
Research Article
Austin J In Vitro Fertili. 2015;2(2): 1016.
Antioxidant Enzyme Levels in ICSI-Treated Patients with and without Uterine Myoma
Artur Wdowiak*
Diagnostic Techniques Unit, Medical University in Lublin, Poland
*Corresponding author: Artur Wdowiak, Diagnostic Techniques Unit, Faculty of Health Sciences, Medical University in Lublin, Poland
Received: April 22, 2015; Accepted: May 20, 2015; Published: May 28, 2015
Abstract
The negative impact of the environment in which the egg cell grows affects the quality of the embryo and the time during which subsequent developmental stages are reached, which ultimately translates into the achievement of pregnancy. The aim of this study was to compare follicular fluid levels of superoxide dismutase and catalase, the rate of embryonic development and treatment efficacy at early perimenopause, at childbearing age and in women with documented uterine myoma and the history of Intra Cytoplasmic Sperm Injection (ICSI). A total of 208 patients were divided into two groups: 150 women of childbearing age (27-35 years), (including 10 females with uterine myoma and ulipristal acetate therapy, 30 untreated females) and 58 women aged 40 to 46 years. Follicular fluid levels of Superoxide Dismutase (SOD) and catalase were determined using spectrophotometry. Embryo culture was assessed every 10 minutes during continuous monitoring. We have demonstrated that the rate of embryonic development decreases with patient’s age, superoxide dismutase levels in follicular fluid increase, while catalase levels decrease. SOD activity reduces the time to achieve the 4-cell stage while catalase activity delays the 5-cell stage and the blastocyst stage at early perimenopause. Uterine myomatosis does not induce changes in the follicular fluid levels of antioxidant enzymes. Ulipristal acetate enhances the ICSI efficiency, without significant effects on the rate of embryonic development or antioxidant enzyme levels.
Keywords: Superoxide dismutase; Catalase; Ulipristal acetate; Real-time embryo observation; ICSI
Introduction
The negative impact of the environment in which the egg cell grows affects the quality of the embryo and the time during which subsequent developmental stages are reached, which ultimately translates into the achievement of pregnancy [1]. The egg cell is probably damaged by the toxic microenvironment during the primordial stage of follicle development and at the time of oocyte maturation [2]. However, the most important cause of oocyte damage has been attributed to the deleterious effects of excess amounts of Reactive Oxygen Species (ROS) [3,4]. The term ROS covers a wide range of metabolites derived from the reduction of molecular oxygen, including free radicals, such as the superoxide anion, hydroxyl radical and powerful oxidants such as hydrogen peroxide (H2O2). The elimination of ROS partially depends on the antioxidant enzyme Superoxide Dismutase (SOD), which eliminates superoxide anions by producing H2O2 and is a first-line defence against ROS toxicity. The enzyme catalase acts as the second-line defence by detoxifying H2O2 to produce H2O [5,6]. Literature reports on the effects of these enzymes on reproduction are contradictory [4,5,7-10].
Achieving and maintaining pregnancy also depends on the uterine receptivity for embryo implantation and its ability to maintain pregnancy till term. The presence of uterine fibroids is one of the factors adversely affecting this ability. The closer they are to the uterine cavity, the more impact they have on the woman’s fertility. Procreation is mainly affected by endometrium-distorting intramural fibroids and sub mucosal fibroids [11]. The fibroids are associated with the disorders in the oxidoreductive system observed within the endometrium [12]. Presently, the ulipristal acetate treatment seems the most promising non-invasive therapy for uterine fibroids [13-15].
