
Review Article
Austin J Infect Dis. 2022; 9(3): 1074.
The Role of the NETosis Phenomena as a Function of Neutrophils in the Pathogenesis of Infection and Cancer
Glukhareva AE1*, Afonin GV1, Melnikova AA1, Grivtsova LY1, Kolobaev IV1, Ivanov SA1,3 and Kaprin AD2,3
1Tsyba Medical Radiological Research Centre – Branch of the National Medical Research Radiological Center, Russia
2National Medical Research Center for Radiology, Russia
3People’s Friendship University of Russia (RUDN University), Russia
*Corresponding author: Anastasia E Glukhareva, Clinical Resident, Tsyba Medical Radiological Research Centre, Branch of the National Medical Research Radiological Center, Russia
Received: September 26, 2022; Accepted: October 27, 2022; Published: November 03, 2022
Abstract
Neutrophils are one of the key barriers to anti-infective protection, an important mechanism of which is NETosis - the formation of neutrophil extracellular traps (NET). In recent years, this ambiguous biological phenomenon has been considered as a factor of unfavorable prognosis in some types of cancer. This review is devoted to the analysis of the role of NETosis in the pathogenesis of autoimmune diseases and other non-communicable diseases. The role of NET in malignant tumors, in particular in metastasis and progression of the tumor process, has been studied and data on the subpopulations of neutrophils – low-density neutrophils (LDN) and high-density neutrophils (HDN) in tumor processes have been analyzed. Further study of the phenomenon of netosis and the characteristics of peripheral blood neutrophils in cancer patients will be useful both for detailing the mechanisms of the metastatic cascade and for identifying their role as a biomarker and a possible therapeutic target.
Keywords: Neutrophil extracellular traps; Low and high density neutrophils; Oncological diseases; Netosis; COVID-19; Lung diseases
Introduction
Heterophilic leukocytes are the largest population of leukocytes, they are one of the first cellular barriers that prevents the penetration and spread of infection in the body. These cells are terminally differentiated, they have a short lifespan and a low level of gene expression. Getting into the bloodstream, they have all the necessary set of proteins for the destruction of microorganisms. In case of infection, neutrophils are sent to infected tissues under the influence of signaling cytokines, where they encounter invading microbes. This collision leads to the activation of neutrophils and the absorption of the pathogen by the phagocytotic vesicle. Antimicrobial activity requires two events in the phagosome. First, the pre-synthesized NADPH oxidase subunits assemble on the phagosomal membrane and transfer electrons to oxygen to form superoxide anions. They spontaneously or catalytically dismute to form carbon dioxide and hydrogen peroxide. Collectively, superoxide anions, carbon dioxide and hydrogen peroxide are called Reactive Oxygen Species (ROS). Secondly, neutrophil granules fuse with the phagosome, releasing antimicrobial peptides and enzymes. Microorganisms are exposed to high concentrations of ROS and cytotoxic granules in the phagosome. Together they are responsible for the destruction of microbes. Humoral nonspecific protective factors produced by neutrophilic leukocytes (complement, lysozyme, interferon, myeloperoxidase, cationic proteins) have a powerful antimicrobial effect. Phagocytosis and intracellular killing of microorganisms are considered to be the classical antibacterial function of neutrophils [1].
NETosis is an important mechanism of anti-infective protection of neutrophils. This is the formation of so-called Neutrophil Extracellular Traps (NETs).
For the first time, a new mechanism of antimicrobial action of neutrophils was described relatively recently, namely in 2004 and demonstrated in the conducted studies. This is the formation of reticular structures in the extracellular space from DNA strands associated with antimicrobial proteins, histones and cytotoxic granules, described relatively recently [2]. The rather late fact of the discovery of this phenomenon is explained by the small size of NET, instability, fragility of traps; the difficulty of detecting them; their almost complete absence in the peripheral blood of healthy people. NETs provide a high local concentration of antimicrobial molecules that effectively kill a wide range of microbes (gram-positive and gram-negative bacteria, fungi) and provide the most important innate protective immune mechanism. The role of NETs in various pathological processes is described. A significant amount of NETs is detected in places of inflammation, as demonstrated in human appendicitis and in an experimental model of shigellosis. Traps have been shown to be important in vivo in human preeclampsia and streptococcal infections causing necrotizing fasciitis and pneumococcal pneumonia.
Although this phenomenon was discovered more than 15 years ago, the specific signaling events leading to the formation of the NETs are still largely unclear.
The fact that the presence of NETs in the blood of healthy people is an extremely rare situation allows us to consider the formation of NETs as a kind of biological marker of various pathological conditions. In this case, it is important to analyze the role of this phenomenon in the development of various pathological conditions, including the oncological process, in which the formation of NETs and the characteristics of neutrophils can be both a prognostic factor and a possible chemotherapeutic target.
Mechanisms of NETs Formation
The morphological sequence of events in the formation of NETs is as follows: activated neutrophils initiate a process in which the classical lobular morphology of the nucleus is first lost and the differences between euchromatin and heterochromatin disappear. Then all the inner membranes are dissolved and the active components of the NETs are mixed. After that, as a result of a process biologically different from both apoptosis and necrosis, the cytoplasmic membrane ruptures and the extracellular part of the trap is formed. This process of neutrophil death is called NETosis. The main form of NETs formation is the so–called suicidal NETosis, which leads to the death of neutrophils and is characterized by the above-mentioned sequential morphological changes [3].
