
Research Article
Austin J In Vitro Fertili. 2015;2(2): 1020.
Tenascin Expression in Human Placentas during FGRAffected Pregnancies and Umbilical Doppler Velocimetry Correlation
Locci M¹, Nazzaro G¹, Miranda M¹, Iazzetta R¹, Patrì A¹, D’Anna M², Castaldo C²* and Montagnani S²
¹Department of Nervous, “Federico II” University of Naples, Italy
²Department of Public Health, “Federico II” University of Naples, Italy
*Corresponding author: Castaldo C, Department of Public Health, “Federico II” University of Naples, via Pansini 5 80131 Naples, Italy
Received: June 03, 2015; Accepted: July 13, 2015; Published: June 15, 2015
Abstract
Objective: The aim of this study was to evaluate the expression of some non collagenous extracellular matrix proteins, in particular tenascin, in human placentas of intrauterine growth restricted fetuses with abnormal umbilical Doppler velocimetry.
Study Design: Study group (group A) consisted of 23 pregnant women with intrauterine growth restricted fetuses, with or without preeclampsia. Control group (group B) consisted of 10 pregnant women with appropriate fetal weight for gestational age. Placental specimens were collected from biopsies obtained after cesarean delivery. Umbilical artery Doppler velocimetry was performed within four hours from delivery in all patients. Tenascin expression was studied by immunohistochemistry and western blot techniques.
Results: A difference in birth weight and placental weight was found in the two groups, being lower in the study group. Umbilical artery Doppler velocimetry showed abnormal patterns in the study group and normal findings in the control one. Tenascin was strongly expressed in placentas from growth restricted fetuses, as shown by immunohistochemistry and by RT-PCR, while it was almost absent in placentas from group B.
Conclusion: A relationship between abnormal Doppler patterns and tenascin distribution in growth restricted fetuses has been observed. The presence of tenascin might be considered as a placental compensatory mechanism in FGR fetuses with abnormal umbilical artery Doppler velocimetry.
Keywords: Fetal growth restriction, Tenascin, umbilical Doppler velocimetry, Extracellular Matrix
Abbreviations
FGR: Fetal Growth Restriction; PE: Preeclampsia; ROS: Reactive Oxygen Species; SOD: Superoxide Dismutase; ECM: Extracellular Matrix; MMPs: Matrix Metalloproteinase’s; AGA: Appropriate for Gestational Age; AC: Abdominal Circumference; LBW: Low Birth Weight; VLBW: Very Low Birth Weight; PI: Pulsatility Index; AEDF: Absent End-Diastolic Flow; REDF: Reversed End-Diastolic Flow; VEGFR: Vascular Endothelial Growth Factor Receptor-2.
Introduction
Fetal Growth Restricted (FGR) fetuses are the fetuses in which growth restriction implies a pathological restriction of the genetic growth potential. As a result, FGR fetuses manifest evidence of fetal compromise i.e. abnormal Doppler velocimetry [1]. FGR with or without Preeclampsia (PE) complicates a significant number of pregnancies [2,3]. This condition is an important risk factor for adverse perinatal outcome and contributes to maternal and perinatal morbidity and mortality. Impaired placental perfusion and angiogenesis seem to be the most common causes of FGR, even if, in some cases, both FGR and PE are related to a failure of immunomodulatory placental functions [4,5]. Moreover, excessive levels of placental oxidative stress lead to PE and FGR, and placental hypoxia-reoxygenation is a potential cause of such stress. High levels of Reactive Oxygen Species (ROS) may induce cellular apoptosis. Thus, ROS-scavenging enzymes, such as Superoxide Dismutase (SOD) enzyme family, are important for preserving fetal growth [6]. Several placental histological and morphological abnormalities such as infarcts, terminal villous fibrosis and impaired trophoblastic invasion have been well described in FGR placentas [7-10]. Many studies have reported an association between abnormal Doppler velocimetry changes in umbilical artery and adverse perinatal outcome in growth restricted fetuses. The correlation between placental morphology and umbilical artery Doppler velocimetry, an indicator of placental vascular resistance, shows that substantial changes in the growth of villi and in fetal vasculature can reduce maternal-fetal exchanges of nutrients and oxygen and contribute to fetal hypoxic stress [11].
