
Short Communication
Austin J Infect Dis. 2022; 9(3): 1075.
The Pathological Changes in Osteoarthritis and Rheumatoid Arthritis Joint Diseases
Pawlak Z¹* and Yusuf KQ²
1Tribochemistry Consulting, Salt Lake City, UT 84117, USA and University of Economy, Biotribology Laboratory, Poland
2College of Medical Rehabilitation Sciences, Department of Prosthetics and Orthotics, Taibah University, Kingdom of Saudi Arabia
*Corresponding author: Zenon Pawlak, Tribochemistry Consulting, Salt Lake City, UT 84117, USA
Received: November 03, 2022; Accepted: November 22, 2022; Published: November 29, 2022
Abstract
The pathological changes observed in Osteoarthritis (OA) and Rheumatoid Arthritis (RA) affects the entire joint structure resulting in pain, surface change, molecules modification and dysfunction. In our study, we report molecular deactivation mechanism surface active phospholipids and cartilage matrix in (OA) and (RA). Deactivated PLs can be related to high friction leading to articular cartilage damage. The interaction occurs between antibodies β2-Glycoprotein I (β2-GPI) protonated amino acid functional group (-NH3+) and the phospholipid functional group (-PO4-):
(β2-GPI) (-NH3+) + PL(-PO4-) → (-NH3+-PO4-)
In a proposed articular cartilage damage of OA and RA, a substantial progress has been made towards understanding the mechanisms of PLs deactivation that lead to the degradation of the cartilage surface.
Keywords: Cartilage; Osteoarthritis and rheumatoid arthritis; Cartilage damage and degeneration; Anti phospholipid syndrome (APS); Glycoprotein I (β2-GPI); Atomic force microscope (AFM)
Introduction
Both Rheumatoid Arthritis (RA) and Osteoarthritis (OA) cause inflammation of joints. Osteoarthritisis caused by mechanical wear and tear on joints. Rheumatoid arthritis is an autoimmune disease in which the body’s own immune system attacks the body’s joints [1]. The surface of a normal healthy articular cartilage is hydrophilic due to the phosphorous functional group (-PO4-) of phospholipids [2,3], but declines in Osteoarthritis (OA) and Rheumatoid Arthritis (RA). A very low coefficient of friction is facilitated by boundary bilayers of phospholipids on the articular surface supported by lubricin and hyaluronan with adsorbed surface-active phospholipids (PLs) and hydrated water [4]. In Osteoarthritis (OA) and Rheumatoid Arthritis (RA), the visual changes in cartilage appear on surface in areas of mechanical sheer stress. In healthy joint, bilayers of phospholipids adsorbed to the surface of articular cartilage are surrounded by components of Synovial Fluid (SF) at pH~ 7.4, such as hyaluronan [5], lubricin [6], and surface-active phospholipids [4]. The surface-active phospholipids promote boundary lubrication [5,7] and have been suggested as a the main lubricant. With degenerative joint diseases, such as OA and RA [8,9,10], the phospholipid bilayers are found to be shifted towards deactivated molecules in SF, (Figure 1) [4]. Deactivated phospholipids molecules are expressed in increased concentration in the synovial fluid. If the cartilage is worn out, the joint becomes painful and stiff, and with reduced range of movement. In severe osteoarthritis, the hydrophilic cartilage surface is completely worn away, leaving the bones to rub against each other.
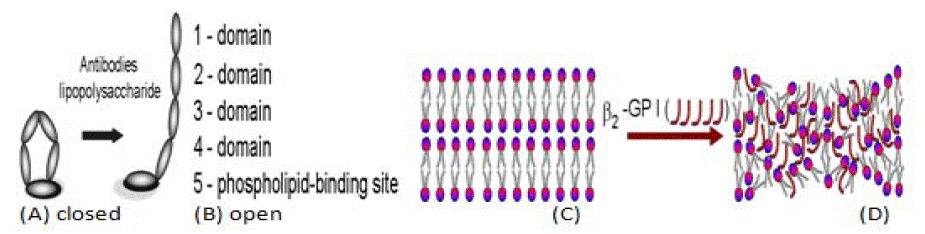
Figure 1: β2-Glycoprotein I in closed form (A) and conversion into an open hockey-stick-like conformation (B) each molecule has five domains (1-5). The antibodybinding site (B5) is accessible to the autoantibodies. Phospholipid bilayers (C), Bilayer and closed (β2-Glycoprotein I) (D). Deactivated PLs molecules by open hockey-stick (β2-Glycoprotein I) (D) removed from cartilage surface.