At the same time, there is also a so-called vital NETosis. This is the process when neutrophils remain viable and release only parts of their nuclear or mitochondrial DNA. Both mechanisms of NETosis, their duration and sequence of events at the neutrophil level are described in sufficient detail in a recent review by A. Palladina et al. [4].
There is no doubt that the leading role in the formation of NETs belongs to the phagocytic coenzyme Nicotinamide Adenine Dinucleotide Phosphate (NADPH) - the oxidase that forms ROS. In infection, the formation of ROS can contribute to both the intraphagosomal destruction of live neutrophils and the postmortem destruction of neutrophils that have already formed NETs. Pretreatment of neutrophils stimulated with forbolmyristate acetate (PMA) or Staphylococcus aureus in combination with the NADPH oxidase inhibitor Diphenylene Iodonium (DPI) prevented NETs formation.
Considering in more detail the mechanism and conditions of formation of NETs at the intracellular level, at this stage of our knowledge we can say the following.
With the participation of Protein Kinase C (PKC), mitochondrial ROS (mtROS) stimulate NADPH oxidase [5], and intracellular kinases (the Src kinase family) that stimulate PKC are activated by ROS [6]. In the study of N. Vorobjeva et al. [7] an increase in the activity of mtROS and NADPH oxidase was demonstrated due to a signal from the G-protein-coupled formyl-methionine-leucinephenylalanine (fMLP) receptor, which induces the release of Ca2+ from the intracellular depot, as well as Ca2+-independent activation of phosphoinoside-3-kinase (PI3K).Ca2+-dependent activation of mtROS formation can serve as one of the main sources of ROS in the case of induction of the formation of NETs caused, in particular, by ionomycin [8]. These data also confirm that two different mechanisms of NETosis are possible, one of which is independent of NADPH oxidase [9]. The generation of mtROS caused by fMLP (formyl-methionine-leucine-phenylalanine, bacterial peptide) and A23187 (calcimycin, calcium ionophore) directly depends on the opening of the mitochondrial pore (mPTP) [10]. Interestingly, when NETosis was activated under the action of fMLP, there was no swelling of mitochondria, characteristic of the long-term opening of mitochondrial pores. It is possible that in this case there was a shortterm opening of the pore caused by an increase in the concentration of calcium ions (Ca2+) in the cytoplasm [10]. On the contrary, when NETosis was activated under the action of A23187, which also depended on the discovery of mPTP, mitochondrial swelling was observed, which coincided with chromatin decondensation and destruction of the nuclear envelope [10]. The high concentration of cytoplasmic Ca2+ formed under the action of calcium ionophore A23187 led to the prolonged discovery of mPTP. This led to the formation of large concentrations of mtROS due to the release of the main components of antioxidant protection from the mitochondria [10]. Thus, the conditions for the formation of NETs depend on the type of initiating agent.
ROS formed by NADPH oxidase penetrate back into the cell, thereby stimulating the opening of ptp and enhancing NETosis [10].
Neutrophil degranulation with the release of proteins from granules and their release into the cytosol is a key moment in NETosis. This primarily concerns the yield of azurophilic granules containing various proteins, some of them are represented by serine proteases – Neutrophil Elastase (NE), cathepsin G and Myeloperoxidase (MPO) [5]. ROS promote the release of serine proteases from granules into the cytosol. Serine proteases migrate to the nucleus, contributing to chromatin decondensation and destruction of the nuclear envelope [11]. In addition to serine proteases, Peptidyl Arginine Deaminase 4 (PAD4) also enters the nucleus, which activates citrullination of histones and promotes the final decondensation of chromatin. Proteoclastic damage of the nuclear plate and chromatin decondensation lead to the destruction of the nuclear envelope and the release of chromatin into the cytoplasm [5]. At the final stage of vital NETosis, pores are formed in the plasma membrane to release chromatin. Pores are formed with the help of a special protein gasdermine D (GSDMD), the cleavage and activation of which occur due to neutrophil elastase. There is an assumption that gasdermin D forms pores not only in the plasma, but also in the nuclear membrane [12].
Under the action of RIP kinases, another pore-forming protein MLKL (Mixed lineage kinase domain like pseudokinase), a key activator of necrosis, is activated, which also leads to the release of NETs, whichare decondensed chromatin and proteins released from granules (Figure 1) [13].
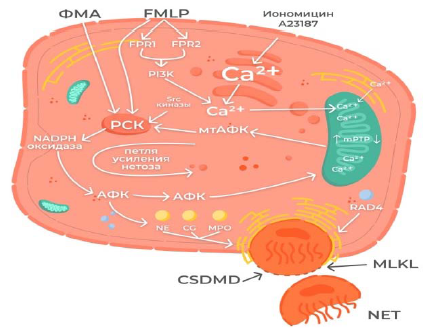
Figure 1: Scheme of the NETosmechanism .
