
Review Article
Austin J In Vitro Fertili. 2015; 2(3): 1022.
Consider Anti-Ovarian Antibody Testing for ART: a Parameter to Improve the Success Rate of Your Clinic!
Eusebio S Pires*
Department of Obstetrics and Gynecology, University of Virginia, USA
*Corresponding author: Eusebio S Pires, Department of Obstetrics and Gynecology, School of Medicine, University of Virginia, Charlottesville, Virginia 22908, USA
Received: September 04, 2015; Accepted: September 21, 2015; Published: September 23, 2015
Abstract
There is a steady and continuing trend towards later childbearing in developed countries, to some extent in developing countries and at the same time several infertile couples seek for Assisted Reproduction Techniques (ART) to conceive. ART has been used in the United States since 1981 to help women become pregnant however; the decision to undergo this expensive and time-consuming treatment can be difficult. ART can also alleviate the burden of infertility on individuals and families. With a chance of helping women get pregnant through IVF; there also are numerous hurdles that come along the way for a successful IVF, such as female age, embryo quality, ovarian response, poor implantation, poor diet and lifestyle. Usually ignored but, a new addition to this list could be the detection of serum Anti-Ovarian Antibodies (AOA) which have been demonstrated to further reduce IVF success rates. This review highlights recent research findings supporting AOA testing in women enrolled for ART prior to initiating them into the IVF program. In doing so it could help these women at the time of counseling, to give them a better perspective of a chance on how to improve their reproductive outcome. This simple counseling which involving a blood work done for testing serum AOA would not only ensure effectiveness of IVF, but also save on the time invested by the treating clinician and the monetary investment done by the patients and thus aim towards restoring fertility.
Keywords: Anti-ovarian antibodies; Ovarian autoimmunity; Infertility in women; IVF successes
Introduction
Infertile couples, by the time they begin In Vitro Fertilization (IVF) treatment, are often desperate for children having been through years of intrusive investigations. Since the birth of Louise Brown, innovations in Assisted Reproductive Techniques (ART) have overcome numerous seemingly undefeatable barriers to allow couples the chance to have babies. Significant developments in the first decade led to greater efficiency and expanded accessibility of IVF to the general public. Efforts continue to focus on potential ways to increase the success of ART using Preimplantation Genetic Diagnosis (PGD) for aneuploidy screening [1]. Of all, improving the efficiency of cryopreservation of oocytes and of ovarian tissue transplantation promises to provide options to modern day women. Despite these major technological advances achieved by ART in the last three decades, intense efforts to follow the long term impact of these technologies have to be undertaken knowing the fact that the fruit of this technology- child conceived with IVF is currently around 33 years of age. Furthermore, many technologies such as ICSI, in vitro maturation, oocyte cryopreservation and vitrification, and PGD have limited research findings on developmental outcomes. Heightened awareness of potential health risks secondary to ovulation inducing medications, in vitro culture conditions, and oocyte/embryo manipulations is paramount to the continuous surveillance of rare complications of ART that may only manifest over time. Some of the known key factors with IVF success rates [2]:
1. Age and IVF. The younger the woman, the higher the IVF success rates. Higher risk for miscarriage, especially after age 40.
2. Type of fertility problem. Recurrent miscarriage, infertility in both the male and female, uterine abnormalities, blocked or absent tubes, ovarian dysfunction, or low sperm quality and motility can result in lower IVF success rates.
3. Lifestyle habits. Women with a history of Sexually Transmitted Diseases (STDs) and Pelvic Inflammatory Disease (PID) may have lower IVF success rates.
4. The fertility center data. At the time of an interview with the treating clinician, it is a good practice to ask for the success rates and stories from the IVF lab where the patient plans to register. This rate varies from clinic to clinic.