The human placenta, as a boundary organ between mother and fetus, plays a dynamic role in establishing and maintaining pregnancy through a multifunctional way involving continuous rearrangement in its structure. It is now widely accepted that the Extracellular Matrix (ECM) is a dynamic and highly specialized structure, involved in several signal transduction pathways [12]. For instance, ECM proteins are degraded by Matrix Metalloproteinases (MMPs), endopeptidase capable of processing a number of bioactive molecules. They are released by placental cells during tissue remodeling processes, cell migration and neoangiogenesis: aberrations in MMPs activity in early pregnancy can play a role in the physiopathology of conditions like FGR [13]. It has been recently suggested that ECM proteins, such as fibronectin, tenascin and laminin, play different roles in cell proliferation, migration and differentiation. Tenascin, in particular, has shown to be able in modulating cellular adhesion by increasing or decreasing it, and seems to be involved in the immunological protection of the embryo during implantation. Moreover, tenascin seems to be involved in villous repairing after placental infarctions and in neoangiogenic mechanisms [14,15]. Also fibronectin, which often co-distributes with tenascin, and laminin, can induce migration of cell populations and metabolites [16]. Cell–matrix interactions represent a so important phenomenon that we can hypothesize that some changes in the structure and in the distribution of these proteins are involved in pregnancies complicated by FGR. Moreover, tenascin, whose expression has been correlated with villous growth, cells proliferation and fibrinoid deposition, may play a critical role in placental homeostasis development throughout pregnancy [14]. Tenascin is a large ECM glycoprotein. Until now five isoforms are known: tenascin-C, or cytotactin, which plays a regulatory role on neuron morphology and adhesion, representing the most abundant form [17], tenascin-X, whose gene is on chromosome 6 and is typically detected in muscles, tenascin-Y distributed on muscletendon junctions, tenascin-J or janusin, exclusive of the nervous tissue and tenascin-R which is synthesized by oligodendrocytes during mielinization [18].
Tenascin-C is mostly expressed in the mesenchymal villi, cell islands and columns. These structures are the proliferating units of the villous trees. Probably this molecule is involved in angiogenesis. In addition, tenascin separates fibrinoid deposits at the surface of the villous trees from the fetal stroma. This location suggests a role of such protein in placental repair mechanisms and in immunoprotection of fetal tissues [19].
In the present study we have investigated the placental expression of non collage nous ECM components such as fibronectin and tenascins in pregnancies complicated by FGR with or without PE, with abnormal umbilical artery Doppler findings. Our attention was particularly appointed on the expression of tenascin-C, as this protein is involved in various aspects of cell and tissue development and in cell-to-cell and cell-to-substrate adhesion.
Our study includes a control group of healthy pregnancies with appropriate fetal gestational age and normal umbilical artery Doppler waveforms.
Patients and Methods
Patients
A number of 33 Caucasian pregnant women were recruited between January 2010 and December 2013. On the total, 23 patients affected by FGR, with or without PE, were considered for the study group (group A). All patients of the group A were submitted to cesarean section. The control group (group B) consisted of 10 pregnant women with appropriate fetal growth, homogeneous for age, BMI (20-25 kg/m2), socioeconomic status and gestational age to the group A (Table 1). In the group B, abnormal presentation and previous cesarean section were the indications for cesarean section. Gestational age was calculated using the crown-rump length ultrasonographic determination in the first trimester, according to the last menstrual period. Ultrasound criteria were applied to assess fetal growth. Fetuses were considered Appropriate for Gestational Age (AGA) if Abdominal Circumference (AC) was found between 10th and 95th percentile. Fetuses were considered affected by FGR, if abdominal circumference was found below the 10th percentile of our standard population fetal growth curves. At delivery, neonates were consisted Low Birth Weight (LBW) if neonatal weight was less than 2,500 g and Very Low Birth Weight (VLBW) if less than 1,500 g.