Late motherhood becomes more common, posing a significant challenge for reproductive medicine [16]. Female fertility declines markedly after the age of 40 [17]. It is a result of many factors, the most important of them being the reduced number and quality of egg cells produced by the ovaries. The decrease in child-bearing potential observed in women over 40 years of age may be ascribed to a number of reasons, including impaired ovarian vascularization, as well as free radical imbalance leading to oxidative stress and genetic dysfunctions [18,19]. The anomalies found in the egg cells produced in females of advanced age are associated with dispersed chromatin, decondensation of chromosomes and abnormalities connected with the spindle apparatus. Ageing oocytes also show abnormal synthesis of proteins associated with mitochondrial function, abnormal expression of genes regulating the cell cycle, cytoskeletal structure and genetic pathways [20].
The aim of the study was to compare follicular fluid levels of superoxide dismutase and catalase, the rate of embryonic development and treatment efficacy at early perimenopause, at childbearing age and in women with documented uterine myoma and the history of Intra Cytoplasmic Sperm Injection (ICSI).
Materials and Methods
The study was conducted in 2013 and 2014 at the Ovum Centre for Infertility Treatment in Lublin. The study group consisted of 208 women receiving infertility treatment via microinjection of sperm into an egg cell. All patients have been classified for ICSI due to moderate male factor infertility which rendered classic In Vitro Fertilization (IVF) impossible, including 188 couples with the history of 4-6 unsuccessful intrauterine inseminations during 1-2 years and 20 women with bilateral tubal obstruction. The patients were divided into the following subgroups : 150 females of childbearing age (27- 35 years), all with Follicle-Stimulating Hormone (FSH) <10 IU/ mL and normal Anti-Müllerian Hormone (AMH) levels (including 110 women without underlying anomalies, 10 women with uterine fibroids and prior ulipristal acetate therapy, and 30 untreated women) and a group of 58 females aged 40 to 46 years, with FSH <17 IU/mL and AMH levels ranging from 0.2 to 1.1 ng/ml. The patients with uterine fibroids who were included in the study had intramural and subserosal fibroids with a diameter <2.5 cm, not distorting the endometrium. Patients with severe endometriosis, Body Mass Index (BMI) <17 or >30 and women with metabolic diseases were excluded from the study. All patients gave their written consent and the study has been approved by the Ethics Committee at the Institute of Rural Health in Lublin.
All patients were treated with ICSI, using fresh oocytes and fresh sperm. Three to ten months before the stimulation of ovulation, 10 patients with uterine fibroids received therapy with 5 mg ulipristal acetate (Esmya: Gedeon Richter) at a dose of 5 mg/day for 84 days. The stimulation of ovulation was performed according to short protocols using GnRh analogues (Gonapeptyl Daily: Ferring) and recombinant FSH (Gonal-F: Merck-Serono), beginning on the third day of the cycle. The puncture was performed 36 hrs after the administration of recombinant human chorionic gonadotropin (r-hCG) (Ovitrelle: Merc-Serono).
Follicular fluid was sampled from the follicles with the diameter exceeding 16 mm. In the case of absence of an egg cell in follicular fluid or its contamination with blood, the sample was excluded from the study. SOD levels were determined with spectrophotometry using SOD Assay Kit (Sigma-Aldrich), while catalase levels were measured with Catalase Assay Kit (Sigma-Aldrich), following manufacturers’ instructions. The final SOD and catalase levels were expressed as enzyme activity unit per mg of protein (U/mg).
The oocytes were separated from the mural granulosa cells and ICSI was performed 3 hours after ovarian puncture; fertilized cells were cultured in 25 μl drops of Cleavage medium (COOK, Sydney IVF, Australia) under mineral oil until day 2 (2-5 cell stage) in an automatic incubator equipped with time-lapse image recording unit (Time-lapse, Primo Vision EVO Microscope, Cryo-Innovation, Hungary), at a 5% CO2 level and 37°C. Fifty hours after ICSI, the culture medium was replaced with Blastocyst medium (COOK, Sydney IVF, Australia).