Autoimmunity of the reproductive system
Ovarian autoimmunity: Apart from the factors discussed above, there is one factor that has not been given substantial consideration while enrolling a candidate for IVF- ‘Ovarian Autoimmunity’. Like all other organs the reproductive tissue also undergoes an autoimmune attack. Although many researchers have enormously contributed towards this field, till date there have been several agreements and differences in opinions. Among the sexes, females are generally more prone to have autoimmune diseases than males. In most endocrine autoimmune diseases, an abnormal level of the regulatory hormone is a primary diagnostic indicator of potential pathology. The diagnosis is confirmed by measurement of specific autoantibodies. Regardless of the mechanisms involved in autoimmune pathology, detection of specific autoantibodies remains the most practical clinical and research marker of autoimmune disease [3]. The relation between regulatory hormones and ovarian autoimmunity is more complex than that of noncyclic endocrine organs. Ovarian function is cyclic and regulated by cyclic changes in gonadotropin levels. During normal function, hormone levels vary, and during the process of ovarian aging associated with menopause, changes are erratic [4]. For example a single measurement of FSH may not be a reliable indicator of ovarian function. Autoimmune and non autoimmune ovarian failures are not readily differentiated by endocrine profiles. The presence of autoantibodies in individuals without end-organ dysfunction is associated with a higher risk for development of autoimmune disease. Ovarian antibodies have been shown to be present in infertile women although showing normal levels of FSH and inhibin-B, suggesting that ovarian antibodies are independent predictors of potential autoimmune ovarian failure [5].
Pathological role of ovarian antibodies: Presence of these antibodies to various cellular components of the ovary can (a) reduce fertilization rates (b) decrease pregnancy rates (c) generate a poor response to gonadotropin stimulation (d) affect egg and embryo development (e) could be responsible for implantation failures [6].
Selection of subjects
There are additional confounding factors in studies involving infertility and ovarian autoimmunity. A potential source of variability is related to differences in treatment history. Cross-sectional study designs are common in infertility. In Vitro Fertilization and Embryo Transfer (IVF-ET) has become a promising treatment for infertility with various underlying causes.
Presence of serum Anti Ovarian Antibodies (AOA) in general circulation of women registered for the IVF-ET program [7-11] has been shown using Enzyme Linked Immunosorbent Assay (ELISA) and Indirect Immunofluorescence (IIF). These AOA do not necessarily appear after follicular aspiration as pre-existing AOA levels have been reported in some studies. In some of these patients an increase in AOA is seen with an increase number of IVF attempts [8]. As the likelihood of pregnancy changes with succeeding cycles, variable treatment history may confound the study result. A question has been raised about possible effects of IVF treatment on the prevalence of ovarian antibodies [8,12,13] and zona pellucida antibodies [14]. It is also possible that individuals with ovarian antibodies have repeated treatment failure and thus undergo multiple treatment cycles [15].
Few studies have assessed women before infertility treatment. The question of treatment effects is not resolved but is a consideration in study design. A major difference among studies is the extreme variation in inclusion and exclusion criteria for infertility study and control groups. Study groups may be defined by diagnostic category, follicular phase FSH, or functional outcomes, such as fertilization, gonadotropin responsiveness, or pregnancy. In addition, comparison/ control groups may include tubal factor, male factor, or normally cycling women. In couples initially classified as “male factor” infertility, the woman may be a poor responder to gonadotropin stimulation and have ovarian antibodies.
Clearly, it is difficult to compare studies with such diverse study groups. Furthermore, use of an infertility subgroup as a control group in which the possibility of ovarian autoimmunity has not been eliminated may confound identification of autoimmunity in the study group. It has been reported that ovarian autoimmunity is primarily associated with unexplained infertility, but this does not rule out ovarian autoimmunity associated with other diagnostic classifications, as women may have more than one etiologic condition contributing to infertility [13].