PMA FMLP IonomycinA23187
FPR1 FPR2
PI3K Ca2+
Src kinases Ca2+ Ca2+ Ca2+
Ca2+
?KC mtROS ↑mPTP↓
NADPH oxidase amplification step of NETosis
ROS ROS Ca2+ Ca2+
NE CG MPO RAD4
CSDMD MLKL
NET
The best evidence of the need for NADPH oxidase for the extrusion of NETs was obtained in experiments conducted on human neutrophils obtained from patients with chronic granulomatous disease (CGB). A biological feature of patients with CGB is mutations in one of the subunits of the NADPH oxidase enzymatic complex, leading to the absence or significant decrease in the respiratory release of neutrophils. In the same study, a mechanism for the formation of NETs independent of NADPH oxidase was found. Thus, neutrophils of patients with CGB do not form NETs in response to PMA or Staphylococcus aureus, but at the same time, the formation of NETs still occurs if NADPH-independent sources of ROS are used. Thus, the addition of an extracellular source of ROS, glucose oxidase and glucose bypassed the need for ROS produced by NADPH oxidase and caused DPI-independent formation of NETs. Data on the formation of NETs by a method independent of NADPH oxidase are gradually accumulating. For example, the formation of NETs in the suicidal form, stimulated by certain bacteria and fungi, requires NADPH oxidase, while the extrusion of NETs in the vital type, induced by other bacteria, parasites and most inflammatory microcrystals, does not include ROS generated by this coenzyme [2,14,15]. It should be noticed that NADPH oxidase cannot be considered an NET-specific protein, since it is also necessary for the implementation of cellicolous phagocytosis by neutrophils.
One of the important events in the formation of NETs is the citrullination of histones by the enzyme PAD4. This enzyme is present in neutrophils in large quantities and moves to the nucleus with an increase in the concentration of cytosolic calcium, mediating the conversion of arginine residues into citrulline in target proteins, primarily in histones. This leads to chromatin decondensation [16].
At the time of the description of this phenomenon, PAD4 was proposed as a specific and universal mediator of the formation of NETs and a marker of their formation, but the results of further studies showed that NETs can also be formed in a way independent of PAD4.
One of the key activators of neutrophils and mediators of the formation of NETs is cytokines: interleukin (IL)-8, IL-18, IL-1Β. They, as mentioned earlier, provide directed chemotaxis of neutrophils into the focus of inflammation, mediating increased formation of NETs. In turn, NETs can stimulate phagocytic cells to produce cytokines and thereby have a damaging effect on tissues. This mechanism has been demonstrated in the study of the causes of damage to the pulmonary epithelium in patients with COVID-19 [17,18].
Inflammatory cytokines, including IL-1Β, are involved in the immunopathogenesis of COVID-19.There is also a reaction when NETs stimulate macrophages to produce IL-1Β.It was this fact that suggested the presence of damage to the alveoli and pulmonary endothelium in patients with severe progression of COVID-19 [17,18]. The participation of IL-8 and IL-18 in the formation of NETs in ovarian cancer has been demonstrated. Their action led to a decrease in the number of NETs and an increase in the NETs index, which may indicate a decrease in the killer function of neutrophils [19].
The Phenomenon of NETs and Its Significance for the Body
The phenomenon of NETs has a twofold significance for the body. On the one hand, it has a protective function, and on the other, it can contribute to the formation and aggravation of the severity of a number of pathological conditions, including thrombosis, autoimmune diseases and malignant neoplasms.
In the case of prolonged formation of NETs by the type of reaction, many pathological processes are stimulated [18]. It is believed that excessively active formation of NETs can cause a cascade of inflammatory reactions that contribute to metastasis of cancer cells, destroy surrounding tissues, promote microthrombosis and lead to irreversible damage to the organs of the pulmonary, cardiovascular and renal systems [20].
As an example of the positive influence of the phenomenon of NETs, the positive role of NETosis in the treatment of fungal keratitis can be cited. In all patients, the formation of NETs was detected, but in patients with a large number of them, fungal keratitis was much easier [21]. In acute inflammatory process, NETs prevent the dissemination of infectious pathogens by their cytotoxic activity, contributing to the migration of granulocytes into the inflammatory focus [22,23].
NETosis makes a special contribution to the development of lung diseases, and here the function of NETs is ambiguous. On the one hand, they participate in the elimination of fungal microbiota from lung tissue and protect the respiratory tract from infection. On the other hand, their excessive accumulation contributes to a decrease in pulmonary function and pulmonary ventilation. It is impossible to ignore the fact that many pathogens produce enzymes capable of cleaving NETs [24].
NETosis can lead to after troubles such as Acute Respiratory Distress Syndrome (ARDS), Chronic Obstructive Pulmonary Disease (COPD), bronchial asthma, so, an increased content of NETs is detected in the expectoration of COPD patients. At the same time, the number of neutrophil traps in expectoration in COPD patients directly correlates with the severity of the course [25,26]. The formation of traps leads to damage to the alveoli and pulmonary endothelium in patients with COVID-19.
Thus, in the case of ARDS, it was found that the levels of NETs in plasma are higher in patients with ARDS associated with blood transfusion than in patients without ARDS. Increased levels of NETs formation in the blood of patients with ARDS associated with pneumonia correlate with the severity of the disease and mortality. There was also an increase in extracellular histones in bronchoalveolar lavages and blood plasma of patients with ARDS. There is convincing experimental evidence supporting the role of histones in ARDS and sepsis. At the same time, in an experimental model of lung injury, it is shown that NETs are formed in response to various stimuli that cause ARDS, and preventing their formation or eliminating NETs reduces lung damage and increases survival.
