
Review Article
Austin J In Vitro Fertili. 2015;2(1): 1012.
Are we Close to Solve the Mystery of Fragile X Associated Premature Ovarian Insufficiency (FXPOI) in FMR1 Premutation Carriers?
Elizur SE, Orvieto R and Cohen Y*
Department of Obstetrics and Gynecology Sheba Medical Center, Ramat Gan and Sackler School of Medicine, Tel Aviv University, Israel
*Corresponding author: Cohen Y, Infertility and IVF Unit, Department of Obstetrics and Gynecology Sheba Medical Center, Ramat Gan, 52621, Israel
Received: November 11, 2014; Accepted: February 11, 2015; Published: February 16, 2015
Abstract
Fragile X Syndrome (FXS), the most common form of inherited mental retardation, is caused by a trinucleotide repeat expansion (CGG >200) in the 5'-untranslated region of the fragile X Mental Retardation 1 (FMR1) gene. Amplification of the CGG triplet number above the normal range (n=5-44) towards the so-called premutation status (n=55-200) is associated with increased risk for Fragile X-Associated Premature Ovarian Insufficiency (FXPOI) in females and Fragile X-Associated Tremor/Ataxia Syndrome (FXTAS) in males. Very little is known about the mechanisms leading to FXPOI. The observation that premutation carriers, both males and females, have increased FMR1 transcript levels, led researchers to suggest a similar molecular pathogenesis in both FXPOI and FXTAS. A variety of models have been proposed as the culprits of FXTAS and FXPOI:
The toxic RNA gain-of-function model: This model suggests that DNA containing CGG expanded repeats leads to the formation of dynamic intranuclear long rCGG RNA aggregates that sequesters specific RNA binding proteins. Proteins such as Sam68 and DGCR8 and its partner DROSHA, directly bind to the double-stranded RNA hairpin structure of long rCGG RNA aggregates resulting in the loss of normal cell function and cell death.
Repeat Associated Non-AUG initiated (RAN) translation: Due to translation of the expansion mutation, as part of a larger open-reading frame (ORF), mutant protein named polyglycine-containing protein (FMRpolyG) is expressed in neuronal cells and granulosa cells, with the consequent disruption of cellular function, leading to cell toxicity.
Keywords: FMR1 premutation; Premature ovarian failure; Fragile X
Introduction
Fragile X Syndrome (FXS), the most common form of inherited mental retardation, is caused by a trinucleotide repeat expansion (CGG) in the 5'-untranslated region of the Fragile X Mental Retardation 1 (FMR1) gene located at Xq27.3. Patients with fragile X-related mental retardation, carry the full mutation CGG-repeat expansions (>200 repeats), which are generally accompanied by hypermethylation of the promoter region, with the consequent transcriptional silencing of the FMR1 gene and absence of the encoded FMR1 protein (FMRP) [1].
Expansion of the CGG triplet number above the normal range (n=5-44) towards the so-called premutation status is associated with increased risk for Fragile X-Associated Premature Ovarian Insufficiency (FXPOI) in females, [2,3] and Fragile X-Associated Tremor/ Ataxia Syndrome (FXTAS) in males [4].
Premature Ovarian Insufficiency (POI), defined by cessation of menses prior to age 40, affects approximately 1% of reproductiveage female population. While in over half of cases, no etiology can be identified, recent studies suggest that premutation repeat length (n=55-200) is associated with overt POI [5,6]. Carriers for the premutation on one allele have a high risk (16-35%) for POI [7], compared with only 1% of females in the general population and enter menopause approximately 5 years earlier, compared to non-carrier [8]. Moreover, fragile X premutation carriers have impaired ovarian function, as evident by abnormal ovarian reserve biomarkers (serum anti-mullerian hormone, FSH, inhibin B, inhibin A) and a reduced ovarian response to Controlled Ovarian Hyperstimulation (COH), as reflected by a higher gonadotropin consumption and a lower oocytes yield, during In-Vitro Fertilization (IVF) treatment cycles [9-15].