Embryo culture was assessed every 10 minutes during continuous monitoring with a camera placed inside the incubator. The embryos were not removed from the incubator throughout the observation period. The monitoring system was turned off between recording time points to avoid the adverse effects of electromagnetic waves. The t0 was defined as the time of ICSI. The tF was defined as the first time point in which the pronuclei were visible and the tC as the last time point in which the pronuclei could be seen. When the pronuclei were disappearing, first, the nucleoli shrank and faded and then, pronuclear membrane became no longer visible. The time point with one-cell embryo after the syngamy was defined as t1 and the subsequent cleavage time points were defined as t2, t3, t4, t5, t6, t7, t8. The tM stood for the beginning of morula formation and the tB was defined as the time point when the first signs of blastocyst cavity became visible. The ASRM (American Society for Reproductive Medicine) and the ESHRE (European Society of Human Reproduction and Embryology) criteria were used for blastocyst evaluation and one of the blastocysts was transferred in order to avoid multiple pregnancy. In the 7th week of pregnancy, ultrasound assessments of embryonic echo and heart rate were performed.
The results were analyzed statistically. Measurable parameters included in the analysis were presented as mean values, median, minimum and maximum values and standard deviation, while nonmeasurable parameters were expressed as size and percentage. For qualitative characteristics, Chi2 test was used to identify the correlation between the achievement of pregnancy and patient’s age. The Shapiro–Wilk normality test was used to verify a normal distribution. The Kruskal-Wallis test was used to analyze the differences between the two groups. The correlation between the duration of embryonic development and SOD and catalase levels was analyzed with Pearson’s r correlation. Multivariate analysis with logistic regression was used to create a model predicting the achievement of pregnancy. The p value of <0.05 indicated the statistical significance of differences or correlations. Statistica 9.1 software (StatSoft, Poland) was used for the database and statistical analysis.
Results
Thirty-four (30.91%) clinical pregnancies were achieved as a result of therapy in patients of childbearing age without co-morbidities; 3 (30%) pregnancies were achieved in the group of females with prior ulipristal acetate therapy; 5 (16.67%) pregnancies were obtained in patients with untreated uterine fibroids (16.67%), while 2 pregnancies (3.45%) were achieved in the group of early perimenopausal women. Statistically significant differences were observed between the number of pregnancies in patients of childbearing age without uterine fibroids and those at early perimenopause (Chi2=15.415, df=1, p=0.0001) (Figure 1). In the case of women with uterine fibroids, the sample size was too small to use the Chi2 test in order to compare the group treated with ulipristal acetate with untreated patients (due to a relatively short time the product has been on the market), but if the same proportions were used in a larger sample, the differences would be statistically significant.
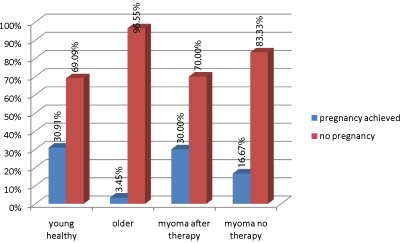
Figure 1: Proportion of pregnancies achieved during therapy in the analyzed
groups of patients.
Statistically significant differences were observed between the group of females of childbearing age and the group of women aged 40-46 years in relation to both, the rate of embryonic development from the time when pronuclei appeared to the 4-cell stage and the time in which the blastocyst stage was achieved. The rate of embryonic development to the stages listed above was higher in females of childbearing age than in older patients. The rate of pronuclear atrophy was higher and the time to achieve the blastocyst stage was shorter in women with prior ulipristal acetate therapy compared to those of childbearing age, without uterine fibroids. No statistically significant differences were observed in other rates of embryonic development in women of childbearing potential between the group without uterine fibroids and the group with uterine fibroids. There were no significant differences in the rates of embryonic development between both groups of women with uterine myoma (Table 1).