The negative role of NETs in cystic fibrosis has been shown, when mucous secretions containing intracellular DNA (NETs) disrupt the gas exchange of the lungs. At the same time, excessive production of nonspecific elastase NETs makes the mucus thick and viscous, which not only worsens ventilation, but also contributes to the colonization of bacteria. In turn, bacterial colonization additionally promotes the recruitment of neutrophils and the formation of NETs, increasing the viscosity of mucus and, consequently, reducing the respiratory function of the patient.
One of the chromatin proteins is High-Mobility Group Protein B1 (HMGB1), or amphoterin, one of the key mediators of inflammation, a prototype of cell danger molecules that induces a special type of cell death – pyroptosis. It was found that the formation of NETs in the case of septic inflammation is associated with the release of NMGB1 and induces pyroptosis of peritoneal macrophages, leading to the activation of nuclear factor NFkB and increasing the secretion of tumor necrosis factor a, IL-1β, IL-6 and IL-12, which again creates a feedback loop and eventually leads to uncontrolled inflammation and death patient [27].
By supporting an excessive inflammatory response, NETs contribute to the formation of blood clots in the vessels [23]. The interaction of NETs with damaged endothelium and platelets plays a very important role in thrombosis and pathogenesis of a number of diseases. Thus, the DNA included in the NETs, when it enters the blood plasma, activates the production of thrombin independently of the tissue factor, also triggers a cascade of reactions that lead to the activation of XIIa, then the activation of XI factor and the generation of thrombin [28]. With the help of DNA, stable complexes of thrombin and fibrin are formed, disrupting the process of fibrinolysis [28]. Histones, which are part of NET, cause the generation of thrombin also in the absence of a tissue factor in platelet-rich plasma. They cause the activation of V factor, increasing the activity of the prothrombinase complex, and activate protein C, leading to the synthesis of thrombin in the presence of thrombomodulin [29]. Following damage to the endothelium, neutrophils are attracted to this zone, which trigger a cascade of thrombotic reactions by forming an NETs and delivering a tissue factor, while neutrophil elastase – Neutrophil Elastase (NE) and Cathepsin G (CG) - play an important role in the formation of a thrombus [30]. Currently, correlations between the activity of NETosis and the severity of infarction and ischemic stroke have been demonstrated. A direct effect on the severity of the process of the amount of formation of NETs and associated platelets has been shown [31].
The role of NETs in the mechanism of coagulation and thrombosis in patients with COVID-19 was studied. It was shown that the severity of the process correlated with the number of NET formed. In the course of the study, data were obtained on the effect of NETosis on this process [32]. The mechanism of thrombosis in this case is standard – the damaged endothelium causes the release of the Willebrand factor, which activates platelets. They, in turn, activate neutrophils and their formation of NETs. They are, as networks, trap platelets, erythrocytes and fibrin, leading to the development of blood clots in patients with COVID-19 [32].
Since the components of NETs cause the production of antibodies, it is natural that NETosis is also involved in the development of autoimmune diseases.
For example, with systemic lupus erythematosus, a special form of neutrophils appears. They are called Low-Density Neutrophils (LDN). Under the action of interferon a/β, they secrete pro-inflammatory cytokines and undergo NETosis faster than conventional neutrophils. In this case, nuclear and mitochondrial DNA, LL-37, HMGB1 are released, which are autoantigens and are recognized by proinflammatory receptors of innate immunity TLR4 [33].
In seropositive rheumatoid arthritis, neutrophils are a source of citrullinated autoantigens in joints and internal organs. Hazard molecules, DAMPs, circulating in the blood of patients interact with autoantibodies and form immune complexes that are captured by NETs and recognized by plasmocytoid dendritic cells [34]. NETs and DAMPs formed during neutrophil death act as factors of arthritis progression [33]. There are observations about the role of NETosis in autoimmune diseases such as vasculitis, antiphospholipid syndrome, multiple sclerosis and psoriasis [35].
A large number of studies conducted on experimental models and in patients with oncological diseases have shown a significant contribution of NETs to the development of tumor-associated venous and arterial thrombosis [36]. Taking into account these facts, NETs and participants in the trap formation chain can be considered as prognostic factors, as well as a possible therapeutic target in the formation of tumor-associated thrombosis.
Thus, NETosis is one of the important regulators of homeostasis and innate immune response and, like any protective immune mechanism, can play both a positive and a negative role for the body [37-39].
Neutrophils and the Role of NETs in Malignant Neoplasms
For a long time, neutrophils were considered fairly simple cells of the innate immune system. Thanks to a detailed study of the functions of neutrophils, interest in this population of cells increases. As already noted, the conducted studies prove the participation of neutrophils in the oncological process, autoimmune diseases, thrombosis and chronic inflammatory reactions [40]. Studies have demonstrated the active participation of neutrophils in the oncological process, namely in angiogenesis, progression, and facilitation of extravasation of cancer cells. The tumor microenvironment is able to reprogram neutrophils, activating both their antitumor activity andtriggering the mechanisms of carcinogenesis [41].