Autoimmunity and IVF
The hypothesis of an underlying autoimmune mechanism has been reported in some cases of repeated, unexplained IVF failure where the presence of AOA has been described [16], particularly in Premature Ovarian Failures (POF) / Primary Ovarian Insufficiency (POI) [17] and after follicular puncture for IVF [18,5]. An earlier study demonstrated a negative correlation between AOA levels in serum samples from IVF patients, the number of oocytes collected, the number of embryos obtained and the pregnancy rate [18]. Subsequently, these results have been confirmed in several other studies [12]. It has been demonstrated in some cases that AOA appear after follicular aspiration while in other cases, pre-existing AOA levels have been shown to increase with the number of IVF attempts [8].
Specific ovarian antigens still remain to be clearly delineated in human ovarian autoimmunity. It is possible that several different target antigens are involved in ovarian autoimmunity [6,10,11,19-21]. The auto antigens identified till date demonstrates the pathological role of AOA, and have been proposed to be involved in human ovarian autoimmunity. These target proteins differ in terms of their molecular identities and cellular localizations respectively. As there is evidence for both oocyte and cellular antigens in ovarian autoimmunity, it is likely that the best predictive value will be obtained with detection of antibodies to multiple antigens. In Figure 1 it has been demonstrated that sera having AOA immunoreactive to various compartments of the ovary. Although the oocyte seems the primary target, the other somatic cells of the ovary are also attacked. The specificity of assays detecting AOA has been questioned [22] and a simple sensitive test was reported to overcome this problem [9].
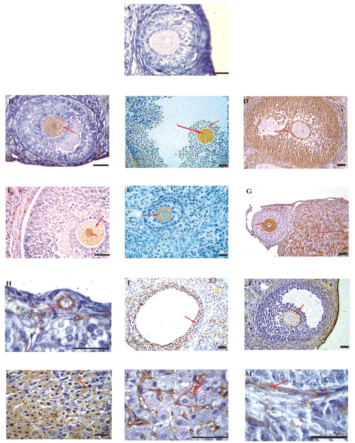
Figure 1: Immunohistochemical localization of serum AOA against 5-mm rat
ovarian sections.
Representative figure using AOA-negative serum and sera from control
women showing no immunoreactivity was seen to any cell type (A). Sera from
patients with POF and IVF-ET patients reacted with different cellular targets
as depicted by the red arrows. Immunoreactivity was seen to ooplasm of the
oocytes (B,C,E–G), cumulus cells (C,D), granulosa cells (D,I), zona pellucida
(J), corpora luteal cells (K), interstitial region of the corpora lutea (G,L), and
the cal cells (E,M). Few of the patient’s sera showed immunoreactivity to
all stages of folliculogenesis from primordial follicles (H) to the antral and
matured follicles (B and C, respectively). Serum from one patient with POF
reacted to the nuclear and cytoplasmic compartments of the oocyte (E). The
oocyte seems to be the immunodominant cellular target in comparison with
the other cell types of the ovary. Bar = 20 mm.
The multiplicity of the above mentioned potential autoimmune targets illustrates the variety of pathological mechanisms in ovarian disease, but their clinical significance and diagnostic relevance still needs to be investigated thoroughly through animal models etc. By way of systematic investigation of these ovarian targets we may lead to: (i) the characterization of new molecules that could be playing a crucial role in reproduction; (ii) a thorough know-how into the pathological mechanisms causing ovarian damage and thereby infertility; (iii) the development of quick and sensitive immunoassays in order to screen large numbers of serum samples from women attending the infertility clinics with an underlying autoimmune etiology of the ovary. Evaluation of AOA can be effective as a prognostic factor in the treatment of infertile patients and for the IVF-ET program.
Role of corticosteroids and tackling AOA positive results
A handful of specialties in Medicine have enjoyed the popular growth and sustained improvements witnessed by physicians and their patients with infertility. However, there is increasing evidence that ART-conceived children may be at greater risk of perinatal complications than naturally conceived children and that knowledge on long-term health effects of ART is incomplete. Hence, all clinicians and researchers involved in the care of these patients must maintain a heightened awareness of these potential issues. As ART approaches its fourth decade, new and existing technologies must be used responsibly to help infertile couples achieve their goals without compromising on general health.