FGR (group A)
AGA (group B)
p
Number of cases
23
10
Age (media +/- DS)
28.8+/-4
21.2 +/- 5
0,525
Parity
0.6+/- 0.3
0.4+/- 0.2
0.338
G. A. (delivery)
35+/- 3.3
36.1+/- 2.1
0.003
Birth weight (g)
1680+/- 530
3002+/- 420
<0.001
Placental weight (g)
298+/- 84
480+/- 95
<0.001
Pre-eclampsia (n)
11/21
-
Perinatal morbidity (n)
20
-
<0.001
PI > 2 DS (n/%)
9/%
-
<0.001
ARED (n/%)
12/%
-
<0.001
Table 1: Clinical Features of Group A (FGR) and group B (AGA).
PE complicated 11 cases out of 23 of the group A. At delivery, placental tissue was collected with the permission of the local research, Ethics Committee and with the informed consent of all patients. Clinical characteristics of the two groups were compared by the one-way ANOVA test.
Doppler evaluation
The Doppler evaluation of umbilical artery resistance was performed within four hours from delivery in both groups. Doppler signals were obtained using an Echo Color Doppler Samsung Medison A30 and an Echo Color Doppler Samsung WS80 combined with a 3.5 MHz convex probe. The Doppler sample volume was placed on a free-floating tract of the umbilical cord during fetal quiet status. A near zero insonation angle was obtained for every Doppler examination. To analyze the Doppler flow velocity waveforms, the Pulsatility Index (PI) was automatically calculated (maximum velocity–minimum velocity/mean velocity) and the average of three consecutive measurements was used for analysis to minimize intraobserver variability. Doppler findings were considered as mild if PI values were above the 2SD of the mean for gestational age based reference standards, moderate when the End-Diastolic Flow Was Absent (AEDF), severe when the End Diastolic Flow was Reversed (REDF). All measurements were performed by a single experienced investigator (G. N.) to minimize inter-observer variability.
Immunohistochemistry
Normal and pathological placental tissue samples were randomly harvested from both maternal and fetal side. Specimens were fixed and embedded in paraffin or cryopreserved at – 80°C. 5 Μm-thick serial sections were cut, mounted on slides, and immunostained for Tenascin (Sigma, St. Louis, MO, USA, mouse monoclonal IgG) and Fibronectin (Sigma, rabbit polyclonal IgG) as previously described [20]. Briefly, immunohistochemistry was performed with indirect immunofluorescence technique: sections were incubated for 30 minutes with 10% serum derived from the species in which the secondary antibody was raised, and subsequently with primary antibody for 60 minutes at 37°C. After PBS washes, slides were incubated with rhodamine or fluoresce in conjugated anti-rabbit or anti-mouse IgG antibody for 60 minutes at 37°C. Nuclei were labeled with DAPI for 15 minutes at room temperature before the final washes in PBS and sections were then mounted in Vectashield. Signal was visualized with a Leica DMLB fluorescence microscope. Negative controls were included for each staining using an isotypematched non-specific antibody. Microscopic analysis was performed by three independent observers, using a four point arbitrary scale ranging from 0 (total absence of immunopositivity) to 3 (very strong immunopositivity), and pictures were taken with digital camera connected to the microscope (Leica DC200).
