
Research Article
Austin J Infect Dis. 2022; 9(2): 1068.
Retrospective Evaluation of COVID-19 Therapeutics
Zhang X1, Peng K2, Zhang R3,4, Li F3,4, Xiao C3,4, Zhai S3,4, Liu C3,4, Hu Q3,4, An L3,4 and Yang C1*
1Health Supervision Offce of Health and Hygiene Burea, Meihekou, Jilin Province, China
2Department of Automation, Hangzhou Dianzi University, China
3Institute of Biopharmaceutics and Health Engineering, Tsinghua Univeristy International Graduate School, China
4Center of Precision Medicine and Healthcare, Tsinghua-Berkeley Shenzhen Institute, China
*Corresponding author: Chengming Yang, 1Southern University of Science and Technology Hospital, Shenzhen, Guangdong Province, 518055, China
Received: July 15, 2022; Accepted: August 10, 2022; Published: August 17, 2022
Abstract
Background: The pandemic outbreak of COVID-19 created panic all over the world. As therapeutics that can effectively wipe out the virus and terminate transmission is not available, supportive therapeutics is the main clinical treatments for COVID-19. Repurposing available therapeutics from other viral infections is the primary surrogate in ameliorating and treating COVID-19. The therapeutics should be tailored individually by analyzing the severity of COVID-19, age, gender, and the underlying conditions. Here, we retrospectively revisit the clinical data collected in China and systematically analyze the efficacy and target patients of different therapeutics and find that Arbidol and Traditional Chinese Medicine (TCM) increase the survival rate significantly, whereas antibacterial treatment is ineffective for viral and bacterial co infection. Multicenter collaboration and large cohort of patients will be required to evaluate therapeutics combinations in the future.
Methods: This study is a single-center retrospective observational study of COVID-19 clinical data in China. We screen 2844 COVID-19 patients from the patients admitted to Tongji Hospital (Wuhan) between January 18, 2020, and April 25, 2020 and exclude cases with missing information or false positive diagnosis. Then the patients’ information with different severity will be study to evaluate the efficacy of treatment, including treatment modalities, past medical records, individual disease history, and clinical outcomes were analyzed. As the severity of illness is correlated with laboratory or clinical data, the information can be used to evaluate disease severity. We divide the patients into three groups with moderate, severe, and critical illness. Kaplan-Meier method, univariate and multivariate Cox regression are used to explore different treatment methods on clinical outcomes.
Results: After screening, 2844 patients are selected for the study. The mean age of all the patients was 58.74 years (Standard Deviation, SD =15.28), and 49.0% is male. It shows that treatment with TCM (Hazard Ratio (HR) 0.191 [95% Confidence Interval (CI), 0.14 – 0.25]; p < 0.0001), antiviral therapy (HR 0.331 [95% CI 0.19 – 0.58]; p =0.000128), or Arbidol (HR 0.454 [95% CI 0.34 – 0.60]; p < 0.0001) is associated with good prognostic of patients. Multivariate Cox regression showed TCM treatment decreased the mortality hazard ratio by 69.4% (p < 0.0001). Larger Mean Platelet Volume (MPV), international standardized ratio of prothrombin (PT-INR), and K+ are associated with poorer survival. In contrast, larger Eosinophil Count (Eos#), Basophil Count (Baso#), Percentage of Basophils (Baso%), Total Calcium (Ca), Albumin/Globulin Ratio (ALB/GLO), Lymphocyte Count (Lymph#), and Percentage of Eosinophils (Eos%) are associated with better survival.
Introduction
The quick spread and highly contagious nature of COVID -19 created a severe crisis worldwide. The absence of specific treatment for this decrease further raises the public concerns. Therefore, governments of various nations utilize all the possible measures to prevent the infection and decrease the disease’s devastating outcomes. Although the current therapeutics and vaccines have made promising progress, supportive therapeutics is the main methods for COVID-19 clinically [1]. There is still a long way for therapeutic optimization and understanding of diverse therapeutic approaches under the high risk of the second COVID-19 wave.
As the diseases caused by the SARS-CoV-2 range from asymptomatic, mild pneumonia to acute severe respiratory distress Research Article Retrospective Evaluation of COVID-19 Therapeutics Zhang X1, Peng K2, Zhang R3,4, Li F3,4, Xiao C3,4, Zhai S3,4, Liu C3,4, Hu Q3,4, An L3,4 and Yang C1* 1Health Supervision Offce of Health and Hygiene Burea, Meihekou, Jilin Province, China 2Department of Automation, Hangzhou Dianzi University, China 3Institute of Biopharmaceutics and Health Engineering, Tsinghua Univeristy International Graduate School, China 4Center of Precision Medicine and Healthcare, Tsinghua-Berkeley Shenzhen Institute, China *Corresponding author: Chengming Yang, 1Southern University of Science and Technology Hospital, Shenzhen, Guangdong Province, 518055, China Received: July 15, 2022; Accepted: August 10, 2022; Published: August 17, 2022 syndromes (ARDS), septic shock, and multiple organ dysfunction syndromes (MODS) [2]. The clinicians widely use antiviral, antibacterial, and TCM therapies to treat patients. Antivirals generally act through two paths: first path directly attacks the virus and interrupts its replication machinery or its ability to attack host cells, and second path blocks the host–viral interactions on the host side. Lopinavir (LPV), a protease inhibitor of 3CLpro, showed an antiviral effect against the SARS-CoV-2 virus with the estimated EC50 (halfmaximal effective concentration) at 26.63 μM. LPV is commonly administered in coformulation with the structurally related ritonavir (LPV/r), a mutagenic guanosine analog that inhibits cytochrome P450 metabolism of LPV and boosts lopinavir concentrations [3]. Arbidol blocks virus replication by inhibiting the fusion of the virus’s lipid membrane with the host cells, which blocks viral entry and post-stages of entry by targeting viral proteins or virus-associated host factors [4]. Arbidol targets the SARS-CoV-2 spike glycoprotein and impedes its trimerization [5]. Arbidol may induce structural rigidity for binding at the RBD/ACE2 interface, which will inhibit the conformational dynamics required during virus entry [6]. Besides, it can also regulate the immune system by promoting interferon release from cells and continuing to play an antiviral role [7].
Fluoroquinolones are broad-spectrum antibiotics [8]; their mechanism of action is by inhibiting the activities of p prokaryotic DNA gyrase–topoisomerase II and topoisomerase IV, which are involved in replication transcription and DNA synthesis [9]. Ciprofloxacin and moxifloxacin may interact with COVID-19 main protease [10]. Fluoroquinolones have limited ability to inhibit the replication of SARS-CoV-2 and MERS-CoV in cultured cells [11]. Azithromycin is an orally active synthetic macrolide antibiotic with a wide range of antibacterial, anti-inflammatory and antiviral properties. Azithromycin increased rhinovirus 1B- and rhinovirus 16-induced interferons and interferon-stimulated gene mRNA expression and protein production, and reduced rhinovirus replication and release [12]. Macrolides’s antibacterial action is through inhibition of protein synthesis via binding to the 50S subunit of bacterial ribosomes [13]. Antibacterial therapy will be adopted to prevent bacterial co-infection and secondary bacterial infection are critical risk factors for the severity and mortality rates of COVID-19. It may increase drug resistance and raise the risks of allergic reactions.
TCM is an important weapon to contain the pandemic in Chinese history, which has been widely used to treat a variety of infectious diseases such as SARS, H1N1, and H5N1 [14,15]. TCM can mitigate clinical symptoms, alleviate fever, shorten average hospitalization time, and slows down mild to severe transition [16]. Some plants have been observed to be effective in laboratory or animal studies; however there is a need to be aware that plant products may interact with other drugs [17]. Natural compounds (such as heparin and vitamin C) are effective natural products and TCM-based therapies for combating the COVID-19 and immune boosters [18]. The compound from Qingfei Paidu Decoction may directly interfere with Toll-like receptor 4 and regulate the downstream signaling pathways, leading to the inhibition of release of proinflammation factors [19]. Lianhuaqingwen exerted its anti-coronavirus activity by inhibiting virus replication, affects virus morphology and reducing the cytokine release from host cells [20]. The mortality rate of patients receiving TCM treatment was lower than those not receiving TCM treatment [21].
Thisstudy explores the factors that correlate with disease severity and hospitalization mortality, and reveals the impact of different therapies on patient clinical outcomes.TCM shows positive effects because early deployment of TCM for moderate cases and antibiotics are incapable of saving patients with coinfection since current antibiotics are not effective for certain bacteria. The physiological parameters of patients such as MPV, PT-INR, K+, EOS#, BASO#, BASO%, Ca, ALB/GLO, Lymph#, and EOS% are closely related to the severity of the disease.
Methods
Study Design and Participants
This study is a retrospective, observational study based on clinical data from Tongji Hospital in Wuhan. The severity of patients’ illness is determined by WHO interim guidance with positive SARS-CoV-2 RNA detection in throat swab specimens. We categorize patients into three groups and analyze the data by statistical methods. Specifically, we analyze the causal relationship of the treatment modalities, past medical history, individual disease history, and clinical outcomes among patients with different disease severity. We study the correlation between the severity of illness and laboratory data.