Immunosuppressive doses of corticosteroids administered for a short period of time to patients undergoing IVF-ET have been shown to significantly improve the implantation and pregnancy rates [23,24]. A pilot study highlighted that an indication for corticosteroid treatment was based only on the identification and follow up of organ-specific antibodies directed against ovarian targets in patients with previous IVF failure. In these selected patients they showed that corticosteroids not only reduced the antiovarian autoimmune response trigged by follicular aspiration but also increased the live birth rate after IVF [24]. Several mechanisms are proposed to explain the beneficial effect of corticosteroids. The immunosuppressive action of these drugs could inhibit potential adverse effects of AOA on oocyte quality or gamete interaction [25]. Furthermore, corticosteroids could also help with implantation and embryonic development and have therefore been proposed for the treatment of recurrent pregnancy loss [26]. Corticosteroids are shown to stimulate estradiol and progesterone productions, which are immunoregulating biomolecules, during pregnancy, as it’s well known that progesterone is needed to maintain pregnancy [27]. There are numerous anecdotal reports of successful treatment of POF/POI and infertility with low-dose corticosteroid immunosuppression [28]. Women with POF/POI were treated with immunosuppression without screening for ovarian autoimmunity and 2 pregnancies occurred in 11 women. In another trial in the same study, without screening for ovarian autoimmunity, danazol was not effective for reversal of POF/POI [29]. However, success with lowdose corticosteroid treatment of POF/POI with autoimmunity has been confirmed by ovarian biopsy [30]. Likewise, infertile women with AOA treated with immunosuppression before IVF stimulation had improved embryo quality and pregnancy rates [31]. Controlled clinical trials to determine dose and side effects have yet not been conducted. Another study was undertaken to analyze the influence of AOA on ovarian responsiveness in IVF-ET cycles. The investigators suggested screening for AOA before initiating the IVF-ET treatment, as it was found to suppress the ovarian function and response during stimulation [32].
To establish the importance of AOA testing in IVF, a clinical reproductive outcome comparative study between two groups of women undergoing IVF-ET was conducted [33,34]. Group 1 consisted of women tested positive for AOA, and were put on corticosteroid therapy, reverted to AOA negative and then taken up for IVF-ET. Group 2 were seronegative for AOA. AOA positive serum samples were sent periodically to re-investigate presence of AOA after corticosteroid therapy and women turned AOA negative were taken up for IVF-ET. Of the 138 women in group 1 who were treated with corticosteroids; 70 turned seronegative for AOA, 22 of these 70 were poor responders and needed independent donor oocyte-recipient cycles and were excluded from the study. Results demonstrated that fertilization and clinical pregnancy rates between both groups were comparable. Nevertheless, it was also observed that there was poor response to stimulation protocol, smaller number of oocytes retrieved and more spontaneous abortions in group 1 women. Hence not all outcomes following the treatment were comparable between the two groups. Table 1 demonstrates a comparable clinical outcome between women seropositive for AOA and reverted to AOA negative after corticosteroid treatment versus women seronegative for AOA.
Parameters
AOA positive reverted to negative
Originally AOA negative
p-value
Group 1
Group 2
Number of subjects
48
121
NA
Mean age (years)
33.4+4.3
32+3.1
0.02
Number of IVF cycles
58
121
NA
Number of cycles dropped
8
6
NA
Suboptimal response (%)
65.5
16.5
0
Normal response (%)
34.5
83.5
0
Average number of oocytes collected (%)
3.2+1.6
6.1+2.2
0
Fertilization rate (%)
73.2
71.8
1
Cleavage rate (%)
99.2
98
1
Clinical pregnancy rate (%)
34.5
39.6
0.67
Abortion rate (%)
35
10.4
0
Take home baby rate (%)
21
35.5
0.08
Table 1: This table illustrates the importance of AOA testing before enrolling and taking up women for ART. The table also shows comparison between the two groups in the study with regards to their clinical reproductive outcome of the IVF-ET. Group 1 tested seropositive for AOA and were placed on corticosteroid therapy and once their sera tested seronegative for AOA they were taken up for the IVF-ET and results were compared to the group 2 women who were primarily seronegative for AOA. From the table, once can appreciate the comparable results between the two groups with regards to their fertilization, cleavage and clinical pregnancy rates. The Statistical analysis was performed using Student’s t-tests on paired series and chi-square Fischer’s exact tests (NA- not applicable).