I) Young healthy
II) Older
III) Myoma after therapy
IV) Myoma no therapy
H
p
Between-group differences
Mean
Mean
Mean
Mean
tF
8.67
11.44
8.30
9.43
31.208
<0.001
I-II, II-III, II-IV
tC
23.03
26.97
19.29
22.47
69.176
<0.001
I-II, I-III, II-III, II-IV
t1
23.79
27.25
21.60
22.68
54.791
<0.001
I-II, II-III, II-IV
t2
25.59
29.82
25.16
25.04
51.207
<0.001
I-II, II-III, II-IV
t3
35.75
40.94
32.58
35.70
65.225
<0.001
I-II, II-III, II-IV
t4
37.15
43.90
35.80
35.81
63.820
<0.001
I-II, II-III, II-IV
t5
51.87
54.10
46.00
49.36
16.451
0.001
II-III, II-IV
t6
55.43
54.98
52.78
53.79
1.219
0.748
–
t7
58.25
57.93
57.43
58.31
0.189
0.979
–
t8
63.35
61.30
72.01
62.39
5.303
0.151
–
t9
75.60
78.37
81.18
73.88
4.907
0.179
–
tM
85.81
84.96
86.48
82.65
3.201
0.362
–
tB
104.55
107.91
103.71
104.40
46.498
<0.001
I-II, I-III, II-III, II-IV
Table 1: The rates of embryonic development in females of child bearing age and those at early perimenopause. The data is expressed in hours and fractions of an hour. t0 - time of ICSI, tF- time of the first frame in which both pronuclei could be observed, tC- the frame with the last observation of both pronuclei, t1- time for the corresponding number of 1 cell, t.2- time for the corresponding number of 2 cells, t.3- time for the corresponding number of 3 cells, t.4- time for the corresponding number of 4 cells, t.5- time for the corresponding number of 5 cells, t.6- time for the corresponding number of 6 cells, t.7- time for the corresponding number of 7 cells, t.8- time for the corresponding number of 8 cells, t.9- time for the corresponding number of 9 cells, tM- the first frame in which the embryos were compacting into the morula stage , tB- the frame in which a crescent-shaped area began to emerge from the morula. p <0.05– statistical significance of difference, H-Kruskal-Wallis test.
Follicular fluid SOD levels were lower in younger patients without uterine fibroids (mean level 34.63 U/mg) than in older women (mean level 43.75 U/mg) and these differences were statistically significant (H=81.418, p<0.001). In the group of patients of childbearing age, no statistically significant differences in SOD levels were observed between women with and without uterine fibroids. On the other hand, catalase levels were statistically significantly higher in all patients of childbearing age compared to women aged 40-46 years (H=60.574, p<0.001). No differences in catalase levels were found in the groups of females of childbearing age (Figure 2).
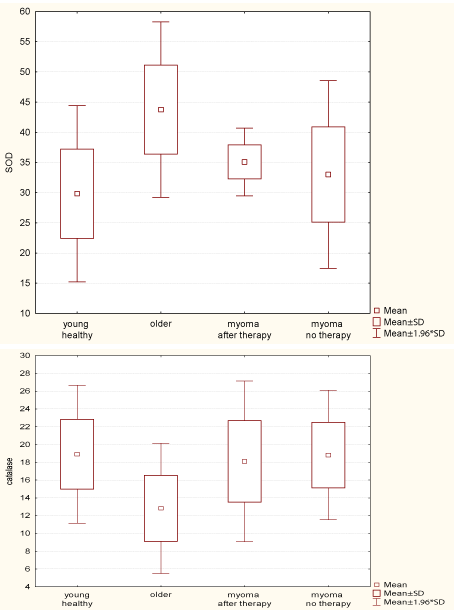
Figure 2: Mean SOD and catalase levels in follicular fluid obtained from the
analyzed groups.
A positive correlation (r- Pearson’s correlation coefficient) between catalase levels and the time to reach the 5-cell stage was observed in patients of childbearing age without uterine fibroids (r=0.195, p=0.035), while no statistical correlations were found for other time points. SOD levels within the same group had no significant effect on the rates of embryonic development. Older subjects reached the 4-cell stage sooner and had higher superoxide dismutase levels (r=-0.342, p=0.008). A reverse correlation, however, was observed between catalase levels and the time to reach the 5-cell stage of the embryo (r=0.282, p=0.032) and the blastocyst stage (r=0.292, p=0.022). Among patients with uterine fibroids, no correlation was observed between antioxidant enzyme levels and the rate of embryonic development (Table 2).