Inclusion and exclusion criteria as follows: we include (1) RCTs or (2) cohort or case-control studies reporting on the adjusted effect estimates of the association between CST use in COVID-19 patients and one of the following a-priori outcomes: (1) in-hospital mortality, (2) mechanical ventilation, (3) ICU admission, (4) viral shedding and (5) composite outcomes if reported.
Data Collection
We collect the clinical data for 3337 COVID-19 patients. Data are ascertained from hospital’s electronic medical record and recorded in a standardized electronic case report form. The data include all the diagnostic, pathological, and therapeutic information. Baseline data (such as demographics, medical history, individual disease history, and physical examination), laboratory, treatment, and outcome data are extracted from electronic medical records. Laboratory tests include routine blood tests, biochemical tests, coagulation tests, blood gas analysis, cytokine tests, ferritin, erythrocyte sedimentation rate, hypersensitive C-reactive protein, procalcitonin, etc. The treatment mainly includes TCM, immunotherapy, antiviral drugs, antibacterial therapy, and supportive therapy.
Statistical Analysis
Descriptive data contains normal and non-normal distributed types. The first type is expressed in terms of mean and standard deviation. Others are presented by median and interquartile range. Categorical variables were presented as percentages. We applied the Analysis of Variance or Kruskal-Wallis rank-sum for two kinds of data, respectively, comparing groups with varying disease severity. The chi-square test was performed to compare count data. We use Kaplan-Meier to plot to visualize survival curves, Log-rank test to compare the survival curves of two or more groups, and Cox proportional hazards regression for survival analysis to describe the effect of variables on survival.
Kaplan-Meier curves and log-rank tests - are examples of univariate analysis. They describe the survival according to one factor under investigation, but ignore the impact of any others. Additionally, Kaplan-Meier curves and log rank tests are useful only when the predictor variable is categorical. They don’t work quickly for quantitative predictors. An alternative method is the Cox proportional hazards regression analysis, which works for both quantitative predictor variables and for categorical variables. Furthermore, the Cox regression model extends survival analysis methods to simultaneously assess several risk factors’ effect on survival time.
Assess the association between different drugs and in-hospital mortality in patients admitted with COVID-19 using a Kaplan-Meier method. The Cox proportional hazards regression analysis was used to extend survival analysis methods to assess the effect of several risk factors for in-hospital mortality simultaneously. All statistical analyses were conducted using the R language.
Results
We collect the clinical data for 3337 COVID-19 patients from Tongji Hospital in Wuhan. The data include all the diagnostic, pathological, and therapeutic information, which is screened to finalize the patients’ cohort for further statistical analysis. Patients were excluded because they have asymptomatic or mild clinical symptoms without pneumonia on CT imaging since they do not need therapeutic intervention for recovery. Patients who are not sick enough to be hospitalized or lack of clinical records are excluded in this study. 2844 (85.23%) patients after screening were grouped into categories in this study: moderate, severe, and critical ill according to the severity of COVID-19 (Figure. 1). The definition of COVID-19 severity follows the WHO standards. The various therapeutics have been used to treat three groups of patients, including 242 moderate, 1995 severe, and 607 critically ill patients.
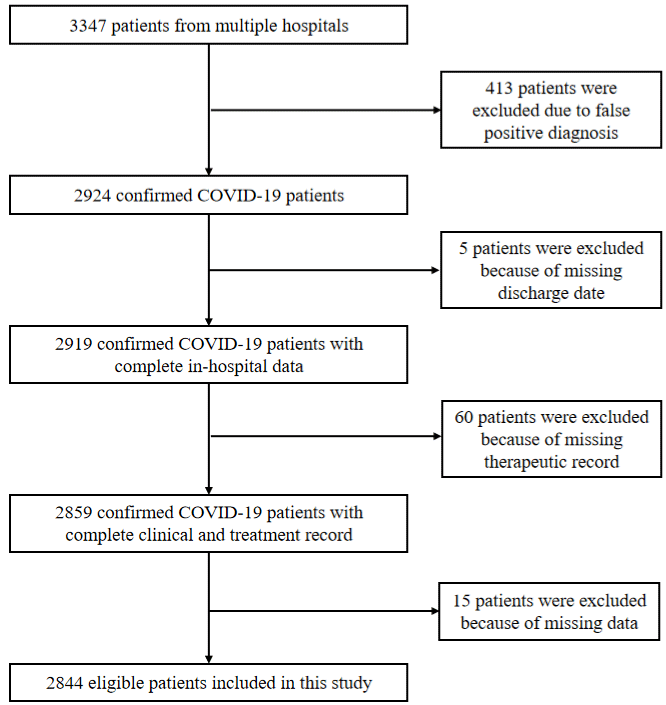
Figure 1: Overview of participant’s selection included in this cohort. Some patients are excluded due to following factors such as missing important information
and non-treatment cases for self-recovery.
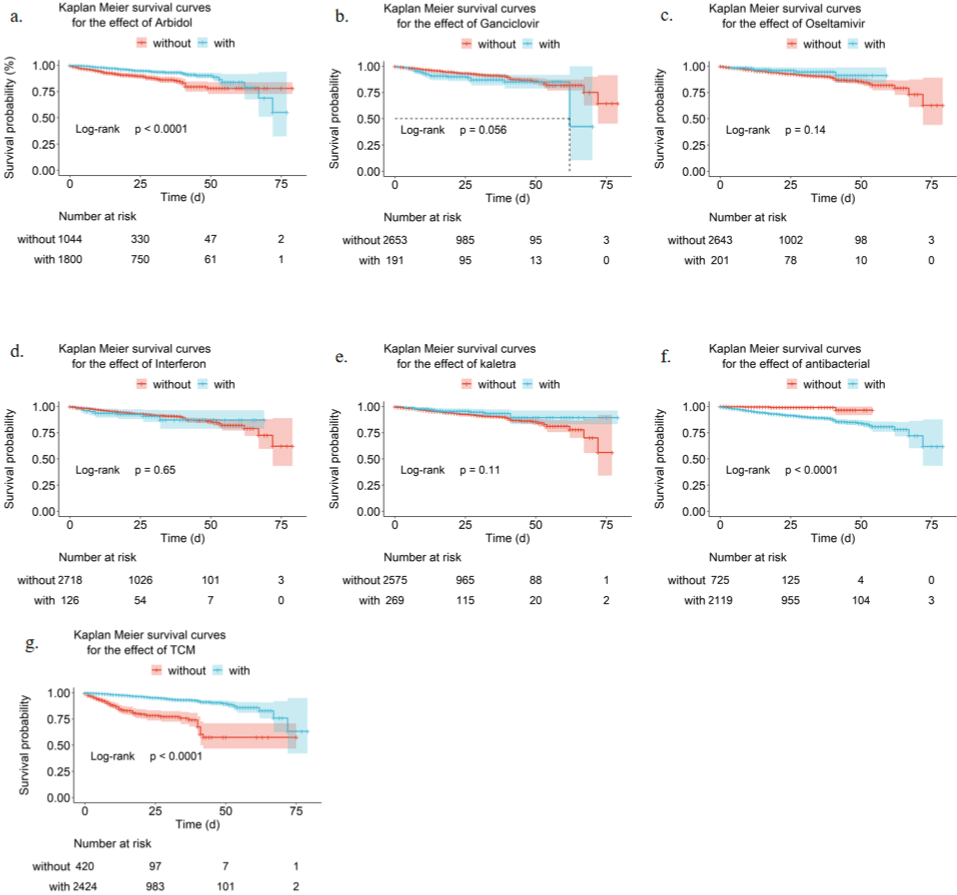
Figure 2: Kaplan-Meier survival curves. Kaplan Meier survival curves and log rank tests for the effect of Arbidol (a), Ganciclovir (b), Oseltamivir (c), Interferon (d),
Kaletra (e), Antimicrobial therapy (f), and TCM (g) treatments on mortality.
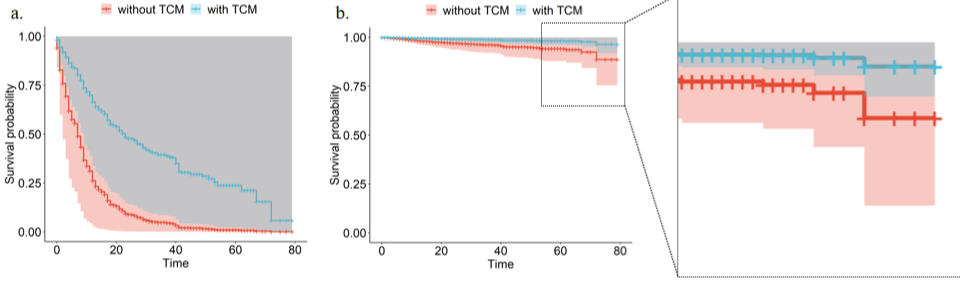
Figure 3: Visualized survival curves for the new data frames. The latest data framework only kept the values of TCM, modified the other parameters to the same
value; the left panel was based on the different treatments all used or had co morbidity, the right panel is the opposite.