2 types of neutrophils were detected and characterized in patients with malignant neoplasms: LDN and high-density neutrophils. Currently, there is a concept that the formation of NETsis possible only from LDN [4]. Despite the rather active discussion in foreign literature, more facts convince us that these types of neutrophils are not separate populations, but under the influence of various factors from one form can pass into another, thereby showing their plasticity. They perform a double function. They can be part of the inflammation contributing to the development of the tumor by triggering angiogenesis, remodeling of the extracellular matrix, metastasis and immunosuppression. Conversely, neutrophils can promote antitumor response by directly destroying tumor cells and participating in cellular networks that mediate antitumor resistance [42]. It should be noticed that neutrophils are the regulators of polarization of M1 and M2 types of macrophages. LDN have a low ability to phagocytosis, migration, and when stimulated by IFN-γ and GM-CSF, they express PDL1, which possibly enhances the suppressor properties of LDN [43]. An increase in the concentration of LDN in cancer patients is a rather late event of carcinogenesis, it is possible that LDN are involved in tumor growth, but this mechanism has not yet been fully studied. The diversity and plasticity of neutrophils underlie the double potential in the tumor microenvironment [42].
It is assumed that the formation of NETs is one of the important events of the metastatic cascade, in which the role of neutrophils is studied in sufficient detail. Thus, the activation of neutrophils with the subsequent formation of NETs in case of ingestion of breast cancer cells into the lungs was demonstrated in experimental models. Network-like structures formed by neutrophils have been described around metastatic lung cancer cells in mice [44]. NETs stimulated migration and invasion of breast cancer cells in vitro.
Possible mechanisms of NETs involvement in metastasis may be different. It is assumed that neutrophil traps can attract circulating tumor cells into tissues or increase vascular permeability, thereby facilitating extravasation to tumor cells [4]. One study showed that inflammation in the lungs and, accordingly, the attraction of neutrophils to the inflammatory focus with the formation of NETs can activate single “dornant” cancer cells and lead to the formation of clinically significant pulmonary metastases [45].
Clinical observations also confirm the significant role of NETs in metastatic tumors. For example, in patients with Ewing’s sarcoma, in the case of a metastatic lesion, a significant amount of NETs is present in the blood [46]. It has also been demonstrated that NETs circulate in large quantities in the blood of patients with various forms of lung, pancreatic, bladder cancer and colorectal cancer [47]. In study B. Hsu et al. it has been shown that LDN, through the formation of NETs, contribute to the formation of breast cancer metastases in the liver (Figure 2) [48].
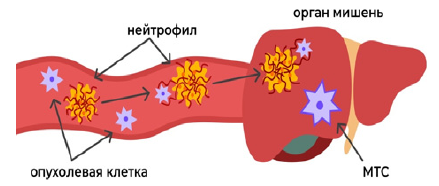
Figure 2: Scheme of the metastasis mechanism using NETs.
Tumor cell neutrophil target organ metastasis
An important role, as already mentioned, NETosis plays in thrombosis, which is one of the frequent causes of death in patients with cancer. It has been demonstrated in various experimental models that an increased number of neutrophils and the NETs formed by them in tumor tissue increase the risk of venous thrombosis [16]. The tumor tissue factor associated with NETs triggers the processes of thrombosis and neoangiogenesis, thereby providing metabolic support to tumor cells [16]. One of the studies demonstrated the relationship of increased biomarkers of the formation of NETs in blood plasma with a high risk of thrombosis in cancer patients [49]. Taking into account these facts of direct involvement of NETs in thrombosis in oncological patients, this process can be considered as an additional target of thromboprophylaxis, and the determination of markers of NETs in the blood plasma of patients can be a predictor factor of thrombosis.
Naturally, a search was carried out for possible blockers of NETosis and, of course, the most convenient antitumor target for NETosis is the extracellular part of DNA. It was found that the use of DNAse blocked the formation of NETs. DNase also contributed to the reduction of lung metastases in mice [50]. However, at the moment, the drug DNAse exists only in one dosage form – inhalation. It is used for cystic fibrosis, reducing the viscosity of sputum due to the destruction of NETs [51]. To deliver DNAse into the bloodstream and tissues, parenteral administration of the drug is required, which is relevant in patients with malignant neoplasms, however, the drug has high toxicity with this route of administration, which makes it difficult to use in this category of patients.
Experimental models have also demonstrated the interruption of the formation of NETs during PAD4 inhibition [52]. Thus, prostaglandin E2 inhibits the formation of NETs caused by tumor cells, as well as chloride, contributes to the inhibition of NET and reduction of thrombosis in cancer patients [53]. Interesting in this aspect is the use of vitamin D3, which has demonstrated a decrease in the formation of NETs. Capillary blood sampling was performed in patients before taking vitamin D3, then 1, 3 and 7 days after the start of taking the drug and 7 days after the end. The formation of NETs was stimulated by the addition of Phorbol-Myristate-Acetate (PMA). Taking vitamin D3 led to the fact that the formation of NETs gradually decreased and stopped completely 7 days after the start of the intake. After the end of vitamin D3 intake, the formation of NETs was completely restored [54].
Thus, the question of a drug that will selectively affect NETs, but at the same time will not have an immunosuppressive effect or an excessive immunostimulatory effect on other mechanisms of the immune system that play an important role in the formation of antitumor immunity remains open.