Young
Old
Myoma after Esmya
Myoma no therapy
SOD
catalase
SOD
catalase
SOD
catalase
SOD
catalase
tF
r
-0.034
0.167
-0.121
-0.128
0.362
0.130
-0.090
0.149
p
0.727
0.082
0.368
0.338
0.304
0.721
0.638
0.432
tC
r
-0.054
0.064
-0.201
-0.094
0.225
0.180
-0.324
0.130
p
0.574
0.506
0.130
0.483
0.533
0.619
0.081
0.495
t1
r
-0.028
0.177
-0.125
0.035
0.071
0.059
0.035
0.010
p
0.775
0.064
0.350
0.797
0.846
0.871
0.855
0.960
t2
r
-0.168
0.070
-0.201
0.245
-0.196
0.434
-0.079
-0.166
p
0.079
0.466
0.129
0.064
0.587
0.211
0.678
0.381
t3
r
0.132
-0.087
0.148
-0.026
-0.132
-0.158
0.263
0.197
p
0.170
0.365
0.267
0.848
0.717
0.662
0.160
0.297
t4
r
-0.141
-0.057
-0.342
0.174
-0.505
0.402
0.247
0.030
p
0.142
0.555
0.008
0.193
0.137
0.249
0.188
0.877
t5
r
-0.107
0.195
-0.033
0.282
-0.215
-0.469
0.015
0.264
p
0.266
0.035
0.807
0.032
0.551
0.172
0.937
0.159
t6
r
0.002
-0.157
-0.085
-0.020
-0.402
-0.312
-0.116
0.259
p
0.980
0.103
0.526
0.882
0.250
0.381
0.543
0.166
t7
r
0.109
-0.046
-0.006
-0.040
-0.041
-0.409
-0.038
0.144
p
0.259
0.630
0,965
0.766
0.911
0.241
0.841
0.449
t8
r
-0.145
-0.004
-0.118
0.015
-0.074
0.155
-0.290
-0.001
p
0.132
0.969
0.378
0.912
0.838
0.669
0.121
0.996
t9
r
-0.104
-0.164
-0.059
-0.046
-0.519
-0.224
-0.142
0.332
p
0.281
0.086
0.662
0.731
0.124
0.533
0.453
0.073
tM
r
-0.021
-0.072
-0.186
-0.089
0.016
-0.271
-0.089
0.164
p
0.830
0.455
0.163
0.506
0.965
0.448
0.641
0.387
tB
r
-0.101
0.122
-0.190
0.292
-0.299
-0.038
-0.256
0.002
p
0.295
0.203
0.153
0.022
0.402
0.918
0.172
0.992
Table 2: Statistically significant correlation (r- Pearson’s correlation coefficient) between Superoxide Dismutase (SOD) and catalase levels and the rate of embryonic development (data is expressed in hours and fractions of an hour; p <0.05– statistical significance of difference).
Discussion
We have shown in our study a significant age-related decrease in the rate of embryonic development. We have also confirmed the increased levels of superoxide dismutase in follicular fluid in early perimenopausal women, as well as the age-related decrease in the levels of catalase. These findings are in line with the results of studies conducted by Carbone et al. who observed that catalase levels in follicular fluid were age-dependent [21]. However, different results were obtained by Matos et al. The researchers discovered that the activity of superoxide dismutase in follicular fluid decreased with age, while it was found to be increased in patients with endometriosis and ovulatory dysfunction [22].
