
Review Article
Austin J Infect Dis. 2021; 8(1): 1042.
Review of the SARS-CoV-2 (COVID-19) Based on Current Evidence
Takele S* and Kedir M
National Institute for Control and Eradication of Tsetse Fly and Trypanosomosis, Ethiopia
*Corresponding author: Samson Takele, National Institute for Control and Eradication of Tsetse Fly and Trypanosomosis, Jimma Branch, Jimma, PO-Box 19917, Ethiopia
Received: December 04, 2020; Accepted: January 07, 2021; Published: January 14, 2021
Abstract
The coronaviruses are a group of RNA-containing agents known to cause respiratory illnesses in humans and animals. This virus has caused two largescale pandemics in humans in the past two decades, SARS and Middle East Respiratory Syndrome (MERS). A novel coronavirus (SARS-CoV-2) that causes the disease Coronavirus Disease 2019 (COVID-19) has been isolated from in a seafood and poultry market in the Chinese city of Wuhan in 2019. Cases have been detected in most countries worldwide, and on March 11, 2020, the World Health Organization characterized the outbreak as a pandemic. The virus spreads from person-to-person via close contact, respiratory droplets, or surface contact. The disease is mild in most people, yet may progress to pneumonia, acute respiratory distress syndrome, multi-organ dysfunction, and even death. Treatment is essentially supportive as the role of antiviral agents is yet to be established. At the moment, is known relatively little about COVID-19, except that it is a highly pathogenic and possibly zoonotic agent. Therefore, the objective of this review paper is to summarize the current published evidence on the genomic structure, pathogenesis, epidemiology, clinical characteristics, diagnosis, and prevention of SARS-CoV-2 (COVID-19).
Keywords: SARS-CoV-2; Epidemiology; Clinical characteristics; Diagnosis; Prevention
Background
Coronaviruses are a large family of viruses, which may cause illness in humans and animals [1]. In humans, several coronaviruses are known to cause respiratory infections ranging from the common cold to more severe diseases such as Middle East Respiratory Syndrome (MERS) and Severe Acute Respiratory Syndrome (SARS). The most recently discovered coronavirus causes coronavirus disease “COVID-19” and was unknown prior to the pandemic causing outbreak in Wuhan, China on December 2019 [2-5]. The most common symptoms of COVID-19 are fever, tiredness, and dry cough, although respiratory disease, and ocular, gastrointestinal, neurological, and dermatological manifestations are being increasingly recognized [6,7]. In a subset of patients, the disease can progress to pneumonia, respiratory failure, and death within one week. The virus is primarily spread between people during close contact, most often via small droplets produced by coughing, sneezing, and talking [8- 11]. As of October 11, 2020 COVID-19 has been diagnosed in more than 37,287,908 patients and associated with over 1,073,675 deaths. Cases have been reported in more than 200 countries and territories. The United States globally leads in COVID-19 cases at 7,718,947, followed by 1,568,091 in Africa, 1,285,084 in Russia, 861,112 in Spain, and 590,844 in the UK [12,13]. Although, in Africa the first case of COVID-19 was reported on February 25th, nearly 1.2 billion people remain at risk given the viruses pathogenicity [14,15]. Currently, there is no treatment specifically approved for COVID-19, and no cure for an infection, although treatments and vaccines are currently under study. Thus, the ability to limit the devastating consequences of the disease to rely on the implementation of effective preventative non-pharmaceutical interventions. Therefore, the objective of this review paper is to summarize the current published evidence on the genomic structure, pathogenesis, epidemiology, clinical characteristics, diagnosis, and prevention of SARS-CoV-2 (COVID-19).
Historical Background of Coronavirus
Coronaviruses were discovered in the early 1930s when an acute respiratory infection of domesticated chickens was shown to be caused by a virus now known as avian Infectious Bronchitis Virus (IBV) [17]. The first Human Coronaviruses (HCoV) were discovered in the 1960s. Research with human volunteers at the Common Cold Unit near Salisbury, UK, showed that colds could be induced by nasal washings that did not contain rhinoviruses. Subsequent invitro experiments, where nasal swabs from these volunteers were inoculated onto organ cultures of the respiratory tract, revealed the presence of enveloped viruses with the characteristic morphology of coronavirus. In 1968 the term coronavirus was adopted. The name “coronavirus” is derived from the Greek Korona, meaning crown [18,19]. In 1975, the Coronaviridae family was established by the International Committee on the Taxonomy of Viruses. At the 10th International Nidovirus Symposium in Colorado Springs, Colo., in June 2005, it was proposed that the Coronaviridae family be divided into two subfamilies, the Coronaviridae, and the toroviridae. Three Epidemic incidents of human coronavirus have been reported in world history. The first, In November 2002, a viral respiratory disease (SARS-CoV) appeared in southern China and quickly spread to other countries, leading to over 8,000 confirmed cases at the end of the epidemic in 2004, with a mortality rate of ~9.6%. The second, MERSCoV has caused two major outbreaks in Saudi Arabia (2012) and South Korea (2015), with the global confirmed cases exceeding 2,000 and a mortality rate of ~35% (10). Similar to SARS-CoV, MERSCoV originated in bats, but it later adapted to dromedary camels as intermediate hosts [20-22]. Currently, a novel and highly pathogenic coronavirus (SARS-CoV-2) has caused an outbreak in Wuhan city, Hubei province of China since December 2019, and soon spread nationwide and spilled over to other countries around the world, and it contributes to causing more than 37 millions of people to suffer and more than one million people to die [23,24].