Previous reports suggest that there is a non-linear association between the number of CGG repeats and ovarian function. While Allen et al. and Ennis et al. [16,17] found a non-linear association between CGG repeats size and decreased reproductive lifespan or menopause age accumulating evidence suggests that mid-range repeat size group (80-100 repeats) carries an increased risk for ovarian insufficiency. Bibi et al. [15] found that premutation carriers with <100 CGG repeats suffer from impaired ovarian response and decreased fertilization rate during In-Vitro Fertilization (IVF) treatment compared to patients with =100 CGG repeats. Similar to previous reports [9-15], we recently showed a significant impairment in ovarian reserve biomarkers [18] in premutation carriers, such as high basal FSH levels and high FSH/LH ratio, as well as a reduced response to COH protocol [19]. Moreover, in agreement with previous studies [16,17], we also demonstrated a significant nonlinear association between the number of CGG repeats and ovarian function with the mid-size range (80-120) having the worst prognosis [19]. In our study [19], women with 80-120 CGG repeats had significantly less oocyte retrieved compared to women with higher and lower repeats (Figure 1).
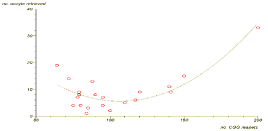
Figure 1: A non-linear association between the number of retried oocytes during IVF cycle and the number of CGG repeats in FMR 1 premutation carriers (55-200 repeats). (p<0.0001).
Premutation carriers have increased FMR1transcript levels [20]. This phenomenon is seen both in male as well as female premutation carriers and lead researchers to postulate that in terms of the molecular mechanism, there may be similarity between the pathogenesis of FXPOI and FXTAS.
Very little is known about the mechanisms leading to FXPOI. It is well established that full mutation carriers, or those with an allele of >200 methylated repeats that leads to silencing of FMR1, do not suffer from FXPOI or FXTAS. Thus, the significant reduction of the FMR1protein product, FMRP, does not appear to be the culprit. However, new advances in understanding the molecular mechanisms underlying FXTAS and other triplet diseases might shed new light on the pathogenesis of FXPOI. These include: RNA toxicity due to abnormal mRNA accumulation and production antisense RNAs, protein sequestration and deregulation of miRNA biogenesis, and repeat-associated protein toxicity via Repeat-associated Non-ATG (RAN) translation, could illuminate the disease biology of FXPOI.
The toxic RNA gain-of-function model
This mechanism has been suggested as a mechanism for triplet repeat-related ataxias, such as Spino Cerebellar Ataxia 8 (SCA8), SCA10, and SCA12, and for Myotonic Dystrophy (DM). This model suggests that DNA containing CGG expanded repeats lead to the formation of dynamic intra-nuclear expanded rCGG RNA aggregates that accumulate over time, resulting in the formation of giant intranuclear inclusions. Continuous enlargement of CGG RNA aggregates suggests that these repeats may constantly recruit proteins. These long rCGG RNA track sequesters specific RNA binding proteins, resulting in the loss of normal cell function and cell death [21]. Various proteins have been identified that may directly bind to CGGRNA and whose sequestration may affect cell viability. One of these proteins is Sam68, which its regulated splicing is altered in FXTAS patients [21]. Sam68 directly binds the mRNAs for the Follicle- Stimulating Hormone (FSH) and the Luteinizing Hormone (LH) receptors (FSHr and LhCGr), which were down regulated in ovaries of adult knockout females [22]. Moreover, it was already shown that Sam68-/- females mice are sub fertile and display a dramatic reduction in the cumulative number of pups delivered during their lifespan. It may be therefore suggested that defects in such protein can strongly reduce ovarian response to gonadotropin exactly as shown in FMR1 premutation carriers. Another protein which was found to bind to CGG RNA aggregates is DGCR8. Expanded CGG repeats form a double-stranded RNA hairpin which mimics the structure of pre-miRNAs recognized by DGCR8. This results in the partial sequestration of DGCR8 and its partner, DROSHA, within CGG RNA aggregates. As a consequence, the level of free DROSHA-DGCR8 microprocessor is decreased, reducing the expression of mature miRNAs. As a result, in cells expressing expanded CGG repeats, such as, in brain samples from patients with FXTAS, the processing of pre-miRNAs is reduced ultimately resulting in neuronal cell dysfunction and degeneration [23]. Recently, published data related to altered miRNA expression in POI patients by miRNA microarray analysis. Ten miRNAs showed increased expression in POI and two showed decreased expression (let-7c and miR-144). In addition, in a rat POI model a total of 63 miRNAs were up-regulated and 20 miRNAs down-regulated (MicroRNA-29a and miRNA-144) [24]. Therefore, the miRNA system may be another common pathway explaining cell death due to the toxic RNA gain-of-function theory both in FXTAS and FXPOI patients.
