
Review Article
Austin J In Vitro Fertili. 2015;2(2): 1018.
Pursuit of Markers to Assess and Select Competence of invitro-Produced Embryos
Rodriguez-Alvarez L¹* and Velasquez AE
Department of Animal Science, Universidad de Concepcion, Chile
*Corresponding author: Rodriguez-Alvarez Ll, Department of Animal Science, Faculty of Veterinary Sciences, Universidad de Concepcion, Avenida Vicente Mendez 595, Chillan, Chile
Received: April 28, 2015; Accepted: June 16, 2015; Published: June 18, 2015
Abstract
Morphological classification has been used as a conventional and noninvasive method to select pre-implantation embryos for transferring to recipients. However, low implantation rate and embryonic mortality after embryo transfer, specially using invitro produced or manipulated embryos, indicate than this method is not reliable enough to reflect the developmental competence of an embryo and it needs to be improved for biological and applied needs. In fact, embryo viability and competence correlate better with gene expression pattern than with embryo morphology. Nevertheless, the analysis of gene expression in pre-implantation embryos is an invasive procedure that most frequently implies the lysis of the embryo or a part of it. In this context, the identification of secreted markers linked to embryo quality and development competence may be a useful tool to classify pre-implantation embryos. Candidates may be studied in order to define suitable markers for embryo selection, widely expressed across species and correlated to developmental capacity and survival up to term. This paper presents a review of the literature on the different methods that may be used for embryo scoring as well of those used to predict embryo competence and quality. The most practical methods are those that consider embryo morphology. However, it seems that the molecular signature of each individual embryo is more predictive of its competence and capability to produce a healthy offspring. Many studies and experiments are required to propose a consistent method for embryo selection applicable in the assisted reproductive technologies in both humans and animals.
Keywords: Embryo competence; Embryo selection; Embryo development; ART
Introduction
Establishment and maintenance of a pregnancy able to produce a healthy offspring is the main goal of any reproductive system. In a natural conception, pregnancy lost before the 20th week of gestation reaches 75% in humans and it is attributed to implantation failure [1]. In farm animals, fertility defined as pregnancy rate per cycle might vary from 50 to 80 % and most of pregnancy loss occurs during the first three weeks due to defective embryo development [2-4]. It has been determined that pregnancy success depends on both embryo quality and uterine environment. In fact, embryonic loss is most likely attributed to maternal conditions such as animal age, nutritional factors, stress and uterine infections [3-9] in naturally occurring conceptions. However, invitro-produced embryos have a reduced developmental potential, resulting in a low implantation rate and an increase in the frequency of early pregnancy loss. Most of the in-vitro produced embryos are not able to function within a normal development schedule so that embryos can stop developing at any stage. Moreover, some of the embryos that reach the implantation stage do not induce a proper signal for pregnancy recognition and embryo-maternal crosstalk.
The development of Assisted Reproductive Technologies (ARTs) represents a great advance in both commercial and basic studies in animals, as well as in the treatment of infertility in humans [10-16]. However, despite continuous efforts to improve embryo development and competence, competent embryos produced invitro nowadays do not exceed 50 % of the total number of fertilized oocytes [17,18], while less than 40 % of transferred embryos produce a healthy offspring [18-21]. In humans, 8 out of 10 transferred embryos will not result in a pregnancy [22]. The low developmental potential of in-vitro produced embryos is mainly due to suboptimal conditions provided during oocyte collection and maturation, fertilization and embryo culture [23-25].
Scoring and selection of invitro-produced embryos
Morphology as a primary criterion for embryo selection: After invitro embryo production, selecting healthy embryos with the best potential to implant and produce an offspring is one of the major tasks for an embryologist. In humans, low embryonic competence is often handled by transferring several embryos to ensure a birth [26]. This practice is frequently the cause of preterm deliveries and other health complications for both the baby and the mother [27]. In farm animals, the main goal of ARTs is to multiply high value animals, which in the long-term contributes to the development of animal agriculture [17]. However, transfers of low competent embryos have a negative economic impact due to the direct costs associated with the maintenance of empty receptors, the price of supplies for embryo transfer and costs of the embryo or fetus lost.