Morphological Structure of SARS-CoV-2 (COVID-19)
All known coronaviruses share a similar structure made of four main structural proteins: Spike (S), Membrane (M), Envelope (E), and Nucleocapsid (N) proteins. More recently, however, it has become clear that some Coronavirus (CoVs) do not require the full ensemble of structural proteins to form a complete, infectious virion, suggesting that some structural proteins might be dispensable or that these CoVs might encode additional proteins with overlapping compensatory functions [6,25,26]. While the exact functions of most accessory proteins are still currently being researched on, it is recognized that the structural proteins aid the viral infection of host cells and subsequent replication [27]. Individually, each protein primarily plays a role in the structure of the virus particle, but they are also involved in other aspects of the replication cycle. The S-protein is responsible for attachment to host receptors, M protein helps shape the virion particles and binding to nucleocapsid, E-protein plays a role in the assembly and release of particles while N-protein aids with the binding of the genome to a replication transcription complex which is required for the replication of genomic material [28,29]. Isolated from a COVID-19 pneumonia patient, a worker in the Wuhan seafood market, the complete genome of Wuhan-Hu-1 coronavirus, one strain of SARS-CoV-2, is 29.9 kb. While SARS-CoV and MERS-CoV have positive-sense RNA genomes of 27.9 kb and 30.1 kb, respectively [26].
Genomic Structure of SARS-CoV-2 (COVID-19)
The genetic information of any life is protected in its genome, and annotation is the initial step to interpret the sequence. The genome of SARS-CoV-2 is a single-stranded positive-sense RNA of 30kb (29891 nucleotides) encoding 9860 amino acids. G+C content varies from 32 to 43% [30]. There are 12 functional Open Reading Frames (ORFs) along with a set of nine sub genomic mRNAs carrying a conserved leader sequence, nine transcriptionregulatory sequences, and 2 terminal untranslated regions [31]. The genome of this virus lacks the haemagglutinin-esterase gene, which is characteristically found in lineage a βCoV. Two-thirds of viral RNA, mainly located in the first ORF translates two polyproteins, pp1a and pp1ab, and encodes 16 Non-Structural Proteins (NSP), while the remaining ORFs encode accessory and structural proteins. The 16 non-structural proteins include two viral cysteine proteases, namely, NSP3 (papain-like protease) and NSP5 (main protease), NSP12 (RNA-dependent RNA polymerase, NSP13 (helicase), and other NSPs which are likely involved in the transcription and replication of the virus The remaining portion of the viral genome codes for four structural proteins E, M, S, and E along with a number of accessory proteins that interfere with the host immune response (Figure 1A & Figure 2B) [32,33]. The organization of the coronavirus genome is 5'-leader-UTR-replicase-S (Spike)-E (Envelope)-M (Membrane)-N (Nucleocapsid)-3'UTR-poly (A) tail with accessory genes interspersed within the structural genes at the 3' end of the genome [34,35]. Given the high sequence similarity between the SARS-CoV-2 and the SARS-like bat CoVs from Hipposideros bats in China, the natural host of the SARS-CoV-2 may be the Hipposideros bat. The discovery that pangolin coronavirus genomes have 85.5 to 92.4% sequence similarity to SARS-CoV-2 suggests pangolins should be considered as possible hosts in the emergence of SARS-CoV-2 [36,37]. SARS-CoV-2 is closer to the SARS-like bat CoVs in terms of the whole genome sequence. However, mutations are observed in NSP2, NSP3 and the spike protein, that play a significant role in infectious capability and differentiation mechanism of SARS-CoV-2 strains, namely L-type and S-type. It was found that L lineage was more prevalent than the S lineage within the limited patient samples that were examined. The implication of these evolutionary changes on disease etiology remains unclear. Coronavirus mainly recognizes the corresponding receptor on the target cell through the S protein on its surface and enters into the cell, then causing the occurrence of infection. A structure model analysis shows that SARS-CoV-2 binds ACE2 with above 10 folds higher affinity than SARS-CoV, but higher than the threshold required for virus infection [38,39]. The detailed mechanism about whether the SARS-CoV-2 would infect humans via binding of S-protein to ACE2, how strong the interaction is for risk of human transmission, and how SARS-CoV-2 causes pathological mechanisms of organ damage remains unknown, which need more studies to elaborate [24] (Table 1).