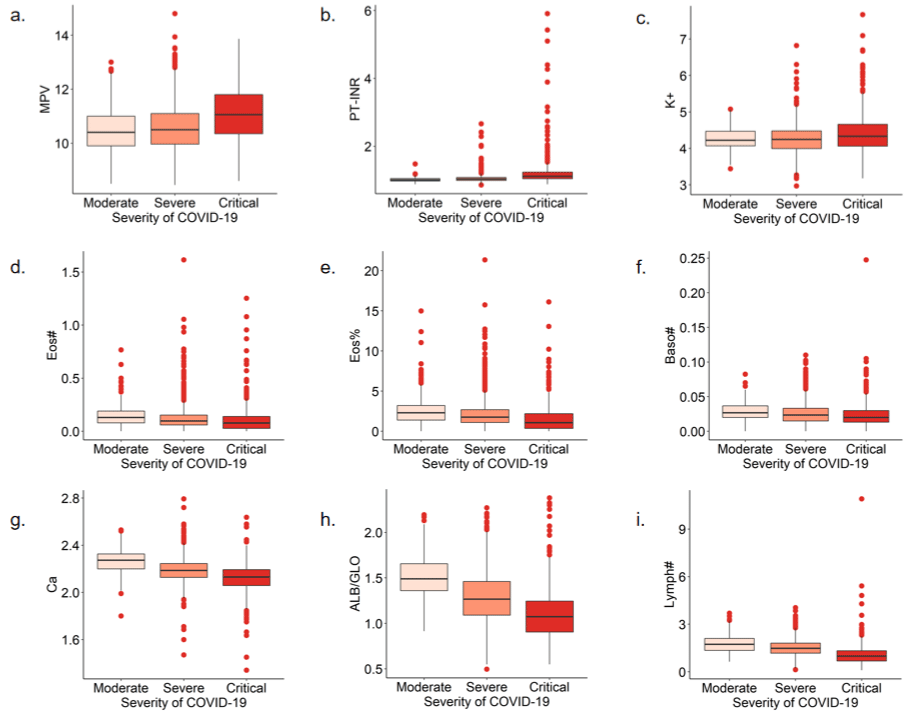
Figure 4: Boxplots of laboratory indices and severity of COVID-19. A-plot to i-plot are boxplots of indicator MPV (a), PT-INR (b), K+ (c), Eos# (d), Eos% (e), Baso#
(f), Ca (g), ALB/GLO (h), and Lymph# (i)for different covid-19 severities, respectively.
Forour study, the most commonly used combination of critically ill patients is ventilator, oxygen-therapy, TCM, hormone therapy, antiviral therapy and antibacterial-therapy. The combination of oxygen therapy, TCM, antiviral therapy and antibacterial therapy is most common for seriously ill patients. Moderate patients used a combination of TCM and antivirals at most.
The main therapeutic methods for moderate patients are antiviral (88.4%) and TCM (79.8%) therapy. Same patients receive a combination of different treatments, which causes the total percentage is greater than 100%. The therapeutics for severe patients is antiviral therapy (97.1%), oxygen therapy (89.3%), and TCM (88.0%), and antibacterial therapy (72.8%). The treatment methods for critically ill patients are: oxygen therapy (98.7%), antiviral therapy (95.4%), antibacterial therapy (92.4%), hormone therapy (80.1%), and TCM (78.4%). Patients in less severity group have less therapeutics since some treatments are invasive, only applicable in severe conditions, and side effects. Oxygen therapy was used in a large proportion of patients (2508, 88.2%), while ventilator, intubate, hemodialysis, ECMO, and CRRT were mainly used in critically ill patients. 848 (29.8%) patients were treated with gamma globulin, 2,424 (85.2%) with TCM, 1,295 (45.5%) with hormone, 455 (16.0%) with immunotherapy, 2,119 (74.5%) with antimicrobial therapy, and 2,731 (96.0%) with antiviral therapy for all three groups. The five most widely used antiviral drugs are Arbidol (1800, 63.3%), Ganciclovir (191, 6.7%), Oseltamivir (201, 7.1%), Interferon (126, 4.4%), and Kaletra (269, 9.5%).
Descriptive data was presented as mean with standard deviation for normally distributed continuous variables, where the standard deviation is in the bracket following mean. For non-normallydistributed data, we list the variable median with interquartile range in the following bracket. Categorical or binary variables were presented as percentages. For a different group of patients, the number of patients is listed followed with the percentage of treatment or nontreatment (Table 1). The mean age was 58.74 years (SD 15.28), and the severe group has higher mean age. Ageing is associated with endothelial dysfunction and weak immune protection, contributing to vascular pathologies and cardiovascular diseases [22]. 1393 cases (49.0%) were male, and the group of critically ill patients comprised more males (57.2%) patients. Sex differences in immune responses underlie COVID-19 disease outcomes [23]. There were no significant differences in height, weight, body mass index, and body surface area among patients with different disease severity within three groups.
Level
Moderate
Severe
Critical
Overall
p
n
242
1995
607
2844
Age (mean (SD))
48.88 (14.42)
57.70 (14.64)
66.10 (14.51)
58.74 (15.28)
<0.001
Inhospital Length (mean (SD))
10.43 (6.94)
22.10 (12.06)
29.03 (16.64)
22.59 (13.66)
<0.001
Sex (%)
Female
126 (52.1)
1065 (53.4)
260 (42.8)
1451 (51.0)
<0.001
Male
116 (47.9)
930 (46.6)
347 (57.2)
1393 (49.0)
Height (median [IQR])
168.00 [160.00, 170.00]
165.00 [160.00, 170.00]
167.00 [160.00, 170.00]
165.00 [160.00, 170.00]
0.067
Weight (median [IQR])
65.00 [59.00, 72.88]
65.00 [57.00, 70.00]
64.00 [55.00, 70.00]
65.00 [57.00, 70.00]
0.322
BMI (median [IQR])
23.66 [21.92, 25.26]
23.44 [21.48, 25.50]
23.03 [20.92, 25.10]
23.44 [21.47, 25.39]
0.163
BSA (mean (SD))
1.81 (0.17)
1.80 (0.34)
1.79 (0.18)
1.80 (0.30)
0.798
Ventilator (%)
No
239 (98.8)
1902 (95.3)
263 (43.3)
2404 (84.5)
<0.001
Yes
3 (1.2)
93 (4.7)
344 (56.7)
440 (15.5)
Intubate (%)
No
241 (99.6)
1978 (99.1)
415 (68.4)
2634 (92.6)
<0.001
Yes
1 (0.4)
17 (0.9)
192 (31.6)
210 (7.4)
Oxygen Therapy (%)
No
115 (47.5)
213 (10.7)
8 (1.3)
336 (11.8)
<0.001
Yes
127 (52.5)
1782 (89.3)
599 (98.7)
2508 (88.2)
Hemodialysis (%)
No
242 (100.0)
1992 (99.8)
524 (86.3)
2758 (97.0)
<0.001
Yes
0 (0.0)
3 (0.2)
83 (13.7)
86 (3.0)
ECMO (%)
No
242 (100.0)
1994 (99.9)
592 (97.5)
2828 (99.4)
<0.001
Yes
0 (0.0)
1 (0.1)
15 (2.5)
16 (0.6)
CRRT (%)
No
242 (100.0)
1994 (99.9)
530 (87.3)
2766 (97.3)
<0.001
Yes
0 (0.0)
1 (0.1)
77 (12.7)
78 (2.7)
Gamma Globulin Therapy (%)
No
220 (90.9)
1521 (76.2)
255 (42.0)
1996 (70.2)
<0.001
Yes
22 (9.1)
474 (23.8)
352 (58.0)
848 (29.8)
TCM (%)
No
49 (20.2)
240 (12.0)
131 (21.6)
420 (14.8)
<0.001
Yes
193 (79.8)
1755 (88.0)
476 (78.4)
2424 (85.2)
Hormone Therapy (%)
No
202 (83.5)
1226 (61.5)
121 (19.9)
1549 (54.5)
<0.001
Yes
40 (16.5)
769 (38.5)
486 (80.1)
1295 (45.5)
Immunotherapy (%)
No
209 (86.4)
1740 (87.2)
440 (72.5)
2389 (84.0)
<0.001
Yes
33 (13.6)
255 (12.8)
167 (27.5)
455 (16.0)
Antiviral Therapy (%)
No
28 (11.6)
57 (2.9)
28 (4.6)
113 (4.0)
<0.001
Yes
214 (88.4)
1938 (97.1)
579 (95.4)
2731 (96.0)
Arbidol (%)
No
150 (62.0)
631 (31.6)
263 (43.3)
1044 (36.7)
<0.001
Yes
92 (38.0)
1364 (68.4)
344 (56.7)
1800 (63.3)
Ganciclovir (%)
No
237 (97.9)
1874 (93.9)
542 (89.3)
2653 (93.3)
<0.001
Yes
5 (2.1)
121 (6.1)
65 (10.7)
191 (6.7)
Oseltamivir (%)
No
227 (93.8)
1848 (92.6)
568 (93.6)
2643 (92.9)
0.627
Yes
15 (6.2)
147 (7.4)
39 (6.4)
201 (7.1)
Interferon (%)
No
229 (94.6)
1914 (95.9)
575 (94.7)
2718 (95.6)
0.338
Yes
13 (5.4)
81 (4.1)
32 (5.3)
126 (4.4)
Kaletra (%)
No