In order to avoid the above-mentioned problems, it is mandatory to perform an accurate embryo selection before transferring. In general, morphological parameters are widely used criteria for embryo selection in all species. Morphology and timing of embryo development (the time of first cell cleavage and when embryos reach the morula or the blastocyst stages) are simple and non-invasive for the embryo. In those species in which embryos are transferred at early stages, the morphological selection is based on the Pronucleus (PN) size and location within either the zygote or the number and size of the blastomeres, as well as the fragmentation percentage in later stages (2-8 cells) [28-30]. In humans, some studies have demonstrated that embryo scoring using the PN characteristics might improve embryo selection. However, the use of this parameter is restricted to those species with visible PN at the zygote stage. Embryos from ruminants or pigs have a dark cytoplasm so that visualizing the PN is almost impossible.
When embryos at more advanced stages are transferred, for instance, at the blastocyst stage, criteria such as blastocyst expansion, quality of the Inner Cell Mass (ICM) and Trophoectoderm (TE) and grade of fragmentation are used for embryo classification [31,32]. Gardner and Schoolcraft [31] determined that the pregnancy rate in humans can be grater than 60 % by transferring a blastocyst with an ICM containing many tightly packed cells and a TE with many cells forming a cohesive layer. However, in some cases, blastocyst scoring might be challenging and depend on the subjective criterion of the embryologist. Moreover, the development schedule of invitroproduced embryos is very heterogeneous in concordance with their competence. In fact, even grade I embryos are often unable to maintain a normal pregnancy [18,21,33-36]. This statement has been demonstrated in several species; transferring grade I embryos produced by invitro fertilization or somatic cell nucleus transfer generate low rate of implantation and development to term [18,21,33- 37]. As a concrete example, we found that transferring bovine cloned blastocyst with a very similar morphology (Figure 1) produced only 33 % of pregnancy (day 35) and 11 % of calving [18]. Furthermore, selection of human embryos based on morphology cannot predict chromosome aneuploidies [38]. It is true that blastocyst morphology correlates with the incidence of aneuploidy. However, a high proportion of good and fair human blastocyst are aneuploidy (32 and 41 %, respectively) [37].
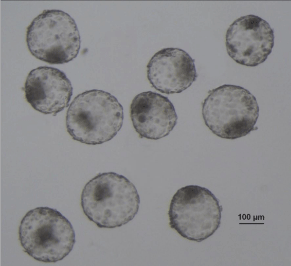
Figure 1: Cloned blastocyst morphologically classified as Grade I, selected to
be individually transferred embryo to synchronized recipients. Embryos were
produced by Hand Made Cloning, using a cell line from a Red Angus cow as a
nucleus donor. The rate of embryo development to blastocyst stage was 62.2
% and 72.8%of the total blastocysts were classified as Grade I. The mean
blastocyst diameter was 216.4 μm [18].
From the literature reviewed, it can be inferred that the cumulative data of the morphological parameters of the embryo at different developmental stages might improve embryo scoring and the success of pregnancy [39]. However, embryo evaluation by morphological tools is usually performed at the stage when embryos are transferred since the continuous disruption of embryo culture for evaluation may be detrimental for embryo development and affect its quality and competence negatively. The recorded data from individual embryos during the complete period of embryo culture using a timelapse system seems to be useful for the selection of human embryos [40,41]. However, this does not constitute a practical tool for invitro embryo production in farm animals with commercial purposes due to the high cost of the system, the reduced number of embryos that are checked at a time and the lack of validated programs to follow embryo development parameters of different species.
Despite the number of studies and experiments to improve the use of morphology as a reliable method for embryo scoring, to date, pregnancy outcome is still low in order to satisfy the reproductive needs especially in animals of economic value. In that sense, new strategies have been developed to be used in combination with morphological criteria.
Emerging strategies for embryo analysis and classification
The competence differences observed in embryos produced invitro can be associated with their molecular signature, including epigenetic modifications what will result in different gene expression patterns, metabolism and response to other manipulation, like cryopreservation [42]. Besides the morphology, the expression patterns of selected genes, the embryonic metabolism and secretome are emerging as interesting methods for embryos scoring and selection.