RT-PCR
RT-PCR was used to analyze target gene expression in the present study. Total RNA was isolated by lysing frozen tissue samples (150- 300 mg) in Trizol solution (Life Technologies, GIBCO BRL) according to the supplier’s protocol. RNA was precipitated and quantified by spectroscopy. 1 Μg of total RNA of each sample was reversely transcribed using the First-Strand cDNA Synthesis Kit (Amersham Pharmacia Biotech) according to the protocol supplied by the manufacturer. The random hexamer primers provided in the kit were used. The same cDNA product obtained from each sample was used for subsequent PCR amplification with the primer sets prepared for the target gene and GAPDH housekeeping gene. The amplification of the GAPDH gene was used as double internal control. The ratio between the samples and the housekeeping gene was calculated to normalize for initial variations in sample concentration and as a control for reaction efficiency. Primer sequences were designed using the software Primer 3 (developed by Steve Rozen, Helen J Skaletsky) available on-line at https://www-genome.wi.mit.edu. Semiquantitative Polymerase Chain Reaction (PCR) was performed using the following conditions: 95°C, 5 min initial denaturing phase; 95°C, 1 min; 55°C, 1 min; 72°C, 1 min for 35 cycles; 72°C, 10 min, final extension. The reaction was carried out in a total volume of 50 ml containing 3 Μl of cDNA, 10-20 pmol of each primer, 200 mM each of dNTP, 1.5 mM MgCl2 and 1 unit of Taq polymerase with the reaction buffer supplied with the kit. In each experiment, possible DNA contamination was determined by a control reaction in which cDNA was omitted from the reaction mixture and replaced by DNAse and RNAse free water. The amplified products (12 Μl of each sample) were analyzed by electrophoresis in a 2% agarose gel containing ethidium bromide, followed by photography under ultraviolet illumination. The levels of mRNA were estimated by densitometric scanning and normalized against GAPDH loading controls. Densitometric analyses of the PCR products were performed using the ImageJ v1.29 software (developed by Wayne Rasband) available on-line at https://rsb.info.nih.gov/ij/. All PCR products were purified using QIAquick PCR purification kit (Qiagen, Santa Clarita, CA, USA) and their identities verified by automated DNA forward and reverse sequencing using adideoxy terminator reaction chemistry for sequence analysis on the Applied biosystem Model 373A DNA sequence.
Results
Patients
Clinical characteristics of the two groups are shown in Table 1. No difference for age, parity and gestational age at delivery was found between the two groups. Both neonatal and placental weight were lower in the study group than in the control group (p<0.001). Increased perinatal morbidity was observed in the study group (p<0.001). Group A babies were further divided in two groups according to birth weight: 10 LBW and 13 VLBW neonates were found. PE was detected in 5 out of 10 pregnancies with LBW infant and in 6 out of 13 pregnancies with VLBW infant.
Doppler evaluation
Normal umbilical artery Doppler pattern was found in the control group (Figure 1a). Increased umbilical artery PI values above 2DS for our normal curves were found in all LBW fetuses (Figure 1b); AEDF (Figure 1c) or REDF (Figure 1d) were detected in all VLBW fetuses.
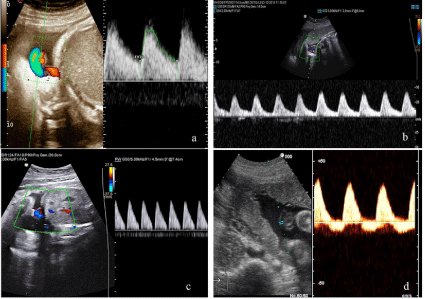
Figure 1: Normal umbilical artery Doppler pattern in the control group (a); increased umbilical artery PI values above 2DS in LBW fetuses (b); AEDF (c) and REDF
(d) in VLBW fetuses.
Immunohistochemistry
Fibronectin showed a normal distribution without significant differences between control and FGR placentas, with or without Doppler anomalies. Immunopositivity for Tenascin was very weak in healthy placentas (Figure 2), when compared with the FGR ones, where Tenascin was strongly expressed in both maternal and fetal side, with a mosaic-like pattern of distribution. To better relate Doppler findings with immunohistochemistry, Tables 2A and 2B show the Immunohistochemical and Doppler findings in the study group. Table 2A, in particular, regards low birth weight FGR. Table 3 shows Immunohistochemical observations as well as Doppler findings in the control group with appropriate birth weight (AGA).