Conclusion
Evaluation of AOA can be effective as a prognostic factor in the treatment of infertile patients and for the IVF-ET program. The following schematic strategy could be employed at an IVF-ET clinical lab to determine the clinical significance of the AOA testing, improve the reproductive outcome there by contributing to an increase in IVF success rates in their clinic: (Flow chart).
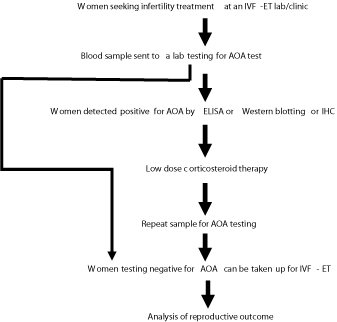
Flow chart:
Take home message
The studies reviewed here clearly indicate the usefulness of the AOA test for follow-up post corticosteroid treatment. It can give the treating clinician an indication as to which patient is likely to benefit post corticosteroid treatment in an IVF treatment cycle. Based on the research findings as reviewed above, it can be proposed that the AOA test could be a part of the battery of tests included for infertility diagnosis and management. The AOA test can allow clinicians to make an early assessment of a possible ‘poor responder’ before initiating a stimulus protocol particularly when women have responded poorly in past IVF cycles. It also gives a strong clue to the clinician to modulate the corticosteroid treatment and to decide when this treatment should be discontinued. Parallely, this will not only save on time utilized in the success of the ART program employed by the clinical team but it would also assist in reducing the cost factor to be incurred by the patient.
Author information
Dr. Eusebio Pires from his Doctoral program days has continuous interest in female infertility and cancers, biomarker discovery, clinical diagnosis, translational medicine and therapeutics.
Acknowledgement
The author thanks the editorial team of the Journal of Histochemistry and Cytochemistry (USA) for allowing reproduction of Figure 1 from his previous publication with no alterations- Eusebio S. Pires, Pervin K. Meherji, Rama R Vaidya, Firuza R Parikh, Manish N Ghosalkar, and Vrinda V Khole. “Specific and Sensitive Immunoassays Detect Multiple Anti-ovarian Antibodies in Women with Infertility”. J Histochem Cytochem, December 2007, 55:1181- 1190, doi:10.1369/jhc.7A7259.200.
The author also thanks the editorial team of the Journal of Assisted Reproduction and Genetics (USA) for allowing reproduction of Table 1 from his previous publication with no modifications- Eusebio Pires, Firuza Parikh, Purvi Mande, Shonali Uttamchandani, Sujata Savkar and Vrinda Khole. “Can anti-ovarian antibody testing be useful in an IVF-ET clinic?” Jour Assis Reprd Genet. 2011 January.
References
- Wang J, Sauer MV. In Vitro Fertilization (IVF): a review of 3 decades of clinical innovation and technological advancement. Ther Clin Risk Manag. 2006; 2: 355-364.
- Fertility authority [https://www.fertilityauthority.com/fertility-issues/ivf-failure]
- Luborsky J. Ovarian autoimmune disease and ovarian autoantibodies. Jour of women’s health & gender-based medicine. 2002; 11: 583-599.
- Burger HG, Dudley EC, Hopper JL, Groome N, Guthrie JR, Dennerstein L, et al. Prospectively measured levels of serum follicle-stimulating hormone, estradiol, and the dimeric inhibins during the menopausal transition in a population-based cohort of women. J Clin Endocrinol Metab. 1999; 84: 4025-4030.