Improvements in technologies for embryo manipulation have facilitated the development of methods for embryo biopsy, which combined with accurate molecular techniques, allow for the use of DNA or RNA from few cells for genetic diagnosis and gene expression analysis. Currently, blastocysts might be biopsied for genomic DNA or gene expression analysis to test their further developmental potential in livestock species and humans [43-49]. However, the challenge of this technique is the identification of genetic markers that correlate with the developmental capability of the embryo and its potential to produce a viable offspring.
During the few last years, most of the efforts to establish a method for a consistent embryo selection have been based on the study and identification of gene markers, predictors of embryo quality, competence and implantation capability [18,45,50]. In addition, research has been focused on identifying genes that are commonly affected by the invitro conditions and that might be responsible for the low competence of invitro-produced embryos. As a result, there is an extensive list of papers that mention genes that are aberrantly expressed during the pre-implantation period as a consequence of the embryo production method, the embryo culture system, among others. However, no convincing markers had been firmly set yet [18,21,35,42,49,50].
For example, it have been demonstrated that canonical pluripotency markers (OCT4 (octamer-binding transcription factor 4), SOX2 (Sex determining Region Y-box 2and NANOG (Homeobox protein NANOG), responsible for the maintenance of the pluripotency of the ICM in bovineblastocyst, might be good candidates to predict embryo competence [18,50]. Bovine blastocysts produced invitro showed a marked deregulation of the expression levels of OCT4 and SOX2, while the expression level of these markers had a high variability between individual embryos regardless of the morphology (all embryos were morphologically classified as grade I) [18]. Moreover, when those embryos are transferred to a cattle recipient, not all of them are able to elongate. Nevertheless, when transferred embryos were recovered at Day-17 of development, all elongated embryos displayed correct expression of both markers [18]. This suggests that only blastocysts expressing normal levels of OCT4 and SOX2 are more likely to reach a further development, at least up to the elongation stage. In another experiment, it has been demonstrated that bovine cloned blastocysts with higher levels of OCT4 expression have a better morphological quality at the blastocyst stage and have a higher implantation rate [18] compared with blastocysts that expressed lower levels of OCT4. These results might indicate that the combination of genetic markers with the morphology parameters could be a good approach to predict embryo competence. However, further research is required to confirm these results since the higher implantation rate obtained is still very low [18]. El-Sayed and coworkers [45] performed a large-transcriptome analysis from blastocyst biopsies to determine candidate genes related to embryo developmental competence judged by the outcome of pregnancy. In this study, authors found a long list of differentially expressed genes between low competent blastocyst (resulting in no pregnancy, resorption and abortion) and competent blastocyst (resulting in calf delivery). Nevertheless, it is difficult to select a suitable marker as a predictor of embryo competence within a long list of candidates and several disrupted signaling pathways.
Apart from the lack of proper gene markers, the analysis of gene expression in pre-implantation embryos is an invasive procedure that implies the manipulation of the embryo for the lysis or a part of it. The invasive nature of this approach may be detrimental for the embryo and does not offer a reliable test for embryo quality.
Nowadays, non-invasive strategies are emerging especially in humans [51-53]. Those methods include the transcriptomic analysis of the cumulus cells at the moment of oocyte retrieval; from that, some gene markers related to embryo competence and successful pregnancy were identified [52]. This method seems to be useful in humans; only few oocytes are collected per patient but in animals, where hundreds of oocytes are processed at a time, may become unpractical. In this sense, the analysis of the embryo secretome (metabolites and proteins secreted to the culture medium) may provide a non-invasive method for assessing embryo quality with practical application. The study of embryo metabolism has revealed exaggerated differences between embryos that result in a pregnancy and those that do not [53,54]. In this context, different molecules have been measured in the culture media of the early embryo, including pyruvate, lactate, glucose, amino acid, oxygen and the proteomic profiling [54-60]. There are many examples in the literature in both humans and animals, supporting the possible use of metabolic profile to select competent embryos by their ability to alter the culture environment. For instance, Gardner and Leese [61] showed that the higher rate of glucose consumption predict the implantation potential of mouse blastocysts. Similar results have been obtained in human embryos, in which the greater glucose utilization positively correlates with the implantation potential of the blastocyst [62]. Lane and Gardner [63] showed that the selection of mouse blastocysts based on their glycolytic rate (around 50%) increases the implantation rate up to 96%.This contrasts with the value obtained when embryos were selected only by the morphology and that reached only20% of implantation.