Cases
Birth weight
AG
FGR
FGR+PE
Tenascin
Fibronectin
Velocimetry Doppler
DCB
LBW
III trimester
·
+++
++
P.I.> 2 DS
GC
LBW
III trimester
·
++
+
P.I.> 2 DS
MM
LBW
III trimester
·
+++
+
P.I.> 2 DS
LM
LBW
III trimester
·
+++
++
P.I.> 2 DS
MR
LBW
III trimester
·
++/+++
+
P.I.> 2 DS
AL
LBW
III trimester
·
++/+++
+
P.I.> 2 DS
RA
LBW
III trimester
·
+++
+
P.I.> 2 DS
AT
LBW
III trimester
·
++
+
P.I.> 2 DS
TF
LBW
III trimester
·
+++
+
P.I. >2 DS
LP
LBW
III trimester
·
++/+++
+
P.I. >2 DS
Table 2a: Perinatal outcome, Doppler velocimetry and Immunohistochemical features in LBW (Group A) fetuses.
Cases
Birth weight
AG
FGR
FGR+PE
Tenascin
Fibronectin
Velocimetry Doppler
PL
VLBW
III trimester
·
+/-
++
A.R.E.D
MS
VLBW
III trimester
·
+
++
A.R.E.D.
GM
VLBW
III trimester
·
+/-
+++
A.R.E.D.
LN
VLBW
III trimester
·
+
++
A.R.E.D.
AS
VLBW
III trimester
·
++
++
A.R.E.D.
DF
VLBW
III trimester
·
-
++
A.R.E.D.
SB
VLBW
III trimester
·
++
++
A.R.E.D.
AR
VLBW
III trimester
·
+/-
++
A.R.E.D.
RF
VLBW
III trimester
·
+/-
++
A.R.E.D.
MA
VLBW
III trimester
·
+/-
+
A.R.E.D.
LT
VLBW
III trimester
·
+
++
A.R.E.D.
GG
VLBW
III trimester
·
+/-
+
A.R.E.D.
LC
VLBW
III trimester
·
+
++
A.R.E.D.
Table 2b: Perinatal outcome, Doppler velocimetry and immunohistochemical features in VLBW fetuses (Group A).
Cases
Birth weight
AG
Tenascin
Fibronectin
Velocimetry
Doppler
PL
AGA
III trimester
+
+++
Regular PI
RT
AGA
III trimester
+/-
++/+++
Regular PI
MG
AGA
III trimester
+/-
++/+++
Regular PI
DA
AGA
III trimester
+
++/+++
Regular PI
MP
AGA
III trimester
+
+++
Regular PI
GG
AGA
III trimester
+/-
++
Regular PI
SR
AGA
III trimester
+/-
+++
Regular PI
ST
AGA
III trimester
+
++
Regular PI
TT
AGA
III trimester
+/-
++/+++
Regular PI
CA
AGA
III trimester
+
+++
Regular PI
Table 3: Perinatal outcome, Doppler velocimetry and immunohistochemical features (Group B).
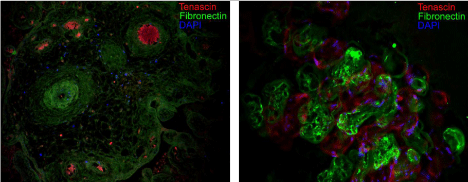
Figure 2: Tenascin (red fluorescence) and fibronectin (green fluorescence)
distribution in control (left) and FGR (right) placentas. Nuclei are
counterstained with DAPI (blue fluorescence).
RT- PCR
RT-PCR analysis allowed the comparison between the two groups of patients in terms of tenascin synthesis (Figure 3). Tenascin-C and tenascin-X were low in placentas from the control group, while they were synthesized in all the placental specimens from the group A. The expression of tenascin subunits was not affected by individual variability in pathological specimens, but it is reasonable that its amount might be related to different gestational age.
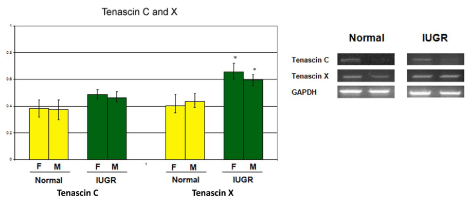
Figure 3: Expression of tenascin as measured by RT-PCR.