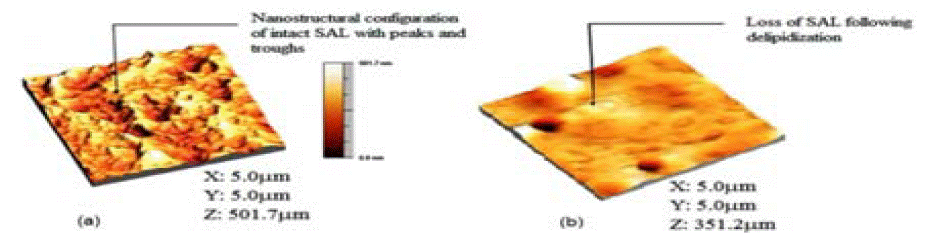
Figure 2a: Microscopic image of (a) healthyand (b) damaged surface of the cartilage.
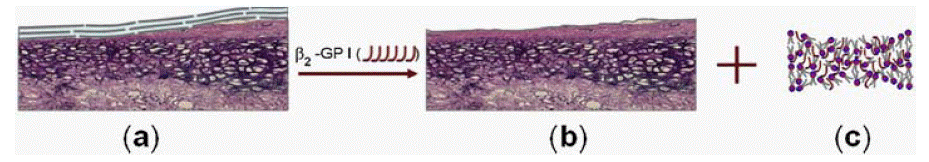
Figure 2b: (a) Healthy cartilage surface with phospholipid bilayers, (b) Phospholipid bilayers degraded by the open hockey stick-like conformation β2- Glycoprotein
I, β2 -GP I, (c) Phospholipids deactivated by β2-Glycoprotein I.
The aim of our study was to identify the degradation bilayers of PLs as well as the deactivation of phospholipid molecules in human SF with common joint diseases, such as OA and RA.
Material and Methods
The articular cartilage bovine animalsamples. The articular cartilage samples used in atomic force microscope (AFM study was obtained from the patellae of 3-4 year old bovine animals. The glued sample 5 mm by 5 mm was submerged in saline solution ready for AFM imaging using the SMENA® head of the NT-MDT P47 Solver scanning probe microscope (SPM) (NT-MDT). In order to simulate the loss of cartilage surface lipids, an artificial lipid extraction process was used (delipidization) [11].
The articular cartilage human samples. Lipids were extracted from cell-free and cell debris-free SF samples of 16 controls and 26 early OA, 22 late OA, and 20 active RA. Extracted phospholipid species were quantified by ESI-MS/MS electrospray ionization tandem mass spectrometry [9,10]. Most of the PLs data for this paper was taken from Kosinska et al. [9,10].
Results and Discussion
Amodel of deactivation of phospholipid bilayersis shown in (Figure 1). At apH around 7 amino acids from β2-Glycoprotein I (β2- GPI) (arginine, lysine and tryptophan) have hydrogen donor atoms in their side chains (-NH3+) and acid–base interaction occurs between protonated amino acid group (β2-GPI) (-NH3+) and the phospholipid function group PL(-PO4-):
(β2-GPI) (-NH3+) + PL(-PO4-) → (-NH3+-PO4-) Kassoc~105 (1)
Electrostatic attraction with association constant has high value Kassoc~105 is high enough to deactivate thePLs bilayer surface [4]. β2- Glycoprotein I (β2-GPI) is a protein that circulates in blood at variable levels (50–500 μg mL-1) with a molecular weight of 50 kDa. β2- Glycoprotein I (β2-GP I) can exist in (a) closed conformation and (b) the open hockey stick-like conformation (Figure 1A and (B). β2-GP I in its hockey stick-like conformation is a strongly adhesive protein, and binds to different receptors on cells [12,13]. Binding of β2-GPI to anionic charged phospholipid functional groups (-PO4-) at pH ~ 7.4, results in a change in conformation.
The content of total phospholipids in SF was calculated as the sum of the concentrations of all lipid species that contained a phosphate group. Compared with control SF [average (Av) 314.2 nmol/ml, the concentrations of phospholipids rose by 2.1-fold in early OA [643.8 nmol/ml], 2.4-fold in late OA [758.8 nmol/ml], and 2.8-fold in RA SF [877.7 nmol/ml] [9].