Interesting information have emerged from these studies, but there some contradictions within the literature specially because different compositions of culture media may provoke stress in the embryo due to an excess of energy substrates or a deficit of essential nutrients [64]. Moreover, the metabolomics experiments require a multidisciplinary team and a proper methodology of analysis and further investigations are required to validate the algorithms used to determine the metabolites in different types and volume culture media [53,62].
The study of proteins produced by the embryo and secreted to the environment is emerging as a powerful tool for the assessment of embryo quality and competence. These studies may contribute to the knowledge of cellular processes of the embryo, including early interaction with the maternal environment. Changes in the transcription profile do not always predict a change in the phenotype (biological process and cellular functions) since not all mRNA will be translated. These facts, along with the advance in proteomic technologies, may expand the non-invasive methods to evaluate embryo competence.
At present, some proteins related to embryo development and competence has been identified. For instance, several reports suggest that leptin might be used as a marker for embryo development. It seems that leptin is involved in the initiation of the establishment of a molecular dialogue between the embryo and the maternal side at the time of implantation in humans and mice [65]. In mice, sheep and pigs, the addition of leptin to the culture medium has a positive effect on pre-implantation embryo development depending on the protein concentration and the embryo stage during the treatment [66-68]. However, previous studies showed that the addition of leptin to the culture media decreases the development of 2-cell mouse embryos in the blastocyst stage [69]. Therefore, inconsistency in the results that describe the beneficial effect of leptin on embryo development might be due to different conditions of the embryo culture and the general protocols for embryo scoring. However, based on the similarities of the leptin system in pre-implantation embryos from mice and humans, it is possible to suggest that leptin presence is required for implantation [66,70-72]. Moreover, it has been shown that competent human blastocysts secrete more leptin compared with developmental arrested embryos [65]. However, there are no functional evidences of the value of leptin to predict embryo implantation and delivery in any species. Therefore, further evidences are needed to propose this protein as a marker for embryo selection.
Apart from leptin, several reports have indicated an association between the presence of soluble HLA-G (human leukocyte antigen G; a non-classical MHC class I molecule that plays a role in immune tolerance in pregnancy) in the spent culture media with the potential of the embryos to produce successful pregnancies. This result suggests a possible use of this marker to predict quality and implantation success of human embryos [73,74]. However, there are contradictory studies since clinical pregnancies have been obtained by transferring sHLA-G–negative embryos [75].
Mains et al. [76] have recently published an interesting work where they performed a serial study of the spent media of invitroproduced human embryos with different morphology. In a first analysis, the comparison of day-4 spent media from good blastocyst and cleavage-arrested embryos, revealed several proteins that differed by at least 1.5 fold (increased or decreased) between both groups. In this experiment APOA1 (apolipoprotein A-I) was within the proteins that increased in good blastocyst. Furthermore, in a second analysis comparing day-5 spent media from the same group of embryos, only APOA1 was identified as higher (1.3 fold) in good quality blastocyst.
Based on the data presented above, it is possible to conclude that very few protein markers are described for embryo selection using a non-invasive method neither in human nor in animals. One of the limitations of this kind of studies is the complexity of the samples, the low amount of proteins in the spent media and the high cost of the experiments and analysis.
This review points to the fact that many studies and experiments are still required to propose a consistent method to select competent embryos produced invitro. On the other hand, combinations of different approaches may be an appropriate manner to perform embryo evaluation. Improvement of embryo selection is mandatory to increase the efficiency of the ARTs and the success of implantation and development of invitro-produced embryos.
References
- Wilcox AJ, Weinberg CR, O'Connor JF, Baird DD, Schlatterer JP, Canfield RE, et al. Incidence of early loss of pregnancy. N Engl J Med. 1988; 319: 189-194.