Coronaviriniae Genera
Strains
Discovery
Cellular Receptor
Host
References
Alpha-coronavirus
HCoV-229E
1966
Human Aminopeptidase N (CD13)
Bats
[35,40]
HCoV-NL63
2004
ACE2
Palm Civets, Bats
[38,42]
HCoV-OC43
1967
9-O-Acetylatedsialicacid
Cattle
[43,44]
HcoV-HKU1
2005
9-O-Acetylated sialic acid
Mice
[40,45]
Beta-coronavirus
SARS-CoV
2003
ACE2
Palm Civets, Bats
[21,30]
MERS-CoV
2012
DPP4
Bats, Camels
[20,22]
SARSCoV-2 (COVID-19)
2019
ACE2
Bats
[37,38]
Table 1: Classification of human coronavirus.

Figure 1: A) Schematic diagram of genomic organization of SARS-CoV,
MERS-CoV and SARS-CoV-2. B) Schematic structure of virion of SARSCoV,
MERS-CoV, and SARS-CoV-2 and its major structural proteins [32].
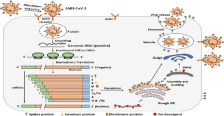
Figure 2: Schematic diagram of phatogenesesis of SARS-CoV-2 ( COVID-19) [30].
Pathogeneses of SARS-CoV-2 (COVID-19)
Angiotensin-Converting Enzyme 2 (ACE2), found in the lower respiratory tract of humans, is known as cell receptor for SARSCoV- 2 and regulates both the cross-species and human-to-human transmission [46]. Isolated from the Bronchoalveolar Lavage Fluid (BALF) of a COVID-19 patient has confirmed that the SARS-CoV-2 uses the same cellular entry receptor, ACE2, as SARS-CoV [47]. The virion S-glycoprotein on the surface of coronavirus can attach to the receptor, ACE2 on the surface of human cells. S glycoprotein includes two subunits, S1 and S2. S1 determines the virus-host range and cellular tropism with the key function domain RBD, while S2 mediates virus-cell membrane fusion by two tandem domains, Heptad Repeats (HR1) and HR2 [48,49]. After membrane fusion, uncoating of the ribonucleocapsid to expose the positive-sense genomic RNA which is translated by the host ribosomes to yield the viral replication complex [50]. The viral replication complex continues with viral transcription and genome replication and, with the aid of host proteins such as hnRNPA1, yields a nested set of positive-sense subgenomic sized mRNAs as well as the full-length virus genome [20]. Sub-genomic sized mRNAs are translated by host ribosomes into viral structural (S, E, M, N) and accessory proteins. The N protein packages the positive-sense genomic RNA into a ribonucleocapsid and is assembled into the virus particles with the help of Β-actin. The newly formed virus particles undergo maturation when passing through the Golgi and exit the host cell via exocytosis [51]. Viral RNA localized to the bronchial and bronchiolar epithelium. Expression of mRNA for angiotensin-converting enzyme 2, The SARS-CoV-2 receptor was detected in the lung following infection [34]. The host innate immune response is a coordinated series of signaling pathways in all nucleated cells that function to thwart an invading pathogen’s replication and disease potential. From Interferon (IFN) induction and secretion to the recruitment of macrophages and DCs to sites of infection, the system functions to restrict tissue tropism and spread, dampen virus replication efficiency, and eliminate virally infected cells [52,53]. In addition to IFN Regulatory Factor 3 (IRF3) in the IFN pathway, another critical signaling protein for the innate immune response is a nuclear factor of kappa light polypeptide gene enhancer in B cells (NF-κB). NF-κB is activated during viral infection from the sensing of viral replication products and via cytokine secretion from macrophages and DCs [54]. This leads to a broad induction of the innate immune response while also fine-tuning the response to remove the virus while not harming the cells. Virus-host interaction, therefore, represents an ongoing evolutionary arms race perfected at the molecular and cellular levels. Evidently, every step of the human Coronavirus including the SARS-CoV-2 replication cycle engages certain host factors and dramatic alterations in cellular structure and physiology activate the host stress response, autophagy, apoptosis, and innate immunity [22,55] (Figure 2).