228 (94.2)
1819 (91.2)
528 (87.0)
2575 (90.5)
0.001
Yes
14 (5.8)
176 (8.8)
79 (13.0)
269 (9.5)
Antibacterial Therapy (%)
No
137 (56.6)
542 (27.2)
46 (7.6)
725 (25.5)
<0.001
Yes
105 (43.4)
1453 (72.8)
561 (92.4)
2119 (74.5)
Smoking (%)
No
242 (100.0)
1976 (99.0)
603 (99.3)
2821 (99.2)
0.265
Yes
0 (0.0)
19 (1.0)
4 (0.7)
23 (0.8)
Past Disease (%)
No
136 (56.2)
956 (47.9)
181 (29.8)
1273 (44.8)
<0.001
Yes
106 (43.8)
1039 (52.1)
426 (70.2)
1571 (55.2)
Infectious Disease (%)
No
231 (95.5)
1934 (96.9)
582 (95.9)
2747 (96.6)
0.269
Yes
11 (4.5)
61 (3.1)
25 (4.1)
97 (3.4)
Allergic History (%)
No
226 (93.4)
1818 (91.1)
571 (94.1)
2615 (91.9)
0.046
Yes
16 (6.6)
177 (8.9)
36 (5.9)
229 (8.1)
Blood Transfusion History (%)
No
242 (100.0)
1980 (99.2)
601 (99.0)
2823 (99.3)
0.313
Yes
0 (0.0)
15 (0.8)
6 (1.0)
21 (0.7)
Past Surgery (%)
No
201 (83.1)
1666 (83.5)
482 (79.4)
2349 (82.6)
0.064
Yes
41 (16.9)
329 (16.5)
125 (20.6)
495 (17.4)
Hypertension (%)
No
203 (83.9)
1448 (72.6)
344 (56.7)
1995 (70.1)
<0.001
Yes
39 (16.1)
547 (27.4)
263 (43.3)
849 (29.9)
Coronary (%)
No
237 (97.9)
1860 (93.2)
544 (89.6)
2641 (92.9)
<0.001
Yes
5 (2.1)
135 (6.8)
63 (10.4)
203 (7.1)
Diabetes (%)
No
222 (91.7)
1733 (86.9)
500 (82.4)
2455 (86.3)
0.001
Yes
20 (8.3)
262 (13.1)
107 (17.6)
389 (13.7)
COPD (%)
No
241 (99.6)
1977 (99.1)
593 (97.7)
2811 (98.8)
0.01
Yes
1 (0.4)
18 (0.9)
14 (2.3)
33 (1.2)
Malignancy (%)
No
238 (98.3)
1950 (97.7)
579 (95.4)
2767 (97.3)
0.004
Yes
4 (1.7)
45 (2.3)
28 (4.6)
77 (2.7)
CKD (%)
No
239 (98.8)
1989 (99.7)
599 (98.7)
2827 (99.4)
0.007
Yes
3 (1.2)
6 (0.3)
8 (1.3)
17 (0.6)
Cerebrovascular Disease (%)
No
237 (97.9)
1954 (97.9)
555 (91.4)
2746 (96.6)
<0.001
Yes
5 (2.1)
41 (2.1)
52 (8.6)
98 (3.4)
Immunodeficiency Disease (%)
No
242 (100.0)
1995 (100.0)
607 (100.0)
2844 (100.0)
NA
Hepatitis (%)
No
234 (96.7)
1963 (98.4)
592 (97.5)
2789 (98.1)
0.107
Yes
8 (3.3)
32 (1.6)
15 (2.5)
55 (1.9)
Tuberculosis (%)
No
238 (98.3)
1961 (98.3)
592 (97.5)
2791 (98.1)
0.458
Yes
4 (1.7)
34 (1.7)
15 (2.5)
53 (1.9)
Trauma History (%)
No
241 (99.6)
1953 (97.9)
583 (96.0)
2777 (97.6)
0.004
Yes
1 (0.4)
42 (2.1)
24 (4.0)
67 (2.4)
Cardiovascular (%)
No
194 (80.2)
1363 (68.3)
305 (50.2)
1862 (65.5)
<0.001
Yes
48 (19.8)
632 (31.7)
302 (49.8)
982 (34.5)
Table 1: Statistics on baseline characteristics, treatment, and comorbidities among patients with different severities and overall. BMI: Body Mass Index; BSA: Body Surface Area; ECMO: Extracorporeal Membrane Oxygenation; CRRT: Continuous Renal Replacement Therapy; COPD: Chronic Obstructive Pulmonary Diseases; CKD: Chronic Kidney Disease.
beta
HR (95% CI for HR)
wald.test
p.value
Ventilator
3.86
47.6 (29-77)
243
9.82E-55
Intubate
2.99
19.9 (15-27)
399
1.07E-88
Oxygen Therapy
17.2
28100000 (0-Inf)
0
0.99
Hemodialysis
2.28
9.75 (7.1-13)
199
4.23E-45
ECMO
1.23
3.43 (1.6-7.4)
9.69
0.00186
CRRT
2.4
11 (8-15)
224
1.17E-50
Gamma Globulin Therapy
1.15
3.17 (2.4-4.2)
60.3
8.15E-15
TCM
-1.66
0.191 (0.14-0.25)
130
3.4E-30
Hormone Therapy
2.07
7.95 (5-13)
76.7
2.01E-18
Immunotherapy
-0.121
0.886 (0.62-1.3)
0.45
0.5
Antiviral Therapy
-1.1
0.331 (0.19-0.58)
14.7
0.000128
Arbidol
-0.79
0.454 (0.34-0.6)
31.2
2.39E-08
Ganciclovir
0.42
1.52 (0.99-2.4)
3.58
0.0584
Oseltamivir
-0.502
0.605 (0.31-1.2)
2.16
0.141
Interferon
0.14
1.15 (0.63-2.1)
0.2
0.653
Kaletra
-0.421
0.656 (0.39-1.1)
2.45
0.117
Antibacterial Therapy
2.18
8.81 (3.6-21)
23
1.62E-06
Smoking
-0.856
0.425 (0.059-3.1)
0.72
0.395
Past Disease
0.361
1.43 (1.1-1.9)
5.78
0.0162
Infectious Disease
0.833
2.3 (1.3-4)
8.41
0.00374
Allergic History
-0.518
0.596 (0.32-1.1)
2.55
0.11
Blood Transfusion History
1.2
3.3 (1.2-8.9)
5.6
0.018
Past Surgery
0.113
1.12 (0.79-1.6)
0.41
0.522
Hypertension
0.178
1.2 (0.9-1.6)
1.48
0.224
Coronary
0.609
1.84 (1.2-2.8)
8.38
0.00379
Diabetes
0.108
1.11 (0.77-1.6)
0.32
0.569
COPD
0.249
1.28 (0.47-3.5)
0.24
0.624
Malignancy
0.743
2.1 (1.2-3.7)
6.66
0.00988
CKD
1.38
3.97 (1.5-11)
7.42
0.00643
Cerebrovascular Disease
0.754
2.13 (1.3-3.5)
8.77
0.00306
Hepatitis
0.551
1.73 (0.77-3.9)
1.76
0.184
Tuberculosis
0.562
1.75 (0.78-4)
1.84
0.175
Trauma History
-0.0835
0.92 (0.38-2.2)
0.03
0.854
Cardiovascular
0.335
1.4 (1.1-1.8)
5.57
0.0183
Table 2: Univariate Cox regression of baseline characteristics, treatment, and comorbidities.
z
p.value
Ventilator
20.213 [11.273, 36.244]
3.006
0.298
10.091
6.08E-24
Intubate
3.267 [2.269, 4.705]
1.184
0.186
6.363
1.98E-10
Hemodialysis
1.511 [0.888, 2.569]
0.413
0.271
1.524
0.127617
ECMO
0.660 [0.296, 1.471]
-0.415
0.409
-1.016
0.309833
CRRT
0.791 [0.465, 1.346]
-0.234
0.271
-0.864
0.387582
Gamma Globulin Therapy
0.725 [0.526, 1.000]
-0.322
0.164
-1.962
0.049746
TCM
0.306 [0.225, 0.414]
-1.186
0.155
-7.633
2.3E-14
Hormone Therapy
1.202 [0.702, 2.060]
0.184
0.275
0.67
0.502639
Antiviral Therapy
0.562 [0.302, 1.045]
-0.576
0.317
-1.821
0.068573
Arbidol
0.794 [0.590, 1.068]
-0.231
0.151
-1.524
0.127407
Antibacterial Therapy
1.438 [0.557, 3.708]
0.363
0.483
0.751
0.452802
Infectious Disease
2.264 [1.260, 4.069]
0.817
0.299
2.732
0.006303
Coronary
1.186 [0.773, 1.822]
0.171
0.219
0.782
0.434423
Malignancy
1.305 [0.722, 2.357]
0.266
0.302
0.882
0.377638
CKD
0.816 [0.293, 2.268]
-0.204
0.522
-0.39
0.696198
Cerebrovascular Disease
0.929 [0.534, 1.616]
-0.074
0.283
-0.262
0.793591
Table 3: Multivariate Cox regression analysis of baseline characteristics, treatment, and comorbidities. exp(coef): the exponentiated coefficients; coef:the regression coefficients; se(coef): standard error of the regression coefficient; z: coef/se(coef).