- Spencer TE. Early Pregnancy: Concepts, Challenges, and Potential Solutions. Animal Frontiers. 2013; 3: 48-55.
- Diskin MG, Morris DG. Embryonic and early foetal losses in cattle and other ruminants. Reprod Domest Anim. 2008; 43: 260-267.
- Hansen PJ. The immunology of early pregnancy in farm animals. Reprod Domest Anim. 2011; 46: 18-30.
- Rizos D, Ward F, Duffy P, Boland MP, Lonergan P. Consequences of bovine oocyte maturation, fertilization or early embryo development in vitro versus in vivo: implications for blastocyst yield and blastocyst quality. Mol Reprod Dev. 2002; 61: 234-248.
- Lonergan P, Rizos D, Gutierrez-Adan A, Moreira PM, Pintado B, de la Fuente J, et al. Temporal divergence in the pattern of messenger RNA expression in bovine embryos cultured from the zygote to blastocyst stage in vitro or in vivo. Biol Reprod. 2003; 69: 1424-1431.
- Hansen PJ, Block J, Loureiro B, Bonilla L, Hendricks KE. Effects of gamete source and culture conditions on the competence of in vitro-produced embryos for post-transfer survival in cattle. Reprod Fertil Dev. 2010; 22: 59-66.
- Lazzari G, Colleoni S, Lagutina I, Crotti G, Turini P, Tessaro I, et al. Short-term and long-term effects of embryo culture in the surrogate sheep oviduct versus in vitro culture for different domestic species. Theriogenology. 2010; 73: 748-757.
- Romaguera R, Morato R, Jimenez-Macedo AR, Catala M, Roura M, Paramio MT, et al. Oocyte Secreted Factors Improve Embryo Developmental Competence of COCs from Small Follicles in Prepubertal Goats. Theriogenology. 2010; 74: 1050-1059.
- Gordon IR. Reproductive Technologies in Farm Animals. CABI Publishing, Oxfordshire; 2004; 332.
- Thibier M. The zootechnical applications of biotechnology in animal reproduction: current methods and perspectives. Reprod Nutr Dev. 2005; 45: 235-242.
- Betteridge KJ. Farm animal embryo technologies: achievements and perspectives. Theriogenology. 2006; 65: 905-913.
- Galli C, Lazzari G. The manipulation of gametes and embryos in farm animals. Reprod Domest Anim. 2008; 43: 1-7.
- Duszewska AM, Trzeciak P, Compa A, Rapala L. Selected Issues Concerning Biotechnology of Farm Animals Breeding - A Review. Anim Sci Pap Rep. 2010; 28: 295-306.
- Figueiredo Freitas VJ, Melo LM. In Vitro Embryo Production in Small Ruminants. Rev Bras Zootec. 2010; 39: 409-413.
- Dang-Nguyen TQ, Somfai T, Haraguchi S, Kikuchi K, Tajima A, Kanai Y, et al. In vitro production of porcine embryos: current status, future perspectives and alternative applications. Anim Sci J. 2011; 82: 374-382.
- Verma OP, Kumar R, Nath A, Sharma M, Dubey PK, Kumar GS, et al. In vivo differentiation potential of buffalo (Bubalus bubalis) embryonic stem cell. In Vitro Cell Dev Biol Anim. 2012; 48: 349-358.
- Rodriguez-Alvarez L, Manriquez J, Velasquez A, Castro FO. Constitutive Expression of the Embryonic Stem Cell Marker OCT4 in Bovine Somatic Donor Cells Influences Blastocysts Rate and Quality after Nucleus Transfer. In Vitro Cell Dev Biol Anim. 2013; 49: 657-667.
- Solter D. Mammalian cloning: advances and limitations. Nat Rev Genet. 2000; 1: 199-207.
- Pomar FJ, Teerds KJ, Kidson A, Colenbrander B, Tharasanit T, Aguilar B, et al. Differences in the incidence of apoptosis between in vivo and in vitro produced blastocysts of farm animal species: a comparative study. Theriogenology. 2005; 63: 2254-2268.