The results obtained by Grøndahl et al. [18] may explain the observations made in the present study that the rate of embryonic development was considerably lower, and the achievement of pregnancy was less successful in older women. The researchers investigated the effects of age on gene expression profile in mature human oocytes. They isolated mRNA for whole genome microarray analysis of gene expression from metaphase II (MII) oocytes from IVF or ICSI patients (10 younger women aged under 36 years, and 5 older women aged 37-39 years) subjected to controlled ovarian stimulation. The researchers observed a considerable difference in the transcriptional level of genes involved in central biological functions of the oocytes between the oocytes from younger and older patients. These findings contribute to the knowledge of processes which may be associated with the phenomenon of ageing as well as to the agerelated reduction in fertility.
Similarly, Stoop et al. explained in their study the age-related reduction in reproductive outcomes. They evaluated the reproductive efficiency of the oocyte based on ovarian ageing and ovarian response [26]. The retrospective analysis involved patients treated during the period 1992-2009, and the number of live births after fresh and cryopreserved embryo transfer per mature oocyte was calculated for 207,267 oocytes retrieved in 23,354 ovarian stimulation cycles. Subsequently, the number of live births per mature oocyte was analyzed according to the ovarian response. It was found that the oocyte utilization rate was constant in women aged up to 37 years, with the mean number of live births per mature oocyte of 4.47% (5%CI: 4.32-4.61), while this rate was significantly lower in women aged over 38 years, and decreased from 3.80% at the age of 38 years to 0.78% at the age of 43 years (p<0.001). Among women aged 38- 43 years, this rate did no longer depend on the ovarian response (p=0.87). The study by Stoop et al. demonstrated that the oocyte utilization rate in women aged 23-37 years depended to a high degree on the ovarian response, and much less on age, whereas in those aged 38 years and more this rate depended to a greater extent on age than on the ovarian response. The study by Stoop et al. seems to be in line with our findings in relation to the observed, significant decrease in ICSI efficacy in older patients.
The present study showed the effects of SOD levels on the time to reach the 4-cell stage of embryonic development in older females; however, it is not clear if this was related with the effectiveness of treatment due to an insufficient number of achieved pregnancies. The study by Combelles at al. conducted in a group of 91 women aged under 40 years did not confirm the effects of the levels of superoxide dismutase on the implantation and quality of embryos in IVF [3]. It seems that the issue will require further investigation.
The results obtained in our study demonstrated that uterine fibroids were not associated with significant changes in the levels of antioxidant enzymes or in the rate of embryonic development. We also found no such differences between the groups treated with ulipristal acetate and untreated patients, which seems to confirm the safety of UA therapy. The safety of Esmya was also shown in the study conducted by Luyckx M. in which 21 patients attempted pregnancy, among whom 15 (71%) succeeded. In total, 18 pregnancies were achieved. Among these 18 pregnancies, 12 resulted in the birth of 13 healthy children and 6 ended in early miscarriage. No regrowth of fibroids was observed during pregnancy [15]. Likewise, a case report under my authorship concerning the pre-treatment with ulipristal acetate prior to ICSI resulting in the birth of a healthy child supported the safety of ulipristal acetate therapy [27,28]. Similar evidence for Esmya safety has been presented in a case report by Monleón J. et al. [29]. Esmya mode of action based on the affinity to Progesterone Receptor (PGR) had provoked many questions about its safety before it was used in the treatment of infertility. We know that in the ovary, PGR is expressed specifically in granulosa cells of preovulatory follicles. It is primarily localized to nuclei and cytosol of mural granulosa cells and is virtually undetectable in cumulus cells or oocytes, consistent with LH receptor expression [30,31]. Although the role of PGR in ovulation is clear, the possible influence of PGR on oocyte developmental competence that is, the ability of the oocyte to produce a viable embryo following fertilization, does not appear to be resolved [32]. Our findings concerning the effectiveness of ulipristal acetate therapy allow us to believe that the effect of progesterone receptor modulation by ulipristal acetate is non-existent as early as 3 months after treatment discontinuation.