Discussion
Placentation requires extensive vasculogenesis and subsequent angiogenesis with deep ECM involvement in tissue remodeling. Impaired placental perfusion and angiogenesis, resulting in placental pathology, are considered the most important causes of FGR [4,5]. According to umbilical Doppler analyses, FGR fetuses have been subdivided in groups of progressive severity [21]. Correlations between acid-base balance and Doppler findings show that only the most severe FGR fetuses have impaired oxygenation and acid-base balance [22].
Many studies documented severe histological and morphological abnormalities in placentas of affected fetuses [7,8,23,24]. In a previous investigation, we approached some factors involved in the balance between the oxidative stress and the expression of molecules preventing and/or protecting tissues, like SOD, MMP-2 and MMP-9, and some receptors for angiogenic factors, i.e. Vascular Endothelial Growth Factor Receptor (VEGFR)-2 and angiopoietin-1 receptor. We found that extracellular SOD, the main anti-oxidant enzyme in vascular wall, was significantly reduced in FGR placentas. As regards angiogenesis, we observed an increased expression of VEGFR-2 (early marker of neoangiogenesis) and a reduction of angiopoietin-1 receptor (marker of mature vessels) in FGR placentas. Moreover, MMP-2 and MMP-9 were constantly reduced in IUGR placentas. We hypothesized that oxygen levels affect ECM remodeling by MMP and neoangiogenesis as a consequence [25]. In another earlier study, we found extracellular matrix abnormalities more evident in fetuses affected by FGR with AEDF or REDF in umbilical artery [22]. These findings prompted us to hypothesize that the ECM structure plays a critical role in umbilical artery Doppler velocimetry regulation. An increased ECM stiffness and the consequential changes in the pressure gradient between fetus and placenta may exert an effect on the development of FGR and on the onset of abnormalities in Doppler findings in umbilical artery. Tenascin is poorly expressed in adult normal tissues, but it is involved in many wound repair mechanisms [14], being a peculiar ECM component mainly expressed during embryonic and tumor growth, but weakly in term placenta. In human placenta tenascin is expressed in a mosaic-like way in the mesenchymal villi, cell islands and cell columns during the developmental placental stages [26].
Castellucci et al. [14,19] have investigated the expression of this molecule in the placenta as related to epitelial-mesenchymal interaction, cells proliferation and fibrin deposition. It is particularly interesting to study its expression and its distribution inside an organ like placenta, which has a very high metabolic activity and homes a lot of different cell population. In fact, some authors have hypothesized an immunomodulation and fetal protection function for tenascin; under this point of view, our observation that this protein strongly increases during PE supports the theory of a maternal immune system involvement in this kind of pathology.
Indeed tenascin distribution through gestation seems to suggest that this molecule plays a pivotal role in placental development. Also a positive modulation of cell migration seems to be due to this protein: it is well known, in fact, that it is involved in the detachment of neoplastic cells during breast cancer and in other kinds of neoplasm, as tenascin rich basal lamina are easily crossed by cells and molecules [35]. All these functions are particularly and characteristically expressed during early Placentation, becoming progressively less evident with placental physiological ageing [28]. Since tenascin C and X immunopositivity is very low in term placenta, it was surprising that tenascin X was often highly expressed in FGR specimens we studied, with a “mosaic” distribution within placental tissue.
Tenascin expression was more weak in the FGR fetuses with severe umbilical Doppler findings (ARED); on the contrary, it was very strong in the early gestation as well as in the FGR fetuses with moderate alteration in Doppler findings (PI> 2SD).
The presence of tenascin in the media of larger blood vessels of tumors and in capillary walls might be related with an angiogenic activity of this molecule, as improved tenascin-mediated angiogenesis and wound repair mechanism might be considered a compensatory mechanism in case of growth restriction with LBW.