Moderate
Severe
Critical
Overall
p
n
242
1995
607
2844
White Blood Cell Count (WBC#)
5.67 [4.82, 6.86]
8.04 [6.00, 10.97]
5.96 [4.95, 7.49]
<0.001
Red Blood Cell Count (RBC#)
4.28 [3.98, 4.72]
4.08 [3.75, 4.45]
3.74 [3.33, 4.14]
4.04 [3.68, 4.42]
<0.001
Mean Corpuscular Volume (MCV)
89.24 [86.59, 91.71]
90.00 [87.35, 92.66]
90.94 [87.90, 93.92]
90.13 [87.37, 92.84]
<0.001
Mean Corpuscular Hemoglobin Concentration (MCHC)
342.00 [336.00, 348.00]
342.00 [335.33, 349.00]
339.73 [331.47, 346.62]
341.33 [334.84, 348.33]
<0.001
Mean Corpuscular Hemoglobin (MCH)
30.60 [29.60, 31.50]
30.87 [29.80, 31.82]
30.85 [29.80, 31.92]
30.83 [29.78, 31.80]
0.021
Red Cell Volume Distribution Width-Coefficient of Variation (RDW-CV)
12.72 [12.13, 13.24]
12.63 [12.10, 13.22]
13.46 [12.70, 14.54]
12.78 [12.20, 13.50]
<0.001
Red Cell Volume Distribution Width-Standard Deviation (RDW-SD)
41.30 [38.92, 43.51]
41.15 [38.98, 43.55]
44.05 [41.29, 48.02]
41.63 [39.30, 44.30]
<0.001
Lymphocyte Percentage (Lymph%)
31.30 [25.87, 36.21]
26.88 [21.28, 32.50]
15.46 [7.41, 21.17]
25.30 [18.71, 31.35]
<0.001
Lymph#
1.74 [1.35, 2.11]
1.49 [1.18, 1.81]
0.99 [0.68, 1.33]
1.41 [1.08, 1.78]
<0.001
Monocyte Percentage (Mono%)
8.52 [7.44, 9.66]
8.90 [7.63, 10.37]
7.39 [5.14, 9.06]
8.64 [7.25, 10.10]
<0.001
Monocyte Count (Mono#)
0.48 [0.41, 0.58]
0.50 [0.41, 0.61]
0.52 [0.40, 0.68]
0.50 [0.41, 0.62]
0.004
Neutrophil Percentage (Neut%)
56.32 [51.56, 62.51]
61.21 [54.97, 67.30]
74.98 [67.39, 86.80]
63.03 [56.00, 70.50]
<0.001
Neutrophil Count (Neut#)
3.27 [2.59, 4.03]
3.46 [2.74, 4.47]
5.99 [4.20, 9.06]
3.73 [2.86, 5.12]
<0.001
Hematocrit (Hct)
38.10 [35.88, 41.70]
36.75 [34.05, 39.42]
33.82 [30.33, 37.13]
36.40 [33.43, 39.22]
<0.001
Eos%
2.30 [1.40, 3.21]
1.77 [1.10, 2.70]
1.06 [0.35, 2.18]
1.70 [0.95, 2.65]
<0.001
Baso%
0.49 [0.30, 0.60]
0.40 [0.28, 0.58]
0.25 [0.16, 0.40]
0.40 [0.24, 0.55]
<0.001
Eos#
0.13 [0.08, 0.19]
0.10 [0.06, 0.15]
0.08 [0.03, 0.14]
0.10 [0.05, 0.15]
<0.001
Baso#
0.03 [0.02, 0.04]
0.02 [0.01, 0.03]
0.02 [0.01, 0.03]
0.02 [0.01, 0.03]
<0.001
Hemoglobin (Hb)
131.50 [122.00, 143.00]
125.50 [115.67, 135.50]
114.87 [102.16, 126.27]
124.00 [113.33, 134.75]
<0.001
Platelet Count (PLT#)
227.00 [199.00, 264.17]
231.38 [192.00, 277.20]
196.47 [135.96, 254.97]
224.83 [183.50, 273.00]
<0.001
MPV
10.40 [9.90, 11.00]
10.50 [9.97, 11.10]
11.06 [10.35, 11.80]
10.60 [10.00, 11.23]
<0.001
Platelet Distribution Width (PDW)
12.00 [10.96, 13.30]
11.93 [10.81, 13.34]
13.03 [11.43, 15.00]
12.13 [10.95, 13.58]
<0.001
Thrombocytocrit
0.24 [0.21, 0.27]
0.24 [0.20, 0.29]
0.22 [0.16, 0.27]
0.24 [0.20, 0.28]
<0.001
Platelet Large Cell Ratio (P-LCR%)
28.02 [23.89, 33.21]
28.52 [24.17, 33.60]
33.08 [27.27, 38.88]
29.38 [24.55, 34.64]
<0.001
Alanine Aminotransferase (ALT)
21.17 [13.88, 36.00]
23.63 [15.24, 37.00]
27.55 [18.22, 42.69]
24.00 [15.67, 38.00]
<0.001
Aspartate Aminotransferase (AST)
20.00 [16.00, 26.50]
22.00 [17.33, 28.85]
29.50 [22.14, 41.90]
23.00 [18.00, 31.20]
<0.001
Gamma-Glutamyltransferase (GGT)
24.58 [16.00, 41.87]
28.33 [18.00, 47.75]
40.75 [25.50, 66.93]
30.00 [19.00, 51.00]
<0.001
Total Bilirubin(TBil)
8.80 [6.93, 11.57]
8.50 [6.68, 11.00]
10.80 [8.02, 15.05]
8.84 [6.90, 11.77]
<0.001
Direct Bilirubin (DBIL)
3.30 [2.71, 4.10]
3.46 [2.80, 4.44]
5.07 [3.60, 7.29]
3.63 [2.90, 4.90]
<0.001
Indirect Bilirubin (IBIL)
5.57 [4.24, 7.40]
4.97 [3.80, 6.60]
5.44 [4.22, 7.56]
5.13 [3.90, 6.82]
<0.001
Albumin (ALB)
41.80 [40.00, 43.80]
37.64 [34.70, 40.65]
33.62 [30.84, 35.80]
37.10 [33.93, 40.50]
<0.001
Globulin (GLO)
27.92 [26.15, 30.31]
30.00 [27.27, 32.92]
32.14 [28.40, 35.72]
30.13 [27.27, 33.40]
<0.001
Total Protein (TP)
69.78 [67.46, 72.93]
68.10 [65.15, 70.95]
65.67 [61.62, 69.26]
67.80 [64.63, 70.85]
<0.001
ALB/GLO
1.49 [1.36, 1.66]
1.26 [1.09, 1.46]
1.07 [0.91, 1.24]
1.25 [1.05, 1.45]
<0.001
Creatinine (Crea)
67.67 [58.00, 77.00]
67.50 [57.00, 79.58]
72.88 [57.67, 93.89]
68.33 [57.00, 82.00]
<0.001
Urea
4.50 [3.90, 5.60]
4.35 [3.60, 5.19]
6.20 [4.70, 9.83]
4.60 [3.78, 5.70]
<0.001
Uric Acid (UA)
321.00 [256.00, 387.15]
271.33 [221.60, 330.42]
233.79 [173.73, 310.33]
268.17 [214.44, 333.35]
<0.001
Total Cholesterol (TC)
4.44 [3.81, 5.07]
4.13 [3.60, 4.71]
3.57 [2.93, 4.31]
4.05 [3.47, 4.68]
<0.001
Potassium Ions (K+)
4.22 [4.07, 4.47]
4.25 [4.00, 4.48]
4.33 [4.06, 4.66]
4.26 [4.02, 4.50]
<0.001
Sodion (Na+)
140.80 [139.70, 141.80]
140.17 [138.75, 141.50]