The role of antioxidant enzymes in the pathogenesis of uterine fibroids remains unclear. Although our study did not show any difference between SOD and catalase levels in women with and without uterine fibroids, we found that the changes in the activity of these enzymes were age-related. On the other hand, Pejic et al. reported elevated SOD levels in the endometrium of patients with uterine fibroids, however, their comparisons were made between SOD levels in females with different pathologies and not in healthy women [12]. This issue requires further studies in the future.
Our study confirmed that patient’s age was a very important factor affecting the successful achievement of pregnancy, raising hopes that ulipristal acetate therapy could enhance the chances of pregnancy in patients with uterine fibroids. Further research is needed in larger groups of patients.
Conclusion
- The rate of embryonic development decreases with woman’s age.
- Superoxide dismutase levels in follicular fluid increase, while catalase levels decrease in early perimenopause.
- In early perimenopause, SOD activity reduces the time in which the 4-cell stage is achieved while catalase activity delays the beginning of 5-cell and blastocyst stages.
- Uterine fibroids are not associated with the changes in antioxidant enzyme levels in follicular fluid.
- Ulipristal acetate used in infertility treatment enhances ICSI efficiency without significant effect on the rate of embryonic development or the activity of antioxidant enzymes.
References
- Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol. 2012; 10: 49.
- Kurus M, Karakaya C, Karalok MH, To G, Johnson J. The control of oocyte survival by intrinsic and extrinsic factors. Adv Exp Med Biol. 2013; 761: 7-18.
- Combelles CM, Holick EA, Racowsky C. Release of superoxide dismutase-1 by day 3 embryos of varying quality and implantation potential. J Assist Reprod Genet. 2012; 29: 305-311.
- Nel-Themaat L, Nagy ZP. A review of the promises and pitfalls of oocyte and embryo metabolomics. Placenta. 2011; 32: S257-263.
- Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev. 1996; 17: 121-155.
- Bausenwein J, Serke H, Eberle K, Hirrlinger J, Jogschies P, Hmeidan FA, et al. Elevated levels of oxidized low-density lipoprotein and of catalase activity in follicular fluid of obese women. Mol Hum Reprod. 2010; 16: 117-124.
- Seleem AK, El Refaeey AA, Shaalan D, Sherbiny Y, Badawy A. Superoxide dismutase in polycystic ovary syndrome patients undergoing intracytoplasmic sperm injection. J Assist Reprod Genet. 2014; 31: 499-504.
- Liu F, He L, Liu Y, Shi Y, Du H. The expression and role of oxidative stress markers in the serum and follicular fluid of patients with endometriosis. Clin Exp Obstet Gynecol. 2013; 40: 372-376.
- Simon L, Murphy K, Shamsi MB, Liu L, Emery B, Aston KI, et al. Paternal influence of sperm DNA integrity on early embryonic development. Hum Reprod. 2014; 29: 2402-2412.
- Ashwood-Smith MJ, Edwards RG. DNA repair by oocytes. Mol Hum Reprod. 1996; 2: 46-51.
- Guo XC, Segars JH. The impact and management of fibroids for fertility: an evidence-based approach. Obstet Gynecol Clin North Am. 2012; 39: 521-533.
- Pejic S, Todorovic A, Stojiljkovic V, Kasapovic J, Pajovic SB. Antioxidant enzymes and lipid peroxidation in endometrium of patients with polyps, myoma, hyperplasia and adenocarcinoma. Reprod Biol Endocrinol. 2009; 7: 149.
- Hoellen F, Griesinger G, Bohlmann MK. Therapeutic drugs in the treatment of symptomatic uterine fibroids. Expert Opin Pharmacother. 2013; 14: 2079-2085.
- Islam MS, Protic O, Giannubilo SR, Toti P, Tranquilli AL, Petraglia F, et al. Uterine leiomyoma: available medical treatments and new possible therapeutic options. J Clin Endocrinol Metab. 2013; 98: 921-934.