- Rodriguez-Alvarez L, Cox J, Tovar H, Einspanier R, Castro FO. Changes in the expression of pluripotency-associated genes during preimplantation and peri-implantation stages in bovine cloned and in vitro produced embryos. Zygote. 2010; 18: 269-279.
- Kovalevsky G, Patrizio P. High rates of embryo wastage with use of assisted reproductive technology: a look at the trends between 1995 and 2001 in the United States. Fertil Steril. 2005; 84: 325-330.
- Wright R, Ellington J. Morphological and Physiological Differences Between In Vivo and In Vitro -Produced Preimplatation Embryo From Livestock Species. Theriogenology. 1995; 44: 1167-1189.
- Lonergan P, Fair T, Corcoran D, Evans AC. Effect of culture environment on gene expression and developmental characteristics in IVF-derived embryos. Theriogenology. 2006; 65: 137-152.
- Metwally M, Ledger WL. Long-term complications of assisted reproductive technologies. Hum Fertil (Camb). 2011; 14: 77-87.
- Reddy UM, Wapner RJ, Rebar RW, Tasca RJ. Infertility, Assisted Reproductive Technology, and Adverse Pregnancy Outcomes: Executive Summary of A National Institute of Child Health and Human Development Workshop. Obstet Gynecol. 2007; 109: 967-977.
- Bromer JG, Seli E. Assessment of embryo viability in assisted reproductive technology: shortcomings of current approaches and the emerging role of metabolomics. Curr Opin Obstet Gynecol. 2008; 20: 234-241.
- Fenwick J, Platteau P, Murdoch AP, Herbert M. Time From Insemination To First Cleavage Predicts Developmental Competence of Human Preimplantation Embryos In Vitro. Hum Reprod. 2002; 17: 407-412.
- Zollner U, Zollner KP, Hartl G, Dietl J, Steck T. The use of a detailed zygote score after IVF/ICSI to obtain good quality blastocysts: the German experience. Hum Reprod. 2002; 17: 1327-1333.
- Nagy ZP, Dozortsev D, Diamond M, Rienzi L, Ubaldi F, Abdelmassih R, et al. Pronuclear Morphology Evaluation With Subsequent Evaluation of Embryo Morphology Significantly Increases Implantation Rates. Fertil Steril. 2003; 80: 67-74.
- Gardner DK, Schoolcraft WB. A randomized trial of blastocyst culture and transfer in in-vitro fertilization: reply Hum Reprod. 1999; 14: 1663-1663.
- Richter KS, Harris DC, Daneshmand ST, Shapiro BS. Quantitative grading of a human blastocyst: optimal inner cell mass size and shape. Fertil Steril. 2001; 76: 1157-1167.
- Bavister B. Chapter 6. Assessment of Mammalian Embryo Quality: Invasive and non-invasive techniques. van Soom A, Boerjan M. editors. 2002; 139-151.
- Rodriguez-Alvarez L, Sharbati J, Sharbati S, Cox JF, Einspanier R, Castro FO. Differential Gene Expression in Bovine Elongated (D-17) Embryos Produced by Somatic Cell Nucleus Transfer and In Vitro Fertilization. Theriogenology. 2010; 74: 45-59.
- Boiani M, Eckardt S, Schöler HR, McLaughlin KJ. Oct4 distribution and level in mouse clones: consequences for pluripotency. Genes Dev. 2002; 16: 1209-1219.
- Wrenzycki C, Herrmann D, Lucas-Hahn A, Gebert C, Korsawe K, Lemme E, et al. Epigenetic Reprogramming Throughout Preimplantation Development And Consequences for Assisted Reproductive Technologies. Birth Defects Res C Embryo Today. 2005; 75: 1-9.
- Phan Vy, Eva Littman, Dee Harris, Antoine La. Correlation Between Aneuploidy and Blastocyst Quality. Asian Pacific Journal of Reproduction. 2014; 3: 253-257.
- Milachich T. New advances of preimplantation and prenatal genetic screening and noninvasive testing as a potential predictor of health status of babies. Biomed Res Int. 2014; 306505.