Several studies have (indicated) that molecules of hyaluronan and lubricin with adsorbed surface-active phospholipids contribute to the boundary lubrication that is provided by SF [5,6,8]. However, in OA and RA conditions, the SF is enriched with deactivated phospholipids with no ability to be absorbed by hyaluronan and lubricin to support cartilage lubrication and this fact remains poorly understood. The interaction between lubricin and surface active PLs and their function in cartilage boundary lubrication remain clear about PLs contribution. Cartilage wear and joint degeneration begin from the destruction of phospholipid bilayers on the cartilage surface with the participation of the phospholipid syndrome. Joint degeneration leads to the destruction of bilayers on the cartilage surface and immobilization of the joint. Synovial fluid with its composition of macromolecules is insufficient to withstand the load and control friction in the joints. It was shown that the phospholipid syndrome after an appearance in the joints leads to the devastation of phospholipid bilayers and inactivation of their molecules. Moreover, it has been experimentally shown that the joint cartilage belongs to intelligent materials and is exposed to destruction, which is graphically expressed in Figure 1 and Figure 2.
Cartilage damage occurred primarily in the form of surface degradation at the interface (cartilage/cartilage) superficial tangential zones. These results suggest that cartilage damage from frictional loading occurs as a result of PLs deactivation.
Conclusion
This study presents the results of cartilage surface damaged by Osteoarthritis (OA) and Rheumatoid Arthritis (RA) by PLs deactivation. Adeactivated PLs molecule has no ability to form bilayers and liposomes or be adsorbed by lubricin and hylurunan molecules,a process considered as antiphospholipid syndrome symptoms. These results extend our current knowledge on cartilage boundarylubricating molecules. Hyaluronan and lubricin are unable to take the function of lubricant without the presence of the surface active phospholipids. The mechanisms leading to articular cartilage surface degradation (measured by PLs deactivation) in RA and OA are still not fully understood, however, since both conditions lead to similar outcomes, it is highly likely that their mechanisms would somehow overlap. Therefore, more studies are required to better understand the similarities and differences between these two debilitating conditions.
References
- Pap T, Korb-Pap A. Cartilage damage in osteoarthritis and rheumatoid arthritis--two unequal siblings. Nat Rev Rheumatol. 2015; 11: 606-15.
- Sarma AV, Powell GL, LaBerge M. Phospholipid composition of articular cartilage boundary lubricant. J Orthop Res. 2001; 19: 671–676.
- Hills BA, Crawford RW. Normal and prosthetic synovial joints are lubricated by surface-active phospholipid: a hypothesis. J Arthroplasty. 2003; 18: 499– 505.
- Pawlak Z. Articular Cartilage: Lamellar-Repulsive Lubrication of Natural Joints. Kindle Direct Publishing. 2018; 161.
- Schmidt TA, Gastelum NS, Nguyen QT, Schumacher BL, Sah RL. Boundary lubrication of articular cartilage: role of synovial fluid constituents. Arthritis Rheum. 2007; 56: 882–891.
- Jay GD, Torre JR, Warman ML, Laderer MC, Breuer KS. The role of lubricin in the mechanical behavior of synovial fluid. Proc Natl Acad Sci. USA. 2007; 104: 6194–6199.
- Mirea A, Trunfio-Sfarghi AM, Matei CI, Munteanu B, Piednoir A, Rieu JP, et al. Role of the biomolecular interactions in the structure and tribological properties of synovial fluid. Tribol Int. 2013; 16: 302–311.
- Schwarz IM, Hills BA. Surface-active phospholipid as the lubricating component of lubricin. Br J Rheumatol. 1998; 37: 21–26.
- Kosinska MK, Ludwig TE, Liebisch G, Zhang R, Siebert HC, et al. Articular Joint Lubricants during Osteoarthritis and Rheumatoid, Arthritis Display Altered Levels and Molecular Species. PLoS One. 2015; 10: e0125192.
- Kosinska MK, Liebisch G, Lochnit G, Wilhelm J, Klein H, et al. A lipidomic study of phospholipid classes and species in human synovial fluid. Arthritis Rheum. 2013; 65: 2323–2333.
- Yusuf KQ, Pai R, Pawlak Z. Atomic force microscope observation of the surfaces of natural articular cartilage. J Surgical Case Reports and Images. 2022: 5.
- de Groot PG, Meijers JC. β2-glycoproteinI: evolution, structure and function. J Thromb Haemost. 2011; 9: 1275–1284.
- de Laat B, Derksen RHWM, van Lummel M, Penningsm MT, de Groot P. Pathogenic anti- β2-glycoproteinI antibodies recognize domain I of β2- glycoproteinI onlyafter a conformational change. Blood. 2006; 107: 1916– 1924.