The weak tenascin immunoreactivity in FGR fetuses with VLBW could be considered as a failed compensative mechanism of placental wound repair and angiogenesis.
Between the 28th and 32nd week of gestation, the reappearance of end diastolic flow in umbilical artery could lead to an improvement of abnormal umbilical artery velocimetry with less severe FGR, or to a persistent ARED flow that lead to the most severe FGR associated with abnormal umbilical artery Doppler velocimetry [29-31]. It is highly suggestive that these fluctuating values depend on wound repair mechanisms which operate during placental development.
Conclusion
Based on our results, strong tenascin immunoreactivity could be considered as a marker of improved wound repair and angiogenesis in these FGR fetuses with better perinatal outcome. Finally, impaired umbilical artery Doppler velocimetry with persistence of end diastolic flow would be possible when the wound repair mechanism in placental tissue does not fail. An ongoing study has been settled to verify if tenascin or related metabolites can be sampled in cervicovaginal fluid. On these bases, tenascin could be a tailor biomarker of placental angiogenesis in FGR fetuses who have the possibility to respond to chronic hypoxia due to placental insufficiency.
References
- The investigation and management of the small for gestational age fetus. Green–top Guideline No. 31, 2nd Edition. 2013.
- Intrauterine growth restriction. ACOG Practice Bulletin. 2000; 12.
- Gaudineau A. [Prevalence, risk factors, maternal and fetal morbidity and mortality of intrauterine growth restriction and small-for-gestational age]. J Gynecol Obstet Biol Reprod (Paris). 2013; 42: 895-910.
- Alfaidy N, Hoffmann P, Boufettal H, Samouh N, Aboussaouira T, Benharouga M, et al. The multiple roles of EG-VEGF/PROK1 in normal and pathological placental angiogenesis. Biomed Res Int. 2014; 451906.
- Helske S, Vuorela P, Carpén O, Hornig C, Weich H, Halmesmäki E. Expression of vascular endothelial growth factor receptors 1, 2 and 3 in placentas from normal and complicated pregnancies. Mol Hum Reprod. 2001; 7: 205-210.
- Nishimura T, Duereh M, Sugita Y, Yoshida Y, Higuchi K, Tomi M, et al. Protective effect of hypotaurine against oxidative stress-induced cytotoxicity in rat placental trophoblasts. Placenta. 2015; 36: 693-698.
- Madazli R, Somunkiran A, Calay Z, Ilvan S, Aksu MF. Histomorphology of the placenta and the placental bed of growth restricted foetuses and correlation with the Doppler velocimetries of the uterine and umbilical arteries. Placenta. 2003; 24: 510-516.
- Viscardi RM, Sun CC. Placental lesion multiplicity: risk factor for IUGR and neonatal cranial ultrasound abnormalities. Early Hum Dev. 2001; 62: 1-10.
- Iskender-Mazman D, Akcoren Z, Yigit S, Kale G, Korkmaz A, Yurdakok M, et al. Placental findings of IUGR and non-IUGR. Turk J Pediatr. 2014; 56: 368-373.
- Zhang S, Regnault TR, Barker PL, Botting KJ, McMillen IC, McMillan CM, et al. Placental adaptations in growth restriction. Nutrients. 2015; 7: 360-389.
- Herrera EA, Krause B, Ebensperger G, Reyes RV, Casanello P, Parra-Cordero M, et al. The placental pursuit for an adequate oxidant balance between the mother and the fetus. Front Pharmacol. 2014; 5: 149.
- Chen CP, Aplin JD. Placental extracellular matrix: gene expression, deposition by placental fibroblasts and the effect of oxygen. Placenta. 2003; 24: 316-325.
- Zhu J, Zhong M, Pang Z, Yu Y. Dysregulated expression of matrix metalloproteinases and their inhibitors may participate in the pathogenesis of pre-eclampsia and fetal growth restriction. Early Hum Dev. 2014; 90: 657-664.
- Castellucci M, Classen-Linke I, Mühlhauser J, Kaufmann P, Zardi L, Chiquet-Ehrismann R. The human placenta: a model for tenascin expression. Histochemistry. 1991; 95: 449-458.