Repeat Associated Non-AUG initiated (RAN) translation
Repeat-expansion disorders, such as FXTAS and FXPOI, can result in protein gain-of-function diseases due to translation of the expansion mutation as part of a larger Open-Reading Frame (ORF), resulting in the expression of a mutant protein that disrupts cellular function and induces toxicity. Recently it has been demonstrated that the CGG repeat expansion in FXTAS triggers RAN translational initiation within the 5'UTR of FMR1 mRNA through an AUG independent mechanism. The translated product, a cryptic polyglycine-containing protein (FMRpolyG), is toxic in Drosophila and in human cell lines, capable of driving intra-nuclear inclusion formation, and is present in FXTAS patient brains [25]. In our laboratory we were recently able to demonstrate in granulosa cells from 4 premutation carriers undergoing IVF-PGD treatments the presence of cellular inclusion bodies containing FMRpolyG, which were not found in granulosa cells from women with normal sized CGG repeat (Elizur et al. unpublished data).
Conclusion
New data is emerging suggesting that both FXPOI and FXTAS are caused by common molecular mechanism pathways. There is evidence that the death of various neuronal cells in FXTAS and granulosa cells (and perhaps oocytes) in FXPOI are related to both RNA and protein toxicity. Expanded rCGG RNA aggregates accumulation may lead to sequestration of various RNA binding proteins such as Sam68, DROSHA and DGCR8, interfering with normal cell physiology. In addition, RAN translation products (polyglycine-containing protein) accumulation in various cells may lead to early cell death. Further studies are required to better understand the mechanism of both RNA and protein toxicity, in order to develop novel remedies or to identify early biomarkers of imminent POI, that will allow patients to attend for fertility preservation procedures, in advance.
References
- Bardoni B, Mandel JL. Advances in understanding of fragile X pathogenesis and FMRP function, and in identification of X linked mental retardation genes. Curr Opin Genet Dev. 2002; 12: 284-293.
- Conway GS, Payne NN, Webb J, Murray A, Jacobs PA. Fragile X premutation screening in women with premature ovarian failure. Hum Reprod. 1998; 13: 1184-1187.
- Wittenberger MD, Hagerman RJ, Sherman SL, McConkie-Rosell A, Welt CK, Rebar RW, et al. The FMR1 premutation and reproduction. Fertil Steril. 2007; 87: 456-465.
- Sellier C, Usdin K, Pastori C, Peschansky VJ, Tassone F, Charlet-Berguerand N. The multiple molecular facets of fragile X-associated tremor/ataxia syndrome. J Neurodev Disord. 2014; 6: 23.
- Bretherick KL, Fluker MR, Robinson WP. FMR1 repeat sizes in the gray zone and high end of the normal range are associated with premature ovarian failure. Hum Genet. 2005; 117: 376-382.
- Bodega B, Bione S, Dalprà L, Toniolo D, Ornaghi F, Vegetti W, Ginelli E. Influence of intermediate and uninterrupted FMR1 CGG expansions in premature ovarian failure manifestation. Hum Reprod. 2006; 21: 952-957.
- Mailick MR, Hong J, Greenberg J, Smith L, Sherman S. Curvilinear association of CGG repeats and age at menopause in women with FMR1 premutation expansions. Am J Med Genet B Neuropsychiatr Genet. 2014; 165: 705-711.
- Sullivan AK, Marcus M, Epstein MP, Allen EG, Anido AE, Paquin JJ, et al. Association of FMR1 repeat size with ovarian dysfunction. Hum Reprod. 2005; 20: 402-412.