Neutrophilic leukocytes are a population that is very sensitive to chemotherapy, which leads to neutropenia. To stimulate the formation of neutrophils in the bone marrow and their release into the bloodstream, preparations of granulocyte colony-stimulating factor are used, but this mainly contributes to the formation of immature forms of neutrophils forming LDN capable of forming NETs [41]. In addition, the secretion of granulocyte colony-stimulating factor by the tumor cells themselves also contributes to an increase in LDN, respectively, this leads to active NETosis and metastasis [48].
Considering all of the above, NETosis can be considered as a new therapeutic target in the complex treatment of an oncological patient. Taking into account the stages of development of NETosis, it is possible to assume possible points of application of pharmacotherapy (Figure 3).
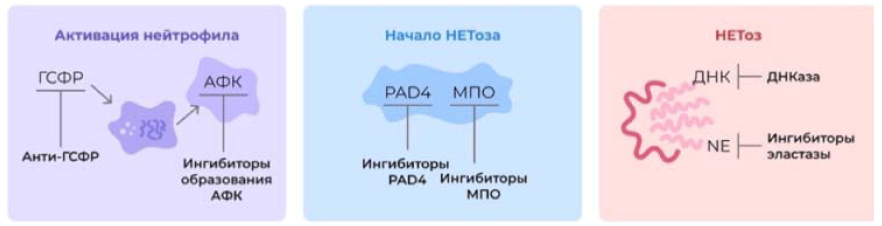
Figure 3: Activation of neutrophils
GSFR-granulocyte-stimulating growth factor ROS
Anti-GSFR Inhibitors of ROS formation
The beginning of NETosis
IPAD 4 MPO
PAD4 Inhibitors MPO Inhibitors
No.
DNA DNAse
NE Elastase Inhibitors
Most of the drugs that suppress the formation of NETs also suppress the mechanisms of the immune system as a whole. Therefore, it is relevant to search for the most effective and safe agents that prevent the development of the phenomenon NETosis. One of the possible drugs may be, for example, an immunomodulator with the properties of an immunoadjuvant – azoximer bromide, which in experimental studies in vitro has shown the ability to inhibit the formation of NETs and NETosis in the late stages [55]. As it is said, it plays both a protective role and it is one of the key mechanisms of the pathogenesis of many diseases.
Conclusion
The discovery of NETosis provides an opportunity to take a fresh look at the participation of neutrophils in the tumor process. A detailed study of each stage of NETosis will allow us to find certain therapeutic targets, the inhibition of which will prevent the progression and metastasis of the tumor process, as well as the development of many other diseases. Assessment of the phenomenon of NETosis and detection of NETs in the blood can be considered as a marker of prognosis in cancer patients. The study of this process is relevant and is considered as another point of application of immunotherapy in the fight against malignant neoplasms.
Disclosure of Interest
The authors declare that they have no competing interests.
Authors’ Contribution
The authors declare the compliance of their authorship according to the international ICMJE criteria. All authors made a substantial contribution to the conception of the work, acquisition, analysis, interpretation of data for the work, drafting and revising the work, final approval of the version to be published and agree to be accountable for all aspects of the work.
Funding
The authors declare that there is no external funding for the exploration and analysis work.
References
- Potapnev MP, Gushchina LM, Moroz LA. Phenotypic and functional heterogeneity of neutrophil subpopulations in normal and pathological conditions. Immunology. 2019; 40: 84-96.
- Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil Extracellular Traps Kill Bacteria. Science. 2004; 303: 1532- 1535.
- Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, et al. Novel cell death program leads to neutrophil extracellular traps. The Journal of Cell Biology. 2007; 176: 231-241.
- Palladina AD, Khomyakova NF. NETosis as a mechanism of cancer progression. Immunology of hematopoiesis. 2019; 17: 39-52.
- Vorobyova NV, Chernyak BV. NETosis: molecular mechanisms, role in physiology and pathology. Biochemistry. 2020; 85: 1383-97.
- Steinberg SF. Mechanisms for redox-regulation of protein kinase C. Frontiers in Pharmacology. 2015; 6.
- Vorobjeva N, Prikhodko A, Galkin I, Pletjushkina O, Zinovkin R, Sud’ina G, et al. Mitochondrial reactive oxygen species are involved in chemoattractantinduced oxidative burst and degranulation of human neutrophils in vitro. European journal of cell biology. 2017; 96: 254-265.
- Douda DN, Khan MA, Grasemann H, Palaniyar N. SK3 channel and mitochondrial ROS mediate NADPH oxidase-independent NETosis induced by calcium influx. Proceedings of the National Academy of Sciences. 2015; 112: 2817-2822.
- Ravindran M, Khan MA, Palaniyar N. Neutrophil Extracellular Trap Formation: Physiology, Pathology, and Pharmacology. Biomolecules. 2019; 9: 365.
- Vorobjeva N, Galkin I, Pletjushkina O, Golyshev S, Zinovkin R, Prikhodko A, et al. Mitochondrial permeability transition pore is involved in oxidative burst and NETosis of human neutrophils. Biochimica et biophysica acta. Molecular basis of disease. 2020; 1866: 165664.