- Minamitani T, Ikuta T, Saito Y, Takebe G, Sato M, Sawa H, et al. Modulation of collagen fibrillogenesis by tenascin-X and type VI collagen. Exp Cell Res. 2004; 298: 305-315.
- Korhonen M, Virtanen I. Immunohistochemical localization of laminin and fibronectin isoforms in human placental villi. J Histochem Cytochem. 2001; 49: 313-322.
- Flück M, Tunc-Civelek V, Chiquet M. Rapid and reciprocal regulation of tenascin-C and tenascin-Y expression by loading of skeletal muscle. J Cell Sci. 2000; 113: 3583-3591.
- Deckner M, Lindholm T, Cullheim S, Risling M. Differential expression of tenascin-C, tenascin-R, tenascin/J1, and tenascin-X in spinal cord scar tissue and in the olfactory system. Exp Neurol. 2000; 166: 350-362.
- Castellucci M, Kosanke G, Verdenelli F, Huppertz B, Kaufmann P. Villous sprouting: fundamental mechanisms of human placental development. Hum Reprod Update. 2000; 6: 485-494.
- Postiglione L, Ladogana P, Montagnani S, di Spigna G, Castaldo C, Turano M, et al. Effect of granulocyte macrophage-colony stimulating factor on extracellular matrix deposition by dermal fibroblasts from patients with scleroderma. J Rheumatol. 2005; 32: 656-664.
- Pardi G, Cetin I, Marconi AM, Lanfranchi A, Bozzetti P, Ferrazzi E, et al. Diagnostic value of blood sampling in fetuses with growth retardation. N Engl J Med. 1993; 328: 692-696.
- Locci M, Nazzaro G, De Placido G, Nazzaro A, Colacurci N, Montagnani S, et al. Correlation of Doppler and placental immunohistochemical features in normal and intrauterine growth-retarded fetuses. Ultrasound Obstet Gynecol. 1993; 3: 240-245.
- Macara L, Kingdom JC, Kaufmann P, Kohnen G, Hair J, More IA, et al. Structural analysis of placental terminal villi from growth-restricted pregnancies with abnormal umbilical artery Doppler waveforms. Placenta. 1996; 17: 37-48.
- Mayhew TM, Ohadike C, Baker PN, Crocker IP, Mitchell C, Ong SS. Stereological investigation of placental morphology in pregnancies complicated by pre-eclampsia with and without intrauterine growth restriction. Placenta. 2003; 24: 219-226.
- Guerra G, Calabrese D, Mele V, Tafuri D, D’Anna M, Di Carlo C. et al. Proceeding of 62nd National Congress of Italian Society of Anatomy, 2008 September 14-16, Italy: Italian Journal of Anatomy and Embriology. 2008.
- Watson AL, Burton GJ. A microscopical study of wound repair in the human placenta. Microsc Res Tech. 1998; 42: 351-368.
- Yoshida T, Akatsuka T, Imanaka-Yoshida K. Tenascin-C and integrins in cancer. Cell Adh Migr. 2015; 9: 96-104.
- Chen CP, Aplin JD. Placental extracellular matrix: gene expression, deposition by placental fibroblasts and the effect of oxygen. Placenta. 2003; 24: 316-325.
- Hanretty KP, Whittle MJ, Rubin PC. Reappearance of end-diastolic velocity in a pregnancy complicated by severe pregnancy-induced hypertension. Am J Obstet Gynecol. 1988; 158: 1123-1124.
- Brar HS, Platt LD. Antepartum improvement of abnormal umbilical artery velocimetry: does it occur? Am J Obstet Gynecol. 1989; 160: 36-39.
- Soregaroli M, Bonera R, Danti L, Dinolfo D, Taddei F, Valcamonico A, et al. Prognostic role of umbilical artery Doppler velocimetry in growth-restricted fetuses. J Matern Fetal Neonatal Med. 2002; 11: 199-203.