139.49 [137.40, 141.57]
140.13 [138.60, 141.53]
<0.001
Chloridion (Cl-)
102.20 [100.90, 103.50]
101.72 [100.10, 103.22]
100.38 [98.04, 102.99]
101.60 [99.72, 103.22]
<0.001
Ca
2.27 [2.20, 2.33]
2.19 [2.13, 2.24]
2.13 [2.06, 2.19]
2.18 [2.12, 2.25]
<0.001
Glucose (Glu)
5.09 [4.75, 5.50]
5.54 [5.03, 6.54]
6.94 [5.87, 9.08]
5.69 [5.07, 6.99]
<0.001
Lactate Dehydrogenase (LDH)
182.00 [162.00, 201.50]
206.00 [178.00, 241.25]
290.20 [234.50, 429.20]
213.85 [181.68, 260.83]
<0.001
Alkaline Phosphatase (ALP)
63.42 [53.50, 76.00]
65.75 [55.40, 78.50]
76.80 [61.36, 99.83]
67.00 [56.40, 81.94]
<0.001
Estimation of Glomerular Filtration Rate (CKD-EPI formula)
98.65 [90.65, 108.77]
93.79 [82.80, 103.90]
86.18 [67.35, 97.70]
93.30 [80.76, 103.50]
<0.001
Thrombin Time (TT)
16.10 [15.40, 16.60]
16.50 [15.90, 17.18]
16.51 [15.76, 17.80]
16.45 [15.80, 17.20]
<0.001
Prothrombin Time (PT)
13.30 [13.00, 13.70]
13.53 [13.10, 14.00]
14.37 [13.67, 15.62]
13.63 [13.20, 14.20]
<0.001
Activated Partial Thromboplastin Time (APTT)
38.10 [35.90, 40.70]
38.20 [35.74, 41.30]
40.10 [36.79, 44.53]
38.50 [35.90, 41.80]
<0.001
PT-INR
1.01 [0.98, 1.05]
1.03 [1.00, 1.08]
1.12 [1.04, 1.24]
1.04 [1.00, 1.10]
<0.001
D-Dimer
0.26 [0.22, 0.39]
0.55 [0.30, 1.11]
2.27 [1.05, 4.27]
0.66 [0.32, 1.59]
<0.001
Fibrinogen (Fbg)
3.10 [2.74, 3.64]
4.06 [3.34, 4.89]
4.32 [3.58, 5.22]
4.03 [3.28, 4.90]
<0.001
Prothrombin Time Activity (PTA)
98.00 [92.75, 103.42]
95.00 [89.00, 101.00]
84.67 [73.00, 93.71]
93.67 [86.33, 100.00]
<0.001
Partial Pressure of Oxygen (PaO2)
110.00 [92.60, 137.00]
116.00 [96.07, 149.33]
102.33 [77.82, 139.07]
112.00 [90.40, 144.00]
<0.001
Oxygen Saturation (SaO2)
98.20 [97.30, 99.00]
98.30 [97.10, 99.10]
96.58 [92.73, 98.60]
97.90 [95.96, 99.00]
<0.001
Partial Pressure of Carbon Dioxide (PaCO2)
42.50 [40.20, 44.80]
41.20 [37.85, 43.90]
38.45 [34.31, 43.61]
40.70 [36.60, 43.80]
<0.001
Actual Bicarbonate (AB)
24.70 [23.20, 25.90]
24.50 [22.92, 25.81]
24.60 [21.90, 26.86]
24.50 [22.70, 26.02]
0.572
Standard Bicarbonate (SB)
24.30 [23.55, 25.10]
24.40 [23.20, 25.40]
24.82 [22.80, 26.75]
24.50 [23.20, 25.86]
0.107
Base Excess (BE)
-0.10 [-1.05, 0.80]
-0.10 [-1.40, 1.10]
0.55 [-1.90, 2.60]
0.10 [-1.50, 1.70]
0.067
Standard Base Surplus (SBE)
0.10 [-0.90, 1.20]
0.10 [-1.40, 1.38]
0.40 [-2.22, 2.60]
0.20 [-1.50, 1.80]
0.317
Blood Carbon Dioxide Content
21.00 [19.90, 21.60]
21.50 [20.30, 23.00]
21.90 [19.40, 23.90]
21.60 [20.00, 23.35]
0.258
Calcium Ion -PH Correction
2.33 [2.19, 2.42]
2.30 [2.19, 2.41]
2.35 [2.23, 2.44]
2.32 [2.20, 2.42]
<0.001
Interleukin-6(IL-6)
2.79 [1.89, 5.26]
5.18 [2.79, 12.50]
24.90 [9.50, 76.53]
6.64 [3.10, 20.44]
<0.001
Interleukin-10 (IL-10)
6.45 [6.12, 7.90]
7.20 [5.90, 9.78]
10.60 [7.66, 18.30]
8.30 [6.30, 12.96]
<0.001
Interleukin-8 (IL-8)
9.00 [7.05, 13.12]
11.00 [7.90, 17.52]
19.60 [12.21, 40.29]
12.28 [8.30, 21.30]
<0.001
Tumor Necrosis Factor-a (TNF-a)
7.30 [6.07, 8.80]
7.80 [6.30, 9.90]
10.59 [8.00, 15.48]
8.25 [6.58, 10.60]
<0.001
Interleukin-1β (IL-1β)
9.75 [7.20, 15.15]
8.70 [6.40, 12.10]
9.11 [6.80, 15.41]
8.80 [6.60, 13.20]
0.074
Interleukin-2 Receptor (IL-2R)
293.25 [234.25, 405.00]
441.50 [310.00, 623.50]
780.61 [519.26, 1179.76]
473.50 [317.00, 705.47]
<0.001
Ferritin (Ferr)
240.30 [121.80, 394.30]
428.80 [232.40, 682.65]
908.59 [496.69, 1620.17]
484.30 [266.97, 864.76]
<0.001
Erythrocyte Sedimentation Rate (ESR)
9.00 [5.50, 17.25]
25.00 [12.00, 46.00]
37.00 [21.25, 60.00]
26.50 [13.00, 49.90]
<0.001
Hypersensitive C-reactive Protein (HS-CRP)
1.23 [0.60, 3.32]
5.87 [1.48, 20.08]
38.49 [16.14, 83.70]
7.91 [1.67, 28.37]
<0.001
Procalcitonin (PCT)
0.05 [0.04, 0.06]
0.06 [0.04, 0.08]
0.15 [0.07, 0.61]
0.06 [0.04, 0.10]
<0.001
N-Terminal Pro-Brain Natriuretic Peptide (NT-ProBNP)
37.50 [18.25, 69.00]
78.25 [34.00, 180.00]
552.12 [187.52, 1923.39]
101.50 [41.00, 312.25]
<0.001
Myoglobin (Mb)
31.20 [26.45, 40.65]
32.30 [25.08, 44.75]
70.00 [38.17, 165.09]
35.03 [26.20, 56.80]
<0.001
Creatine Kinase (CK)
66.25 [48.56, 90.88]
52.00 [37.00, 74.75]
59.92 [36.38, 126.54]
54.63 [37.60, 83.00]
<0.001
Creatine Kinase Isoenzyme MB (CK-MB)
0.60 [0.40, 0.82]
0.60 [0.40, 0.90]
1.15 [0.63, 2.53]
0.67 [0.40, 1.10]
<0.001
Hypersensitive Cardiac Troponin I (HS-CTNI)
3.15 [2.40, 4.65]
4.60 [2.90, 8.50]
15.56 [5.61, 71.00]
5.65 [3.20, 12.90]
<0.001
Table 4: Statistics on laboratory indices among patients with different severities and overall.