- Metzler KD, Goosmann C, Lubojemska A, Zychlinsky A, Papayannopoulos V. A Myeloperoxidase-Containing Complex Regulates Neutrophil Elastase Release and Actin Dynamics during NETosis. Cell Reports. 2014; 8: 883-896.
- Chen KW, Monteleone M, Boucher D, Sollberger G, Ramnath D, Condon ND, et al. Noncanonical inflammasome signaling elicits gasdermin D–dependent neutrophil extracellular traps. Science Immunology. 2018; 3.
- D’Cruz AA, Speir M, Bliss-Moreau M, Dietrich S, Wang S, Chen AA, et al. The pseudokinase MLKL activates PAD4-dependent NET formation in necroptotic neutrophils. Science Signaling. 2018; 11.
- Rada B, Leto TL. Oxidative innate immune defenses by Nox/Duox family NADPH oxidases. Contributions to microbiology. 2008; 15: 164-187.
- Li P, Li M, Lindberg MR, Kennett MJ, Xiong N, Wang Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. The Journal of Experimental Medicine. 2010; 207: 1853-1862.
- Demers M, Krause DS, Schatzberg D, Martinod K, Voorhees JR, Fuchs TA, et al. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proceedings of the National Academy of Sciences. 2012; 109: 13076-13081.
- Yaqinuddin A, Kashir J. Novel therapeutic targets for SARS-CoV-2-induced acute lung injury: Targeting a potential IL-1β/neutrophil extracellular traps feedback loop. Medical Hypotheses. 2020; 143: 109906.
- Schönrich G, Raftery MJ, Samstag Y. Devilishly radical NETwork in COVID-19: Oxidative stress, neutrophil extracellular traps (NETs), and T cell suppression. Advances in Biological Regulation. 2020; 77: 100741.
- Abakumova TV, Gening TP, Dolgova DR, et al. Influence of the levels of the pro-inflammatory cytokines on the formation of extracellular neutrophilic traps in disseminated ovarian cancer. Russian Journal of Immunology. 2019; 22: 704-6.
- Barnes BJ, Adrover JM, Baxter-Stoltzfus A, Borczuk A, Cools-Lartigue J, Crawford JM, et al. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. The Journal of Experimental Medicine. 2020; 217.
- Jin X, Zhao Y, Zhang F, Wan T, Fan F, Xie X, et al. Neutrophil extracellular traps involvement in corneal fungal infection. Molecular Vision. 2016; 22: 944- 952.
- Hwang JW, Kim JH, Kim HJ, Choi IH, Han HM, Lee KJ, et al. Neutrophil extracellular traps in nasal secretions of patients with stable and exacerbated chronic rhinosinusitis and their contribution to induce chemokine secretion and strengthen the epithelial barrier. Clinical & Experimental Allergy. 2019; 49: 1306-1320.
- Sollberger G, Tilley DO, Zychlinsky A. Neutrophil Extracellular Traps: The Biology of Chromatin Externalization. Developmental cell. 2018; 44: 542-553.
- Twaddell SH, Baines KJ, Grainge C, Gibson PG. The Emerging Role of Neutrophil Extracellular Traps in Respiratory Disease. Chest. 2019; 156: 774-782.
- Dicker AJ, Crichton ML, Pumphrey EG, Cassidy AJ, Suarez-Cuartin G, Sibila O, et al. Neutrophil extracellular traps are associated with disease severity and microbiota diversity in patients with chronic obstructive pulmonary disease. The Journal of Allergy and Clinical Immunology. 2018; 141: 117- 127.
- Uddin M, Watz H, Malmgren A, Pedersen F. NETopathic Inflammation in Chronic Obstructive Pulmonary Disease and Severe Asthma. Frontiers in Immunology. 2019; 10.
- Chen L, Zhao Y, Lai D, Zhang P, Yang Y, Li Y, et al. Neutrophil extracellular traps promote macrophage pyroptosis in sepsis. Cell Death & Disease. 2018; 9.
- Ivanov I, Shakhawat R, Sun M, Dickeson SK, Puy C, McCarty OJT, et al. Nucleic acids as cofactors for factor XI and prekallikrein activation: Different roles for high-molecular-weight kininogen. Thrombosis and haemostasis. 2017; 117: 671-681.
- Noubouossie DF, Whelihan MF, Yu Y, Sparkenbaugh E, Pawlinski R, Monroe DM, et al. In vitro activation of coagulation by human neutrophil DNA and histone proteins but not neutrophil extracellular traps. Blood. 2017; 129: 1021-1029.
- Okeke EB, Louttit C, Fry C, Najafabadi AH, Han K, Nemzek J, et al. Inhibition of neutrophil elastase prevents neutrophil extracellular trap formation and rescues mice from endotoxic shock. Biomaterials. 2020; 238: 119836.
- Novotny J, Oberdieck P, Titova A, Pelisek J, Chandraratne S, Nicol P, et al. Thrombus NET content is associated with clinical outcome in stroke and myocardial infarction. Neurology. 2020; 94: e2346-e2360.
- Aitbayev KA, Murkamilov IT, Fomin VV, et al. Coronavirus disease 2019 (COVID-19): not a toz- not a pelvis regulating the formation of neutrophilic entrapment traps (nets). Actabiomedicascientifica. 2021; 6: 64-73.