Epidemiology of SARS-CoV-2 (COVID-19)
The ability of Coronavirus to obtain mutations which facilitate the transmission between animal to humans has made it a zoonotic pathogen of concern. In fact, the recent emergence of human Coronavirus capable of causing respiratory failure, such as SARS-CoV, MERS-CoV and SARS-CoV-2 have had the origins traced back to animals such as bats [56-58]. SARS-CoV-2 is a new virus responsible for an outbreak of respiratory illness known as COVID-19, which has spread to several countries around the world. The disease was first identified in December 2019 in Wuhan, the capital of China’s Hubei province, and has since spread globally, resulting in the ongoing 2019-20 coronavirus pandemic [59,60]. As of 11 October, 2020, more than 37,287,908 cases and 1,073,675 deaths have been reported across 200 countries and territories (Table 2) [12- 14].This virus belongs to the β-coronavirus family, a large class of viruses that are prevalent in nature. Similar to other viruses, SARSCoV- 2 has many potential natural hosts, intermediate hosts, and final hosts. This poses major challenges for the prevention and treatment of viral infection [40]. Understanding of the transmission risk is still incomplete. Epidemiologic investigation in Wuhan at the beginning of the outbreak identified an initial association with a seafood market that sold live animals, where most patients had worked or visited and which was subsequently closed for disinfection [61,24]. However, as the outbreak progressed, person-to-person spread became the main mode of transmission. But the exact mode of person-to-person spread is unclear. It is thought to occur mainly spread between people during close contact, often via small droplets produced by coughing, sneezing, or talking. While these droplets are produced when breathing out, they usually fall to the ground or onto surfaces rather than remain in the air over long distances. People may also become infected by touching a contaminated surface and then touching their eyes, nose, or mouth [5,11]. The interval during which an individual with COVID-19 is infectious is uncertain. It appears that SARSCoV- 2 can be transmitted prior to the development of symptoms and throughout the course of illness [62,63]. The virus is highly contagious and threatens everybody in terms of its infectiousness. That said, there are some groups that are particularly vulnerable to developing serious complications such as older adults (over 65), and people who have chronic medical conditions like HIV, heart disease, diabetes, and lung disease may have a higher risk of complications from COVID-19 [64,65]. Front-line health workers can be initially at risk and infected when they examine and treat patients who present with a respiratory infection; if hand washing or other infection prevention and control measures are not in place, these health workers are at great risk of infection and become the inadvertent carriers to patients who are in hospital for other diseases and treatments, family members, and the community [2] (Table 2).
Region
Total Confirmed case
Total death
Case Fatality Rate (CFR)
Globally
37 287 908
1 073 675
2.88%
Americas
17 971 661
592 222
3.30%
Asia
11 670 553
210 313
1.80%
Europe
6 041 693
232 281
3.84%
Africa
1 568 091
37 849
2.41%
Oceania
35 214
1 003
2.85%
Table 2: SARS-CoV-2 (COVID-19) Situation update worldwide, as of 11October, 2020 [12].
COVID-19 in African Countries
Africa is expected to be greatly impacted by COVID-19 despite the late arrival of the pathogen to the continent. Egypt was the first African country to experience COVID-19, on February 25th, followed by Algeria on the 27th [66]. South Africa (690,896 cases and 17,673 death), Morocco (146,398 case and 2,530 death), and Egypt (104,387 case and 6,040 death) continue to have the most confirmed cases and deaths in the continent (Table 3). Just like other countries, Ethiopia is also suffering from this pandemic. As the Ethiopian Minister of Health report should that till October 11, 2020 83,429 confirmed cases, and 1,277 death is recorded. The Ethiopian government has instituted several control measures, limiting large gatherings and closing several facilities, in an attempt to mitigate further spread of COVID-19. However, cases continue to raise and may be underrepresented due to limited testing capacities. The true epidemiology of COVID-19 on the continent continue to be challenging to predict due to these limitations [16]. In general, due to inadequate testing capacity for COVID-19, the true number of cases may remain undetected, which makes it challenging to predict or conclude the true epidemiology of COVID-19 on the continent. Certainly, several major factors, such as late arrival of the pandemic, weak diagnostics including inadequate COVID-19 testing, lack of essential medical supplies, and a largely susceptible population will significantly affect and change the epidemiology of COVID-19 in the continent [67,68] (Table 3).
Name of Country
Total Confirmed case
Total death
Case Fatality Rate (CFR)
South Africa
690 896
17 673
2.56%
Morocco
146 398
2 530
1.73%
Egypt
104 387
6 040
5.79%
Ethiopia
83 429
1 277
1.53%
Nigeria
60 103.
1115
1.86%
Algeria
52940
1795
3.39%
Ghana
47005
306
0.65%
Libya
41686
623
1.49%
Kenya
41158
760
1.85%
Tunisia
31259
456
1.46%
Table 3: African countries most affected by COVID-19, as of 11 October 2020 [14].