beta
HR (95% CI for HR)
wald.test
p.value
WBC#
0.29
1.34 (1.3-1.4)
679
<0.001
RBC#
0.000851
1 (1-1)
3.43
0.0641
MCV
0.0679
1.07 (1-1.1)
19.6
<0.001
MCHC
-0.0152
0.985 (0.97-1)
6.85
0.00889
MCH
0.0592
1.06 (1-1.1)
3.34
0.0677
RDW-CV
0.198
1.22 (1.2-1.3)
99.2
<0.001
RDW-SD
0.0917
1.1 (1.1-1.1)
160
<0.001
Lymph%
-0.279
0.756 (0.74-0.78)
391
<0.001
Lymph#
-3.97
0.0188 (0.013-0.028)
384
<0.001
Mono%
-0.671
0.511 (0.48-0.54)
511
<0.001
Mono#
0.00404
1 (1-1)
3.65
0.056
Neut%
0.195
1.22 (1.2-1.2)
466
<0.001
Neut#
0.000191
1 (1-1)
4.5
0.0338
Hct
-0.0989
0.906 (0.88-0.93)
44.3
<0.001
Eos%
-2.89
0.0553 (0.039-0.079)
245
<0.001
Baso%
-9.33
8.91e-05 (2.6e-05-0.00031)
218
<0.001
Eos#
-28.5
4.2e-13 (5.8e-15-3.1e-11)
170
<0.001
Baso#
-26.5
3.01e-12 (1.8e-17-5e-07)
18.7
<0.001
Hb
-0.0284
0.972 (0.96-0.98)
48.6
<0.001
PLT#
-0.0205
0.98 (0.98-0.98)
370
<0.001
MPV
1.09
2.97 (2.6-3.4)
247
<0.001
PDW
0.378
1.46 (1.4-1.5)
283
<0.001
thrombocytocrit
-18.4
1.06e-08 (1.3e-09-8.7e-08)
294
<0.001
P-LCR%
0.137
1.15 (1.1-1.2)
229
<0.001
ALT
0.00245
1 (1-1)
36
<0.001
AST
0.00416
1 (1-1)
149
<0.001
GGT
0.00434
1 (1-1)
20.5
<0.001
TBil
0.0148
1.01 (1-1)
203
<0.001
DBIL
0.021
1.02 (1-1)
218
<0.001
IBIL
0.0286
1.03 (1-1)
98.2
<0.001
ALB
-0.351
0.704 (0.68-0.73)
518
<0.001
GLO
0.0849
1.09 (1.1-1.1)
38.5
<0.001
TP
-0.163
0.849 (0.83-0.87)
171
<0.001
ALB/GLO
-4.75
0.00866 (0.0044-0.017)
186
<0.001
Crea
0.00239
1 (1-1)
110
<0.001
Urea
0.108
1.11 (1.1-1.1)
631
<0.001
UA
0.00146
1 (1-1)
3.65
0.0561
TC
-1.29
0.275 (0.23-0.32)
243
<0.001
K+
1.44
4.21 (3.2-5.5)
115
<0.001
Na+
0.15
1.16 (1.1-1.2)
129
<0.001
Cl-
0.119
1.13 (1.1-1.2)
57.8
<0.001
Ca
-7.05
0.000871 (0.00044-0.0017)
417
<0.001
Glu
0.182
1.2 (1.2-1.2)
319
<0.001
LDH
0.00494
1 (1-1)
856
<0.001
ALP
0.00486
1 (1-1)
99
<0.001
CKD-EPI formula
-0.0361
0.965 (0.96-0.97)
195
<0.001
TT
0.0452
1.05 (1-1.1)
56.6
<0.001
PT
0.17
1.19 (1.2-1.2)
505
<0.001
APTT
0.112
1.12 (1.1-1.1)
191
<0.001
PT-INR
1.31
3.69 (3.3-4.2)
433
<0.001
D-Dimer
0.21
1.23 (1.2-1.3)
422
<0.001
Fbg
0.0398
1.04 (0.92-1.2)
0.38
0.539
PTA
-0.0874
0.916 (0.91-0.92)
717
<0.001
PaO2
-0.0282
0.972 (0.97-0.98)
61.1
<0.001
SaO2
-0.108
0.897 (0.88-0.91)
147
<0.001
PaCO2
-0.0153
0.985 (0.96-1)
0.96
0.327
AB
-0.143
0.867 (0.82-0.92)
25
<0.001
SB
-0.189
0.828 (0.78-0.88)
35.3
<0.001
BE
-0.152
0.859 (0.82-0.9)
42.5
<0.001
SBE
-0.148
0.862 (0.82-0.91)
35.7
<0.001
Blood carbon dioxide content
-0.127
0.88 (0.83-0.94)
15.7
<0.001
Calcium ion -PH correction
0.959
2.61 (0.9-7.6)
3.13
0.0769
IL-6
0.00279
1 (1-1)
302
<0.001
IL-10
0.00571
1.01 (1-1)
48.8
<0.001
IL-8
0.00132
1 (1-1)
88.8
<0.001
TNF-a
0.0454
1.05 (1-1.1)
232
<0.001
IL-1β
0.0179
1.02 (1-1)
14.5
<0.001
IL-2R
0.000974
1 (1-1)
318
<0.001
Ferr
0.000183
1 (1-1)
148
<0.001
ESR
0.00563
1.01 (1-1)
3.27
0.0704
HS-CRP
0.0208
1.02 (1-1)
823
<0.001
PCT
0.147
1.16 (1.1-1.2)
207
<0.001
NT-ProBNP
9.06E-05
1 (1-1)
252
<0.001
Mb
0.00532
1.01 (1-1)
461
<0.001
CK
0.000887
1 (1-1)
116
<0.001
CK-MB
0.0348
1.04 (1-1)
147
<0.001
HS-CTNI
0.000605
1 (1-1)
179
<0.001
Table 5: Univariate Cox regression of laboratory indices.
We applied the Analysis of Variance or Kruskal-Wallis rank-sum (variables with non-normal distribution) for continuous variables between groups with different disease severity. The chi-square test was performed to compare count data. There were significant differences (p < 0.01) in the proportion of patients with previous disease history in different disease severity. Patients in COVID-19 with comorbidities of hypertension, coronary, diabetes, chronic obstructive pulmonary diseases, malignancy, cerebrovascular disease, trauma history, or cardiovascular were more likely to be critically ill, associated with poorer outcomes in COVID-19 patients. Diabetes (13.7% overall) is associated with immunological dysregulation, which is potentially equivalent to accelerated ageing, and could therefore potentially explain the poor prognosis in patients with diabetes mellitus and COVID-19. Preexisting cardiovascular diseases is an essential factor for myocardial injury as approximately 30% and 60% of patients with cardiac injury have coronary heart disease and hypertension previously [3,4]. Patients with underlying cardiovascular disease, including hypertension, coronary heart disease, and cardiomyopathy are more likely to develop more severe adverse outcomes when myocardial injury occurs after COVID-19 infection and face a higher risk of death [24].
It is known that there is no effective therapeutics against COVID-19, and it will be informative to compare the available supportive treatment in the reduction of mortality and hospitalization time. We use Kaplan-Meier plots to visualize and Log-rank test to compare the impact on mortality by Arbidol, Ganciclovir, Oseltamivir, Interferon, Kaletra, antimicrobial therapy, and TCM. There were significant differences (p < 0.0001) in patients survival rate treated with or without Abidiol, antimicrobial, or TCM. Patients who had been treated with either Arbidol or TCM had improved survival likelihood, whereas antimicrobial therapy had reduced survival rate. The phenomenon about patients who receive antibiotic therapy shows bacterial infection symptoms reacts SARS-CoV-2 infection weaken the immune system that increases the risk of bacterial infection. However, the current antibiotics are not effective for the inhibition of bacterial infection.
A separate univariate Cox regression evaluated each factor to show the statistical significance of each variable with overall survival. Univariate Cox regression yielded similar results as survival analysis. Besides, we found differences in the characteristics of the patients’ ventricle, intubate, hemodynamics, ECMO, CRRT, gamma globulin therapy, hormone therapy, infectious disease, coronary, malignant, CKD, or cerebrovascular disease had a significant impact (p < 0.01) on survival. Treatment with TCM (HR 0.191 [95% CI 0.14 – 0.25]; p < 0.0001), antiviral therapy (HR 0.331 [95% CI 0.19 – 0.58]; p =0.000128), or Arbidol (HR 0.454 [95% CI 0.34 – 0.60]; p < 0.0001) is associated with good prognostic of patients, and others were associated with poor outcome or a higher risk of death.
Besides, we used multivariate Cox regression analysis to describe how these factors work together to influence survival. Multivariate Cox regression analysis showed that treatment with TCM decreased the mortality hazard ratio by 69.4% (p<0.0001), while supportive treatment ventilator or intubate use was statistically associated with a higher risk of mortality due to COVID-19.
We constructed new data frames with two rows according to TCM treatment or not, and other covariates were fixed as used (not used). The resulting survival curve again indicates a strong relationship between TCM therapy and decreased risk of death.
A total of 77 indicators derived from laboratory tests including routine blood tests, biochemistry, coagulation, blood gas, cytokines, ferritin, erythrocyte sedimentation rate, hypersensitive C-reactive protein, and procalcitonin were included in this study. Except for Mean Corpuscular Hemoglobin (MCH), Monocyte count (Mono#), Actual Bicarbonate (AB), Standard Bicarbonate (SB), Base Excess (BE), Standard Base Surplus (SBE), Blood carbon dioxide content, and Interleukin-1β(IL-1β), the other 69 indices were significantly different(p<0.001) between patients with varying severities of disease.
By univariate Cox regression, several indexes including RBC#, MCH, Mono#, Neut#, UA, Fbg, PaCO2, Calcium ion -PH correction, and ESR showed lower statistical significance (p > 0.01). Larger MPV, PT-INR, and K+ are associated with lower survival, whereas larger Eos#, Baso#, Baso%, Ca, ALB/GLO, Lymph#, and Eos% are associated with better survival.
Discussion
Aging is associated with endothelial dysfunction, contributing to vascular disease and cardiovascular disease in the elderly [22]. Sex differences in immune response are the basis of COVID-19 disease outcomes [23]. High physiological concentrations of the steroid hormones 17β-estradiol and progesterone are powerful immune modulators [25]. The combination of 17β-estradiol and progesterone is a potential therapeutic approach. Diabetes is associated with immune disorders, which may equate to accelerated aging. Patients with underlying cardiovascular disease, including hypertension, coronary heart disease, and cardiomyopathy are more likely to develop more severe adverse outcomes when myocardial injury occurs after COVID-19 infection and face a higher death risk [24]. Consider prioritizing and more aggressive treatment for COVID-19 patients based on the presence of underlying cardiovascular disease.