- Grieshaber-Bouyer R, Nigrovic PA. Neutrophil Heterogeneity as Therapeutic Opportunity in Immune-Mediated Disease. Frontiers in Immunology. 2019; 10.
- Chatfield SM, Thieblemont N, Witko-Sarsat V. Expanding Neutrophil Horizons: New Concepts in Inflammation. Journal of Innate Immunity. 2018; 10: 422-431.
- Fousert E, Toes R, Desai J. Neutrophil Extracellular Traps (NETs) Take the Central Stage in Driving Autoimmune Responses. Cells. 2020; 9: 915.
- Wolach O, Sellar RS, Martinod K, Cherpokova D, McConkey M, Chappell RJ, et al. Increased neutrophil extracellular trap formation promotes thrombosis in myeloproliferative neoplasms. Science Translational Medicine. 2018; 10.
- Cabrini M, Nahmod K, Geffner J. New insights into the mechanisms controlling neutrophil survival. Current Opinion in Hematology. 2010; 17: 31-35.
- Köckritz-Blickwede MV, Nizet V. Innate immunity turned inside-out: antimicrobial defense by phagocyte extracellular traps. Journal of Molecular Medicine (Berlin, Germany). 2009; 87: 775-783.
- Yost CC, Cody MJ, Harris ES, Thornton NL, McInturff AM, Martinez ML, et al. Impaired neutrophil extracellular trap (NET) formation: a novel innate immune deficiency of human neonates. Blood. 2009; 113: 6419-6427.
- Liew PX, Kubes P. The Neutrophil’s Role During Health and Disease. Physiological reviews. 2019; 99: 1223-1248.
- Xiong S, Dong L, Cheng L. Neutrophils in cancer carcinogenesis and metastasis. Journal of Hematology & Oncology. 2021; 14.
- Jaillon S, Ponzetta A, Mitri DD, Santoni A, Bonecchi R, Mantovani A. Neutrophil diversity and plasticity in tumour progression and therapy. Nature Reviews Cancer. 2020; 20: 485-503.
- Sagiv JY, Michaeli J, Assi S, Mishalian I, Kisos H, Levy L, et al. Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell reports. 2015; 10: 562-573.
- Rocks N, Vanwinge C, Radermecker C, Blacher S, Gilles C, Marée R, et al. Ozone-primed neutrophils promote early steps of tumour cell metastasis to lungs by enhancing their NET production. thoraxjnl. 2019; 74: 768-779.
- Albrengues J, Shields MA, Ng D, Park CG, Ambrico A, Poindexter ME, et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science. 2018; 361.
- Berger-Achituv S, Brinkmann V, Abed UA, Kühn LI, Ben-Ezra J, Elhasid R, et al. A proposed role for neutrophil extracellular traps in cancer immunoediting. Frontiers in Immunology. 2013; 4.
- Oklu R, Sheth RA, Wong KHK, Jahromi AH, Albadawi H. Neutrophil extracellular traps are increased in cancer patients but does not associate with venous thrombosis. Cardiovascular diagnosis and therapy. 2017; 7: S140-S149.
- Hsu BE, Tabariès S, Johnson RM, Andrzejewski S, Senecal J, Lehuédé C, et al. Immature Low-Density Neutrophils Exhibit Metabolic Flexibility that Facilitates Breast Cancer Liver Metastasis. Cell reports. 2019; 27: 3902- 3915.e6.
- Grilz E, Mauracher L, Posch F, Königsbrügge O, Zöchbauer-Müller S, Marosi C, et al. Citrullinated histone H3, a biomarker for neutrophil extracellular trap formation, predicts the risk of mortality in patients with cancer. British Journal of Haematology. 2019; 186: 311-320.
- Park J, Wysocki RW, Amoozgar Z, Maiorino L, Fein MR, Jorns J, et al. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Science Translational Medicine. 2016; 8: 361ra138-361ra138.
- Vorobjeva NV, Pinegin BV. Neutrophil Extracellular Traps: Mechanisms of formation and role in health and disease. Biochemistry (Moscow). 2014; 79: 1286-1296.
- Lewis HD, Liddle J, Coote JE, Atkinson SJ, Barker MD, Bax BD, et al. Inhibition of PAD4 activity is sufficient to disrupt mouse and human NET formation. Nature chemical biology. 2015; 11: 189-191.
- Volkov DV, Tetz GV, Rubtsov YP, Stepanov AV, Gabibov AG. Neutrophil Extracellular Traps (NETs): Opportunities for Targeted Therapy. Acta Naturae. 2021; 13: 15-23.
- Gusev SA, Batyreva LYu, Maksimov DI, etal. Vitamin D3 blokirue tobrazovanieneitrofil’nykhvnekletochnykhlovushek v tsel’noikrovi. Molekuliarnye, membrannyeikletochnyeosnovyfunktsionirovaniiabiosistem: Tezisydokladovmezhdunarodnoinauchnoikonferentsii, Chetyrnadtsatogos ”ezdaB elorusskogoobshchestvennogoob” edineniiafotobiologovibiofizikov, Minsk, 17–19 iiunia 2020 goda. Minsk: Belarusian State University, 2020; 114.
- Pinegin BV, DogelYuA, Vorobyova NV, et al. The effect of azoximer bromide on the formation of extracellular neutrophil traps. Breast cancer. 2019; 1: 1-6.