- Murray A, Webb J, MacSwiney F, Shipley EL, Morton NE, Conway GS. Serum concentrations of follicle stimulating hormone may predict premature ovarian failure in FRAXA premutation women. Hum Reprod. 1999; 14: 1217-1218.
- Hundscheid RD, Braat DD, Kiemeney LA, Smits AP, Thomas CM. Increased serum FSH in female fragile X premutation carriers with either regular menstrual cycles or on oral contraceptives. Hum Reprod. 2001; 16: 457-462.
- Welt CK, Smith PC, Taylor AE. Evidence of early ovarian aging in fragile X premutation carriers. J Clin Endocrinol Metab. 2004; 89: 4569-4574.
- Platteau P, Sermon K, Seneca S, Van Steirteghem A, Devroey P, Liebaers I. Preimplantation genetic diagnosis for fragile Xa syndrome: difficult but not impossible. Hum Reprod. 2002; 17: 2807-2812.
- Gleicher N, Weghofer A, Barad DH. A pilot study of premature ovarian senescence: II. Different genotype and phenotype for genetic and autoimmune etiologies. Fertil Steril. 2009; 91: 1707-1711.
- Gleicher N, Weghofer A, Oktay K, Barad D. Relevance of triple CGG repeats in the FMR1 gene to ovarian reserve. Reprod Biomed Online. 2009; 19: 385-390.
- Bibi G, Malcov M, Yuval Y, Reches A, Ben-Yosef D, Almog B, et al. The effect of CGG repeat number on ovarian response among fragile X premutation carriers undergoing preimplantation genetic diagnosis. FertilSteril. 2010; 94: 869-874.
- Allen EG, Sullivan AK, Marcus M, Small C, Dominguez C, Epstein MP, et al. Examination of reproductive aging milestones among women who carry the FMR1 premutation. Hum Reprod. 2007; 22: 2142-2152.
- Ennis S, Ward D, Murray A. Nonlinear association between CGG repeat number and age of menopause in FMR1 premutation carriers. Eur J Hum Genet. 2006; 14: 253-255.
- Shrim A, Elizur SE, Seidman DS, Rabinovici J, Wiser A, Dor J. Elevated day 3 FSH/LH ratio due to low LH concentrations predicts reduced ovarian response. Reprod Biomed Online. 2006; 12: 418-422.
- Elizur SE, Lebovitz O, Derech-Haim S, Dratviman-Storobinsky O, Feldman B, Dor J, Orvieto R. Elevated levels of FMR1 mRNA in granulosa cells are associated with low ovarian reserve in FMR1 premutation carriers. PLoS One. 2014; 9: 105121.
- Allen EG, He W, Yadav-Shah M, Sherman SL. A study of the distributional characteristics of FMR1 transcript levels in 238 individuals. Hum Genet. 2004; 114: 439-447.
- Sellier C, Rau F, Liu Y, Tassone F, Hukema RK, Gattoni R, et al. Sam68 sequestration and partial loss of function are associated with splicing alterations in FXTAS patients. EMBO J. 2010; 29: 1248-1261.
- Bianchi E, Barbagallo F, Valeri C, Geremia R, Salustri A, De Felici M, et al. Ablation of the Sam68 gene impairs female fertility and gonadotropin-dependent follicle development. Hum Mol Genet. 2010; 19: 4886-4894.
- Sellier C, Freyermuth F, Tabet R, Tran T, He F, Ruffenach F, et al. Sequestration of DROSHA and DGCR8 by expanded CGG RNA repeats alters microRNA processing in fragile X-associated tremor/ataxia syndrome. Cell Rep. 2013; 3: 869-880.
- Imbar T, Eisenberg I . Regulatory role of microRNAs in ovarian function. Fertil Steril. 2014; 101: 1524-1530.
- Todd PK, Oh SY, Krans A, He F, Sellier C, Frazer M, et al. CGG repeat-associated translation mediates neurodegeneration in fragile X tremor ataxia syndrome. Neuron. 2013; 78: 440-455.