Clinical Characteristics of SARS-CoV-2 (COVID-19)
The clinical features of COVID-19 are varied, ranging from an asymptomatic state to acute respiratory distress syndrome and multiorgan dysfunction. Common clinical features include fever, cough, sore throat, headache, fatigue, headache, myalgia, and breathlessness. In a subset of patients, by the end of the first week, the disease can progress to pneumonia, respiratory failure, and death [69]. The median time from symptom onset to the development of pneumonia is approximately 5 days, and the median time from symptom onset to severe hypoxemia and ICU admission is approximately 7 to 12 days. Acute hypoxemic respiratory failure with severe hypercapniad from Acute Respiratory Distress Syndrome (ARDS) is the most common complication (in 60 to 70% of patients admitted to the ICU), followed by shock (30%), myocardial dysfunction (20 to 30%), and acute kidney injury (10 to 30%) [70]. Common CT findings are ground-glass opacities and consolidation. Hematological findings include lymphocytopenia, thrombocytopenia, and leukopenia. Most of the patients had elevated levels of C-reactive protein; less common were elevated levels of alanine aminotransferase, aspartate aminotransferase, creatine kinase, and d-dimer. Patients with severe disease had more prominent laboratory abnormalities (including lymphocytopenia and leukopenia) than those with non-severe disease [71]. Mortality has been found to be markedly higher in patients with elevated troponin T levels (TnT) levels than in patients with normal TnT levels (59.6% vs. 8.9%). 27 Exuberant elevation of IP-10, MCP-3, and IL-1ra during SARS-CoV-2 infection is associated with disease severity and fatal outcome [72]. Patients infected with SARS-CoV-2 can present with symptoms of conjunctivitis, including eye redness, ocular irritation, foreign body sensation, tearing, and chemosis. These symptoms have more commonly affected patients with severe systemic symptoms of COVID-19, though they can rarely present as an initial manifestation of the disease. [73]. Researcher’s analyzed data collected from 116 SARS-CoV-2 patients at Stanford Health Care who tested positive for the coronavirus gastrointestinal symptoms were reported by 31.9% of the patients. The majority of that group described the symptoms as mild. Twenty-two percent said they experienced a loss of appetite, 22% had nausea and vomiting, and 12% had diarrhea. In one national registry of 125 patients with COVID-19 and neurological or psychiatric disease reported over a 3-week period, 39 (31%) patients had altered mental status, which included 16 (13%) with encephalopathy, and 23 (18%) with a neuropsychiatric diagnosis [74]. Cutaneous manifestations of the COVID-19 pandemic gain increasing attention since they might be useful in the early diagnosis, triage of COVID-19-positive patients, and their risk stratification. Chilblain-like acral eruptions and purpuric and erythema multiforme-like lesions have been associated with children and young adult patients who are either asymptomatic or develop the mild disease. In contrast, acro-ischemic lesion and a maculopapular rash are often seen among adult patients who run a more severe course. Urticaria with pyrexia has diagnostic significance since this combination is an early symptom of an otherwise not confirmed SARS-CoV-2 infection [75].
Diagnosis of SARS-CoV-2 Infected Patients
Coronavirus Disease-2019 tracking and diagnostic testing are critical to understand the epidemiology, inform on case management, and to suppress transmission. The United States Centers for Disease Control and Prevention has developed criteria to use for a person under investigation. Ideally, if an individual is under investigation, immediate control and management measures are commenced [76]. Simultaneously, clinical factors are utilized to evaluate the necessity for testing. This involves close interaction with a disease-confirmed client within fourteen days of symptoms. Also, it may include travel history to an infected region within fourteen days of symptoms beginning [77]. RNA tests can confirm the diagnosis of COVID-19 cases with real-time RT-PCR or next-generation sequencing. At present, nucleic acid detection techniques, like RT-PCR, are considered an effective method for confirming the diagnosis in clinical cases of COVID-19. When the test outcome shows positive, it is suggested to repeat the test for the purpose of verification. On the other hand, if the test confirms negative, these warrants repeat testing. Also, chest X-ray and CT imaging are used to identify COVID-19 in suspect individuals with adverse molecular diagnosis [78].
Treatment and Prevention of SARS-CoV-2 (COVID-19)
Currently, there are no proven therapies for the treatment of COVID-19 [79,80]. There are ongoing trials on remdesivir, lopinavir-ritonavir, chloroquine, Hydroxychloroquine (HCQ), intravenous immunoglobulin, convalescent plasma, tocilizumab, favipiravir, and traditional Chinese medicines. No peer-reviewed, published safety data is available for SARS-CoV-2 on HCQ though it continues to be widely used [81,82]. Prone Ventilation is suggested for patients with refractory hypoxemia due to progressive COVID-19 pneumonia. Extracorporeal membrane oxygenation is suggested for patients with refractory hypoxemia due to progressive COVID-19 pneumonia if prone ventilation fails [83,84]. There is a large global effort to develop vaccines for protection against COVID-19 and at least ten vaccine candidates have, as of early June 2020 entered clinical trials, including phase II trials [85,86]. Safety and immunogenicity data have been reported in the scientific literature for the first-inhuman trial assessing a vector-based SARS-CoV-2 vaccine candidate conducted in China and merit further studies [87,88]. The European Medicines Agency (EMA) has been in discussion with developers of 33 potential SARS-CoV-2 vaccines since May 26, 2020. However, the EMA expects that it may take at least one year before a vaccine is approved and available for widespread use in the EU/EEA [89,90]. Thus, since at this time there are no approved treatments and vaccines for this infection, prevention is crucial. The best way to prevent the transmission of infection is to avoid or limit contact with people who are showing symptoms of COVID-19 or any respiratory infection. The next best thing you can do is practice good hygiene and physical distancing to prevent viruses from being transmitted [91].
Conclusion
The current COVID-19 pandemic is clearly an international public health problem. The disease has caused millions of infections and deaths. The virus is primarily spread between people during close contact, most often via small droplets produced by coughing, sneezing, and talking. Elderly and immunocompromised patients are at the greatest risk of fatality. Due to rapid transmission, countries around the world should increase attention to intense surveillance and isolation protocols to prevent further transmission. Although many treatments have been proposed, there are currently no specific options for treating COVID-19 or preventing 2019-nCoV infection. Thus, individuals need to take measures such as isolation, proper ventilation, hand hygiene, and the use of personal protective equipment.