- Racowsky C, Ohno-Machado L, Kim J, Biggers JD. Is there an advantage in scoring early embryos on more than one day? Hum Reprod. 2009; 24: 2104-2113.
- Machtinger R, Racowsky C. Morphological systems of human embryo assessment and clinical evidence. Reprod Biomed Online. 2013; 26: 210-221.
- Iwata K, Yumoto K, Sugishima M, Mizoguchi C, Kai Y, Iba Y, et al. Analysis of compaction initiation in human embryos by using time-lapse cinematography. J Assist Reprod Genet. 2014; 31: 421-426.
- Urrego R, Rodriguez-Osorio N, Niemann H. Epigenetic disorders and altered gene expression after use of Assisted Reproductive Technologies in domestic cattle. Epigenetics. 2014; 9: 803-815.
- Picard L, King WA, Betteridge KJ. Production of sexed calves from frozen-thawed embryos. Vet Rec. 1985; 117: 603-608.
- Bredbacka P, Velmala R, Peippo J, Bredbacka K. Survival of biopsied and sexed bovine demi-embryos. Theriogenology. 1994; 41: 1023-1031.
- El-Sayed A, Hoelker M, Rings F, Salilew D, Jennen D, Tholen E, et al. Large-scale transcriptional analysis of bovine embryo biopsies in relation to pregnancy success after transfer to recipients. Physiol Genomics. 2006; 28: 84-96.
- Zoheir KM, Allam AA. A rapid method for sexing the bovine embryo. Anim Reprod Sci. 2010; 119: 92-96.
- Cenariu M, Pall E, Cernea C, Groza I. Evaluation of Bovine Embryo Biopsy Techniques According to Their Ability to Preserve Embryo Viability. J Biomed Biotechnol. 2012.
- Herrera C, Morikawa MI, Bello MB, von Meyeren M, Centeno JE, Dufourq P, et al. Setting Up Equine Embryo Gender Determination by Preimplantation Genetic Diagnosis in A Commercial Embryo Transfer Program. Theriogenology. 2014; 81: 758-763.
- Velasquez AE, Castro FO, Veraguas D, Cox JF, Lara E, Briones M, et al. Splitting of IVP bovine blastocyst affects morphology and gene expression of resulting demi-embryos during in vitro culture and in vivo elongation. Zygote. 2014.
- Khan DR, Dube D, Gall L, Peynot N, Ruffini S, Laffont L, et al. Expression of Pluripotency Master Regulators During Two Key Developmental Transitions: EGA and Early Lineage Specification in The Bovine Embryo. PLoS One. 2012; 7: 34110.
- Pearson H. Safer embryo tests could boost IVF pregnancy rates. Nature. 2006; 444: 12-13.
- Assou S, Haouzi D, Mahmoud K, Aouacheria A, Guillemin Y, Pantesco V, et al. A non-invasive test for assessing embryo potential by gene expression profiles of human cumulus cells: a proof of concept study. Mol Hum Reprod. 2008; 14: 711-719.
- Botros L, Sakkas D, Seli E. Metabolomics and its application for non-invasive embryo assessment in IVF. Mol Hum Reprod. 2008; 14: 679-690.
- Muñoz M, Uyar A, Correia E, Ponsart C, Guyader-Joly C, Martinez-Bello D, et al. Metabolomic Prediction of Pregnancy Viability in Superovulated Cattle Embryos and Recipients With Fourier Transform Infrared Spectroscopy. Biomed Res Int. 2014; 608579.
- Sakkas D, Gardner DK. Noninvasive methods to assess embryo quality. Curr Opin Obstet Gynecol. 2005; 17: 283-288.
- Urbanski JP, Johnson MT, Craig DD, Potter DL, Gardner DK, Thorsen T. Noninvasive metabolic profiling using microfluidics for analysis of single preimplantation embryos. Anal Chem. 2008; 80: 6500-6507.
- Dominguez F, Gadea B, Esteban FJ, Horcajadas JA, Pellicer A, Simon C. Comparative protein-profile analysis of implanted versus non-implanted human blastocysts. Hum Reprod. 2008; 23: 1993-2000.
