenic H5N1 avian influenza in Korea, 2008. Vet Microbiol. 2010; 141: 362-366.
- Ku KB, Park EH, Yum J, Kim JA, Oh SK, Seo SH. Highly pathogenic avian influenza A(H5N8) virus from waterfowl, South Korea, 2014. Emerg Infect Dis. 2014; 20: 1587-1588.
- Lee CW, Suarez DL, Tumpey TM, Sung HW, Kwon YK, Lee YJ, et al. Characterization of highly pathogenic H5N1 avian influenza A viruses isolated from South Korea. J Virol. 2005; 79: 3692-3702.
- Wee SH, Park CK, Nam HM, Kim CH, Yoon H, Kim SJ, et al. Outbreaks of highly pathogenic avian influenza (H5N1) in the Republic of Korea in 2003/04. Vet Rec. 2006; 158: 341-344.
- Tatár-Kis T, Dán Á, Felföldi B, Bálint Á, Rónai Z, Dauphin G, et al. Virus- Like Particle Based Vaccine Provides High Level of Protection Against Homologous H5N8 HPAIV Challenge in Mule and Pekin Duck, Including Prevention of Transmission. Avian Dis. 2018; 63: 193-202.
- Ali A, Safwat M, Kilany WH, Nagy A, Shehata AA, El-Abideen MA, et al. Combined H5ND inactivated vaccine protects chickens against challenge by different clades of highly pathogenic avian influenza viruses subtype H5 and virulent Newcastle disease virus. Vet World. 2019; 12: 97-105.
- Palya V, Tatár-Kis T, Walkóné Kovács E, Kiss I, Homonnay Z, Gardin Y, et al. Efficacy of a Recombinant Turkey Herpesvirus AI (H5) Vaccine in Preventing Transmission of Heterologous Highly Pathogenic H5N8 Clade 2.3.4.4b Challenge Virus in Commercial Broilers and Layer Pullets. J Immunol Res. 2018; 3143189.
- Jin M, Jang Y, Seo T, Seo SH. Inactivated H5 Antigens of H5N8 Protect Chickens from Lethal Infections by the Highly Pathogenic H5N8 and H5N6 Avian Influenza Viruses. J Vet Res. 2018; 62: 413-420.
- Hwang S.D, Kim HS, Cho SW, Seo SH. Single dose of oil-adjuvanted inactivated vaccine protects chickens from lethal infections of highly pathogenic H5N1 influenza virus. Vaccine. 2011; 29: 2178-2186.
- Santos JJS, Obadan AO, Garcia SC, Carnaccini S, Kapczynski DR, Pantin- Jackwood M, et al. Short- and long-term protective efficacy against clade 2.3.4.4 H5N2 highly pathogenic avian influenza virus following prime-boost vaccination in turkeys. Vaccine. 2017; 35: 5637-5643.
- Peng C, Hou G, Li J, Wang S, Wang Y, Cheng S, et al. Protective efficacy of an inactivated chimeric H7/H5 avian influenza vaccine against highly pathogenic avian influenza H7N9 and clade 2.3.4.4 H5 viruses. 2018; 223: 21-26.
- Lu J, Wu P, Zhang X, Feng L, Dong B, Chu X, et al. Immunopotentiators Improve the Efficacy of Oil-Emulsion-Inactivated Avian Influenza Vaccine in Chickens, Ducks and Geese. PLoS One 2016; 11.