References
- Schoeman D, Fielding BC. Coronavirus envelope protein: current knowledge Virology Journal. 2019; 16: 69.
- Cheng VCC, Lau SKP, Woo PCY, Yuen KY. Severe Acute Respiratory Syndrome Coronavirus as an Agent of Emerging and Reemerging Infection Clin. Microbiol Rev, 2007; 20: 660-694.
- Lojkic I, Kresic N, Simic I, Bedekovic T. Detection and molecular characterisation of bovine corona and toroviruses from Croatian cattle Veterinary Research. 2015; 11: 202.
- Vijgen L, Keyaerts E, Moes E, Thoelen I, Wollants E, Lemey P, et al. Complete Genomic Sequence of Human Coronavirus OC43: Molecular Clock Analysis Suggests a Relatively Recent Zoonotic Coronavirus Transmission Event Journal Of Virology. 2005; 79: 1595-1604.
- Guo Y-R, Cao Q-D, Hong Z-S, Tan Y-Y, Chen S-D, Jin H-J, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak an update on the status Military Medical Research. 2020; 7: 11.
- Seah I, Agrawal R. Can the Coronavirus Disease 2019 (COVID-19) Affect the Eyes? A Review of Coronaviruses and Ocular Implications in Humans and Animals Ocular Immunology and Inflammation. 2020; 28: 391-395.
- Sipulwa LA, Ongus JR, Coldren RL, Bulimo WD. Molecular characterization of human coronaviruses and their circulation dynamics in Kenya, 2009-2012 Virology Journal (2016) 13:18 Virology Journal. 2016; 13: 18.
- Zhou P, Yang X-L, Wang X-G, Shi Z-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin | Nature. 2020; 579: 270-273.
- Koo JR, Cook AR, Park M, Sun Y, Sun H, Lim JT, et al. Interventions to mitigate early spread of SARS-CoV-2 in Singapore: a modelling study Interventions to mitigate early spread of SARS-CoV-2 in Singapore: a modelling study Lancet Infect Dis. 2020.
- Chen X, Yu B. First two months of the 2019 Coronavirus Disease (COVID-19) epidemic in China: realtime surveillance and evaluation with a second derivative model. Global Health Research and Policy. 2020; 5: 7.
- Boulos MNK, Geraghty EM. Geographical tracking and mapping of coronavirus disease COVID-19/severe acute respiratory syndrome coronavirus 2 (SARSCoV- 2) epidemic and associated events around the world: how 21st century GIS technologies are supporting the global fght against outbreaks and epidemics Int J Health Geogr. 2020; 19: 8.
- World health Organization (WHO). Coronavirus disease (COVID-2019) situation reports. 2020.
- Central for Disease Control and Prevention (CDC) coronavirus disease (COVID-19). 2020.
- European Centre for Disease Prevention and Control, COVID-19 Situation update for the EU/EEA and the UK. 2020.
- El-Sadr WM, Justman J. Africa in the Path of Covid-19 2020, the New England Journal of Medicine. 2020.
- Ethiopia Ethiopian Public health Institute Notification Not on COVID-19 Situational Update 2. 2020.
- Peiris JSM, in Medical Microbiology Book (Eighteenth Edition). 2012.
- Vabret A, Mourez T, Dina J, van der Hoek L, Gouarin S, Petitjean J, et al. Human Coronavirus NL63, France Emerging Infectious Diseases. 2005; 11: 1225-1229.
- Qian X, Ren R, Wang Y, Guo Y, Fang J, Wu Z-D, et al., and Members of Steering Committee, Society of Global Health, Chinese Preventive Medicine Association. Fighting against the common enemy of COVID-19: a practice of building a community with a shared future for mankind Infectious Diseases of Poverty 2020; 9: 34.
- Weiss SR, Navas-Martin S. Coronavirus Pathogenesis and the Emerging Pathogen Severe Acute Respiratory Syndrome Coronavirus Microbiology and Molecular Biology Reviews, Dec. 2005; 69: 635-664.
- Fenner F. The Classification and Nomenclature of Viruses Summary of Results of Meetings of the International Committee on Taxonomy of Viruses in Madrid. J gen Viral. I976; 31: 463-470.
- Fung TS, Liu DX. Human Coronavirus: Host-Pathogen Interaction Annu Rev Microbiol. 2019; 73: 529-557.
- Roussel Y, Giraud-Gatineau A, Jimeno MT, Rolain J-M, Zandotti C, Colson P, et al. SARS-CoV-2: fear versus data International Journal of Antimicrobial Agents. 2020.
- Singhal T. A Review of Coronavirus Disease-2019 (COVID-19) The Indian Journal of Pediatrics. 2020; 87: 281-286.
- Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020; 579: 265- 269.