- Luyckx M, Squifflet JL, Jadoul P, Votino R, Dolmans MM, Donnez J. First series of 18 pregnancies after ulipristal acetate treatment for uterine fibroids. Fertil Steril. 2014; 102: 1404-1409.
- Aanesen A, Westerbotn M. Prospective study of a Swedish infertile cohort 2005-08: population characteristics, treatments and pregnancy rates. Fam Pract. 2014; 31: 290-297.
- Johnson J, Keefe DL. Ovarian aging: breaking up is hard to fix. Sci Transl Med. 2013; 5: 172fs5.
- Tatone C, Amicarelli F, Carbone MC, Monteleone P, Caserta D, Marci R, et al. Cellular and molecular aspects of ovarian follicle ageing. Hum Reprod Update. 2008; 14: 131-142.
- Grondahl ML, Yding Andersen C, Bogstad J, Nielsen FC, Meinertz H, Borup R. Gene expression profiles of single human mature oocytes in relation to age. Hum Reprod. 2010; 25: 957-968.
- Szafarowska M, Jerzak M. [Ovarian aging and infertility]. Ginekol Pol. 2013; 84: 298-304.
- Carbone MC, Tatone C, Delle Monache S, Marci R, Caserta D, Colonna R, et al. Antioxidant enzymatic defences in human follicular fluid: characterization and age-dependent changes. Mol Hum Reprod. 2003; 9: 639-643.
- Matos L, Stevenson D, Gomes F, Silva-Carvalho JL, Almeida H. Superoxide dismutase expression in human cumulus oophorus cells. Mol Hum Reprod. 2009; 15: 411-419.
- Ashwood-Smith MJ, Edwards RG. DNA repair by oocytes. Mol Hum Reprod. 1996; 2: 46-51.
- Gawecka JE, Marh J, Ortega M, Yamauchi Y, Ward MA, Ward WS. Mouse zygotes respond to severe sperm DNA damage by delaying paternal DNA replication and embryonic development. PLoS One. 2013; 8: e56385.
- Campbell A, Fishel S, Bowman N, Duffy S, Sedler M, Hickman CF. Modelling a risk classification of aneuploidy in human embryos using non-invasive morphokinetics. Reprod Biomed Online. 2013; 26: 477-485.
- Stoop D, Ermini B, Polyzos NP, Haentjens P, De Vos M, Verheyen G, et al. Reproductive potential of a metaphase II oocyte retrieved after ovarian stimulation: an analysis of 23 354 ICSI cycles. Hum Reprod. 2012; 27: 2030-2035.
- Wdowiak A. Pre-treatment with ulipristal acetate before ICSI procedure: a case report. (Zastosowanie octanu uliprystalu przed procedura ICSI: opis przypadku.) Prz Menopauz. 2013; 12: 496-500.
- Wdowiak A. Commentary on the article "Pre-treatment with ulipristal acetate before ICSI procedure: a case report" published in Menopause Review 6/2013 (Przeglad Menopauzalny 2013; 6: 496-500). Prz Menopauz 2014; 18: 150-151.
- Monleón J, Martínez-Varea A, Galliano D, Pellicer A. Successful pregnancy after treatment with ulipristal acetate for uterine fibroids. Case Rep Obstet Gynecol. 2014; 2014: 314587.
- Ismail PM, Li J, DeMayo FJ, O'Malley BW, Lydon JP. A novel LacZ reporter mouse reveals complex regulation of the progesterone receptor promoter during mammary gland development. Mol Endocrinol. 2002; 16: 2475-2489.
- Robker RL, Russell DL, Espey LL, Lydon JP, O'Malley BW, Richards JS. Progesterone-regulated genes in the ovulation process: ADAMTS-1 and cathepsin L proteases. Proc Natl Acad Sci U S A. 2000; 97: 4689-4694.
- Akison LK, Robker RL. The critical roles of progesterone receptor (PGR) in ovulation, oocyte developmental competence and oviductal transport in mammalian reproduction. Reprod Domest Anim. 2012; 47: 288-296.