The P value of antiviral therapy was not less than 0.01 in multivariate COX analysis results, and reached significant level in univariate COX, which may be affected by the difference of multiple antiviral drugs. Kaplan-Meier plots showed a significant improvement in the likelihood of survival of patients treated with Arbidol. Arbidol showed beneficial effects on fever recovery, viral clearance and shorter hospital stay in these patients, especially in males [26]. Arbidol monotherapy is more effective than lopinavir/ritonavir in treating COVID-19 [27]. Arbidol significantly contributed to clinical and laboratory improvement compared to Kaletra in a recent randomized controlled trial (IRCT 20180725040596N2) [28].
We used the Kaplan-Mayer method and Cox regression analysis to show the positive effect of TCM treatment on the patients’ prognosis. This indication may increase the testing of the efficacy of TCM in clinical trials. Early combination of Lianhua qingwen and Arbidol significantly accelerated recovery in patients with moderate COVID-19, but not in patients with severe COVID-19 [29]. SARSCoV- 2 infection weakens the immune system and increases the risk of bacterial infections. Patients treated with antibiotics showed a symbiosis of bacterial infections. However, current antibiotics are ineffective at suppressing bacterial infections, which may be influenced by the very high rate (74.5%) the use of antimicrobial therapy in critically ill patients [30]. For example, prescription drugs are significantly higher than the estimated prevalence of mixed bacterial infections; unnecessary antibiotic use may be high in patients with COVID-19; and antimicrobial resistance may be associated with harm to patients [31]. All these results indicate a more rational use of antibiotic drugs.
We found that larger MPV, PT-INR, and the higher K+ concentration were associated with lower survival, while larger EOS#, BASO#, BASO%, Ca, ALB/GLO, Lymph#, and EOS% were associated with better survival, indicating greater attention to these physiological parameters during the patient’s disease course.
To sum up, from this perspective studies, the doctors need to increase attention to elderly patients and patients with comorbidities, expand the use of TCM and rationalize the use of antimicrobial drugs in clinical practice, and pay attention to the changes of physiological parameters such as MPV, PT-INR, K+, EOS#, BASO#, BASO%, Ca, ALB/GLO, Lymph#, and EOS%.
Funding
Science, Technology, Innovation Commission of Shenzhen Municipality JCYJ20190809180003689, JSGG20200225150707332, JSGG20191129110812708, WDZC20200820173710001, Shenzhen Bay Laboratory Open Funding, SZBL2020090501004, China Postdoctoral Science Foundation 2020M680023, General Administration of Customs of the People’s Republic of China 2021HK007.
References
- Naja M, Wedderburn L, Ciurtin C. COVID-19 infection in children and adolescents. British journal of hospital medicine. 2020; 81: 1-10.
- Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet (London, England). 2020; 395: 507-513.
- Chu CM, Cheng VCC, Hung IFN, Wong MML, Chan KH, Chan KS, et al. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004; 59: 252-256.
- Yang C, Ke C, Yue D, Li W, Hu Z, Liu W, et al. Effectiveness of Arbidol for COVID-19 Prevention in Health Professionals. Frontiers in Public Health. 2020; 8.
- Vankadari N. Arbidol: A potential antiviral drug for the treatment of SARSCoV- 2 by blocking trimerization of the spike glycoprotein. International Journal of Antimicrobial Agents. 2020; 56: 105998 - 105998.
- Padhi AK, Seal A, Khan JM, Ahamed M, Tripathi T. Unraveling the mechanism of arbidol binding and inhibition of SARS-CoV-2: Insights from atomistic simulations. European Journal of Pharmacology. 2020; 894: 173836.
- Blaising J, Polyak SJ, Pécheur E. Arbidol as a broad-spectrum antiviral: An update. Antiviral Research. 2014; 107: 84-94.
- Wolfson JS, Hooper DC. The fluoroquinolones: structures, mechanisms of action and resistance, and spectra of activity in vitro. Antimicrobial Agents and Chemotherapy. 1985; 28: 581-586.
- Correia S, Poeta P, Hébraud M, Capelo JL, Igrejas G. Mechanisms of quinolone action and resistance: where do we stand?. Journal of medical microbiology. 2017; 66: 551-559.
- Marciniec, K., et al., Ciprofloxacin and moxifloxacin could interact with SARSCoV- 2 protease: preliminary in silico analysis. Pharmacol Rep, 2020. 72(6): p. 1553-1561.
- Scroggs SLP, Offerdahl DK, Flather DP, Morris CN, Kendall BL, Broeckel RM, et al. Fluoroquinolone Antibiotics Exhibit Low Antiviral Activity against SARS-CoV-2 and MERS-CoV. Viruses. 2020; 13: 8.
- Schögler A, Kopf BS, Edwards MR, Johnston SL, Casaulta C, Kieninger E, et al. Novel antiviral properties of azithromycin in cystic fibrosis airway epithelial cells. European Respiratory Journal. 2015; 45: 428-439.
- Gielen V, Johnston SL, Edwards MR. Azithromycin induces anti-viral responses in bronchial epithelial cells. European Respiratory Journal. 2010; 36: 646-654.
- Wang C, Cao B, Liu Q, Zou Z, Liang Z, Gu L, et al. Oseltamivir Compared With the Chinese Traditional Therapy Maxingshigan–Yinqiaosan in the Treatment of H1N1 Influenza. Annals of Internal Medicine. 2011; 155: 217.
- Zhou Z, Li X, Liu J, Dong L, Chen Q, Liu J, et al. Honeysuckle-encoded atypical microRNA2911 directly targets influenza A viruses. Cell Research. 2015; 25: 39-49.
- Zhang D, Zhang B, Lv J, Sa R, Zhang X, Lin Z. The clinical benefits of Chinese patent medicines against COVID-19 based on current evidence. Pharmacological Research. 2020; 157: 104882.
- Akalin E, Ekici M, Alan Z, Elevli E, Bucak AY, Aobuliaikemu N, et al. Traditional Chinese medicine practices used in COVID-19 (Sars-cov 2/ Coronavirus-19) treatment in clinic and their effects on the cardiovascular system. Turk Kardiyoloji Dernegi arsivi : Turk Kardiyoloji Derneginin yayin organidir. 2020; 48: 410-424.
- Nile SH, Kai G. Recent Clinical Trials on Natural Products and Traditional Chinese Medicine Combating the COVID-19. Indian Journal of Microbiology. 2020; 61: 10-15.
- Yang R, Liu H, Bai C, Wang Y, Zhang X, Guo R, et al. Chemical composition and pharmacological mechanism of Qingfei Paidu Decoction and Ma Xing Shi Gan Decoction against Coronavirus Disease 2019 (COVID-19): In silico and experimental study. Pharmacological Research. 2020; 157: 104820.
- Runfeng, L., et al., Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2). Pharmacol Res. 2020; 156: 104761.
- Shu Z, Zhou Y, Chang K, Liu J, Min X, Zhang Q, et al. Clinical features and the traditional Chinese medicine therapeutic characteristics of 293 COVID-19 inpatient cases. Frontiers of Medicine. 2020; 14: 760-775.
- Ungvari Z, Tarantini S, Kiss T, Wren JD, Giles CB, Griffin CT, et al. Endothelial dysfunction and angiogenesis impairment in the ageing vasculature. Nature Reviews Cardiology. 2018; 15: 555-565.
- Klein SL, Marriott I, Fish EN. Sex-based differences in immune function and responses to vaccination. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2015; 109: 9-15.
- Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, et al. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA Cardiology. 2020; 5: 811.
- Mauvais-Jarvis F, Klein SL, Levin ER. Estradiol, Progesterone, Immunomodulation, and COVID-19 Outcomes. Endocrinology. 2020; 161.
- Gao W, Chen S, Wang K, Chen R, Guo Q, Lu J, et al. Clinical Features and Efficacy of Antiviral Drug, Arbidol in 220 Nonemergency COVID-19 Patients from East-West-Lake Shelter Hospital in Wuhan: A Retrospective Case Series. Virology Journal. 2020.
- Zhu Z, Lu Z, Xu T, Chen C, Yang G, Zha T, et al. Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID-19. The Journal of Infection. 2020; 81: e21-e23.
- Nojomi M, Yassin Z, Keyvani H, Makiani MJ, Roham M, Laali A, et al. Effect of Arbidol (Umifenovir) on COVID-19: a randomized controlled trial. BMC Infectious Diseases. 2020; 20.
- Fang J, Li H, Du W, Yu P, Guan Y, Ma S, et al. Efficacy of Early Combination Therapy With Lianhua qingwen and Arbidol in Moderate and Severe COVID-19 Patients: A Retrospective Cohort Study. Frontiers in Pharmacology. 2020; 11.
- Langford BJ, So M, Raybardhan S, Leung V, Soucy JR, Westwood D, et al. Antibiotic prescribing in patients with COVID-19: rapid review and metaanalysis. Clinical Microbiology and Infection. 2021; 27: 520-531.
- Majumder MAA, Rahman S, Cohall D, Bharatha A, Singh K, Haque M, et al. Antimicrobial Stewardship: Fighting Antimicrobial Resistance and Protecting Global Public Health. Infection and Drug Resistance. 2020; 2020: 4713-4738.