- Katz-Jaffe MG, McReynolds S, Gardner DK, Schoolcraft WB. The role of proteomics in defining the human embryonic secretome. Mol Hum Reprod. 2009; 15: 271-277.
- Gardner DK, Wale PL, Collins R, Lane M. Glucose consumption of single post-compaction human embryos is predictive of embryo sex and live birth outcome. Hum Reprod. 2011; 26: 1981-1986.
- Sugimura S, Akai T, Hashiyada Y, Somfai T, Inaba Y, Hirayama M, et al. Promising system for selecting healthy in vitro-fertilized embryos in cattle. PLoS One. 2012; 7: 36627.
- Gardner DK, Leese HJ. Assessment of embryo viability prior to transfer by the noninvasive measurement of glucose uptake. J Exp Zool. 1987; 242: 103-105.
- Gardner DK, Wale PL. Analysis of metabolism to select viable human embryos for transfer. Fertil Steril. 2013; 99: 1062-1072.
- Lane M, Gardner DK. Selection of viable mouse blastocysts prior to transfer using a metabolic criterion. Hum Reprod. 1996; 11: 1975-1978.
- Devreker F, Hardy K, Van den Bergh M, Winston J, Biramane J, Englert Y. Noninvasive Assessment of Glucose and Pyruvate Uptake by Human Embryos after Intracytoplasmic Sperm Injection and During the Formation of Pronuclei. FertilSteril. 2000; 73: 947-954.
- Cervero A, Horcajadas JA, Dominguez F, Pellicer A, Simon C. Leptin system in embryo development and implantation: a protein in search of a function. Reprod Biomed Online. 2005; 10: 217-223.
- Kawamura K, Sato N, Fukuda J, Kodama H, Kumagai J, Tanikawa H, et al. Leptin promotes the development of mouse preimplantation embryos in vitro. Endocrinology. 2002; 143: 1922-1931.
- Herrid M, Nguyen VL, Hinch G, McFarlane JR. Leptin has concentration and stage-dependent effects on embryonic development in vitro. Reproduction. 2006; 132: 247-256.
- Li XX, Lee DS, Kim KJ, Lee JH, Kim EY, Park JY, et al. Leptin and Nonessential Amino Acids Enhance Porcine Preimplantation Embryo Development In Vitro by Intracytoplasmi Sperm Injection. Theriogenology. 2013; 79: 291-298.
- Fedorcsak P, Storeng R. Effects of leptin and leukemia inhibitory factor on preimplantation development and STAT3 signaling of mouse embryos in vitro. Biol Reprod. 2003; 69: 1531-1538.
- Chehab FF. A broader role for leptin. Nat Med. 1996; 2: 723-724.
- Mounzih K, Lu R, Chehab FF. Leptin treatment rescues the sterility of genetically obese ob/ob males. Endocrinology. 1997; 138: 1190-1193.
- Malik NM, Carter ND, Murray JF, Scaramuzzi RJ, Wilson CA, Stock MJ. Leptin requirement for conception, implantation, and gestation in the mouse. Endocrinology. 2001; 142: 5198-5202.
- Desai N, Filipovits J, Goldfarb J. Secretion of Soluble HLA-G by Day 3 Human Embryos Associated With Higher Pregnancy and Implantation Rates: Assay of Culture Media Using a New ELISA Kit. Reprod Biomed Online. 2006; 13: 272-277.
- Warner CM, Lampton PW, Newmark JA, Cohen J. Symposium: Innovative Techniques in Human Embryo Viability Assessment. Soluble Human Leukocyte Antigen-G and Pregnancy Success. Reprod Biomed Online 2008; 17: 470-485.
- Vercammen MJ, Verloes A, Van de Velde H, Haentjens P. Accuracy of Soluble Human Leukocyte Antigen-G for Predicting Pregnancy Among Women Undergoing Infertility Treatment: Meta-Analysis. Hum Reprod Update. 2008; 14: 209-218.
- Mains LM, Christenson L, Yang B, Sparks AE, Mathur S, Van Voorhis BJ. Identification of apolipoprotein A1 in the human embryonic secretome. Fertil Steril. 2011; 96: 422-427.