- de Wit E, van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016; 14: 523-534.
- Qiang X-L, Xu P, Fang G, Liu W-B, Kou Z. Using the spike protein feature to predict infection risk and monitor the evolutionary dynamic of coronavirus Infectious Diseases of Poverty. 2020; 9: 33.
- Chen Y, Liu Q, Guo D. Coronaviruses: genome structure, replication, and pathogenesis. J Med Virol. 2020; 92: 418-423.
- Tekes G, Thiel HJ. Feline coronaviruses: pathogenesis of feline infectious peritonitis. Adv Virus Res. 2016; 96: 193-218.
- Shereen MA, Khan S, Kazmi A, Bashira N, Siddique R. COVID-19 infection: Origin, transmission, and characteristics of human coronaviruses Journal of Advanced Research. 2020; 24: 91-98.
- Woo PCY, Huang Y, Lau SKP, Yuen K-Y. Coronavirus Genomics and Bioinformatics Analysis Viruses. 2010; 2: 1804-1820.
- Wang N, Shang J, Jiang S, Du L. Subunit Vaccines Against Emerging Photogenic human Coronaviruses Front. Microbiol. 2020; 11298.
- Narayanan K, Huang C, Makino S. SARS coronavirus accessory proteins Virus Research. 2008; 133: 113-121.
- Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor Recognition by the Novel Coronavirus from Wuhan: an Analysis Based on Decade-Long Structural Studies of SARS Coronavirus Journal of Virology. 2020; 94: e00127-20.
- Khailanya RA, Safdarb M, Ozaslanc M. Genomic characterization of a novel SARS-CoV-2 Gene Reports. 2020; 19: 100682.
- Li M-Y, Li L, Zhang Y, Wang X-S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues Infectious Diseases of Poverty. 2020; 9: 45.
- Wang L, Wang Y, Ye D, Liu Q. A review of the 2019 Novel Coronavirus (COVID-19) based on current evidence, International Journal of Antimicrobial Agents. 2020; 55: 105948.
- Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, et al. Structure of the SARS-CoV-2 spike receptorbinding domain bound to the ACE2 receptor, Nature. 2020; 581: 215-220.
- McBride R, Fielding BC. The Role of Severe Acute Respiratory Syndrome (SARS)-Coronavirus Accessory Proteins in Virus Pathogenesis Viruses. 2012; 4: 2902-2923.
- Lim YX, Ng YL, Tam JP, Liu DX. Human Coronaviruses: A Review of Virus–Host Interactions, Diseases 2016; 4: 26.
- Vijgen L, Keyaerts E, Moes E, Maes P. Duson G, van Ranst M. Development of One-Step, Real-Time, Quantitative Reverse Transcriptase PCR Assays for Absolute Quantitation of Human Coronaviruses OC43 and 229E. J Clin Microbiol. 2005; 43: 5452-5456.
- Jones BA, Grace D, Kock R, Alonso S, Rushton J, Said MY, et al. Zoonosis emergence linked to agricultural intensification and environmental change. Proc Natl Acad Sci USA. 2013; 21: 8399-8340.
- Van der Hoek L. Human coronaviruses: What do they cause? Antivir Ther. 2007; 12: 651-658.
- Walsh EE, Shin JH, Falsey AR. Clinical Impact of Human Coronaviruses 229E and OC43 Infection in Diverse Adult Populations. J Infect Dis. 2013; 208: 1634-1642.
- Gorse GJ, O’Connor TZ, Hall SL, Vitale JN, Nichol KL. Human Coronavirus and Acute Respiratory Illness in Older Adults with Chronic Obstructive Pulmonary Disease. J Infect Dis. 2009; 199: 847-857.
- Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS. J Virol. 2020.
- Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020.
- Zhang N, Jiang S, Du L. Current advancements and potential strategies in the development of MERS-CoV vaccines. Expert Rev Vaccines. 2014; 13:761- 774.
- Xia S, Zhu Y, Liu M, Lan Q, Xu W, Wu Y, et al. Fusion mechanism of 2019- nCoV and fusion inhibitors targeting HR1 domain in spike protein. Cell Mol Immunol. 2020; 17: 765-767.
- van Hemert MJ, van den Worm SHE, Mommaas KKAM, Alexander E. Snijder GEJ. SARS-Coronavirus Replication/Transcription Complexes Are Membrane-Protected and Need a Host Factor for Activity In Vitro PLoS Pathog. 2008.
- Yu F, Du L, Ojcius DM, Pan C, Jiang S. Measures for diagnosing and treating infections by a novel coronavirus responsible for a pneumonia outbreak originating in Wuhan, China. Microbes Infect. 2020; 22: 74-79.
- Frieman M, Baric R. Mechanisms of Severe Acute Respiratory Syndrome Pathogenesis and Innate Immunomodulation Microbiology And Molecular Biology Reviews. 2008; 72: 672-685.
- Frieman M, Heise M, Baric R. SARS coronavirus and innate immunity. Virus Res. 2008; 133: 101-112.
- Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 1998; 16: 225-260.
- Rothana HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak, Journal of Autoimmunity. 2020; 109: 102433.
- Ye ZW, Yuan S, Yuen KS, Fung SY, Chan CP, Jin DY. Zoonotic origins of human coronaviruses. 2020; 16: 1686-1697.
- Guan Y, Zheng BJ, He YQ, Liu XL, Zhuang ZX, Cheung CL, et al. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science. 2003; 302: 276-278.
- Poon LL, Chu DK, Chan KH, Wong OK, Ellis TM, Leung YH, et al. Identification of a novel coronavirus in bats. J Virol. 2005; 79: 2001-2009.
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention, J Am Med Assoc. 2020.
- Jiang F, Deng L, Zhang L, Cai Y, Cheung CW, Xia Z. Review of the Clinical Characteristics of Coronavirus Disease 2019 (COVID-19), J Gen Intern Med. 2020; 35: 1545-1549.
- World Health Organization. Novel coronavirus situation report-2. 2020.
- Zou L, Ruan F, Huang M, Liang L, Haung H, Hong Z, et al. SARS-CoV-2 Viral Load in Upper Respiratory Specimens of Infected Patients. N Engl J Med. 2020; 382: 1177-1179.
- To KK, Tsang OT, Leung WS, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020.
- Tu C, Crameri G, Kong X, Chen J, Sun Y, Yu M, et al. Antibodies to SARS coronavirus in civets. Emerg Infect Dis. 2004; 10: 2244-2248.
- Lau SK, Woo PC, Li KS, Huang Y, Tsoi HW, Wong BH, et al. Severe acute respiratory syndrome coronavirus-like virus in Chinese horseshoe bats. Proc Natl Acad Sci USA. 2005; 102: 14040-14045.
- WHO. A second COVID-19 case is confirmed in Africa. 2020.
- Africa Center for Strategic Studies. Mapping risk factors for the spread of COVID-19 in Africa. 2020.
- The World Economic Forum. Why Sub-Saharan Africa needs a unique response to COVID-19. 2020.
- Zhao L, Jha BK, Wu A, Elliott R, Ziebuhr J, Gorbalenya AE, et al. Antagonism of the interferon-induced OAS-RNase L pathway by murine coronavirus ns2 protein is required for virus replication and liver pathology. Cell host & microbe. 2012; 11: 607-616.
- Jason P, Li W, Lowell L, Moritoki E, ChaeMan L, Jigeeshu VD, et al. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med 2020; 8: 506-517.
- Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J. The New England J Med. 2020; 382:1708-1720.
- Fan W, Aojie W, Mei L, Qimin W, Jun C, Shuai X, et al. Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered 2 patient cohort and their implications. medRxiv. 2020.
- Hu K, Patel J, Patel BC. Ophthalmic Manifestations of Coronavirus (COVID-19). 2020.
- Varatharaj A, Thomas N, Ellul M, Davies NWS, Pollak T, Tenorio EL, et al. UK-wide surveillance of neurological and neuropsychiatric complications of COVID-19: the first 153 patients. SSRN. 2020.
- Wollina U, Karadag AS, Rowland-Payne C, Chiriac AS, Lotti T. Cutaneous signs in COVID-19 patients: A review. 2020.
- Coronavirus disease 2019 (COVID-19) Mayoclinic. 2019.
- WHO Coronavirus disease (COVID-19) technical guidance: Laboratory testing for 2019-nCoV in humans.
- Trafton A, Chu J. Covid-19 diagnostic based on MIT technology might be tested on patient samples soon. MIT News Office March. 2020.
- A randomized, open-label, blank-controlled trial for the efficacy and safety of lopinavir-ritonavir and interferon-alpha 2b in hospitalization patients with novel coronavirus infection. 2020.
- Liu K, Fang YY, Deng Y, Liu W, Wang MF, Ma JP, et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl). 2020.
- Richardson P, Griffin I, Tucker C, Smith D, Oechsle O, Phelan A, et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020.
- Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020.
- Phan LT, Nguyen TV, Luong QC, Nguyen TV, Nguyen HT, Le HQ, et al. Importation and human-to-human transmission of a novel coronavirus in Vietnam. N Engl J Med. 2020.
- CDC. Everything You Should Know About the 2019 Coronavirus and COVID-19.
- Joint Prevention and Control Mechanism of the State Council. 2020.
- World Health Organization (WHO). “Solidarity” clinical trial for COVID-19 treatments: WHO. 2020.
- INSERM. Launch of a European clinical trial against COVID-19. 2020.
- World Health Organisation (WHO). Clinical management of COVID-19. Interim guidance: WHO. 2020.
- Zhu FC, Li YH, Guan XH, Hou LH, Wang WJ, Li JX, et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020.
- European Medicines Agency (EMA). Treatments and vaccines for COVID-19. 2020.
- Diamond MS, Pierson TC. The Challenges of Vaccine Development against a New Virus during a Pandemic. Cell Host Microbe. 2020; 27: 699-703.