- Luborsky J, Pong R. Pregnancy outcome and ovarian antibodies in infertility patients undergoing controlled ovarian hyperstimulation. Am J Reprod Immunol. 2000; 44: 261-265.
- Pires ES. Multiplicity of molecular and cellular targets in human ovarian autoimmunity: an update. J Assist Reprod Genet. 2010; 27: 519-524.
- Monnier-Barbarino P, Jouan C, Dubois M, Gobert B, Faure G, Béné MC. [Anti-ovarian antibodies and in vitro fertilization: cause or consequence?]. Gynecol Obstet Fertil. 2003; 31: 770-773.
- Gobert B, Barbarino-Monnier P, Guillet-May F, Béné MC, Faure GC. Anti-ovary antibodies after attempts at human in vitro fertilization induced by follicular puncture rather than hormonal stimulation. J Reprod Fertil. 1992; 96: 213-218.
- Pires ES, Parte PP, Meherji PK, Khan SA, Khole VV. Naturally occurring anti-albumin antibodies are responsible for false positivity in diagnosis of autoimmune premature ovarian failure. J Histochem Cytochem. 2006; 54: 397-405.
- Pires ES, Meherji PK, Vaidya RR, Parikh FR, Ghosalkar MN, Khole VV. Specific and sensitive immunoassays detect multiple anti-ovarian antibodies in women with infertility. J Histochem Cytochem. 2007; 55: 1181-1190.
- Pires ES, Choudhury AK, Idicula-Thomas S, Khole VV. Anti-HSP90 autoantibodies in sera of infertile women identify a dominant, conserved epitope EP6 (380-389) of HSP90 beta protein. Reprod Biol Endocrinol. 2011; 9: 16.
- Barbarino-Monnier P, Gobert B, Guillet-Rosso F, Béné MC, Landes P, Faure G. Antiovary antibodies, repeated attempts, and outcome of in vitro fertilization. Fertil Steril. 1991; 56: 928-932.
- Luborsky J, Llanes B, Davies S, Binor Z, Radwanska E, Pong R. Ovarian autoimmunity: greater frequency of autoantibodies in premature menopause and unexplained infertility than in the general population. Clin Immunol. 1999; 90: 368-374.
- Ulcová-Gallová Z, Mardesic T. Does In Vitro Fertilization (IVF) influence the levels of sperm and zona pellucida (ZP) antibodies in infertile women? Am J Reprod Immunol. 1996; 36: 216-219.
- Geva E, Fait G, Lerner-Geva L, Lessing JB, Swartz T, Wolman I, et al. The possible role of antiovary antibodies in repeated in vitro fertilization failures. Am J Reprod Immunol. 1999; 42: 292-296.
- Anasti JN. Premature ovarian failure: an update. Fertil Steril. 1998; 70: 1-15.
- Fenichel P, Sosset C, Barbarino-Monnier P, Bene MC, Hieronimus S, Harter M, et al. Prevalence, specificity and significance of ovarian antibodies during spontaneous premature ovarian failure. Hum Reprod. 1997; 2: 2623–2628.
- Gobert B, Barabarino-Monnier P, Guillet-Rosso F, Bene MC, Faure GC. Ovarian antibodies after IVF. Lancet. 1990; 24: 335.
- Sundblad V, Bussmann L, Chiauzzi VA, Pancholi V, Charreau EH. Alpha-enolase: a novel autoantigen in patients with premature ovarian failure. Clin Endocrinol (Oxf). 2006; 65: 745-751.
- Pires ES, Khole VV. A block in the road to fertility: autoantibodies to heat-shock protein 90-beta in human ovarian autoimmunity. Fertil Steril. 2009; 92: 1395-1409.
- Edassery SL, Shatavi SV, Kunkel JP, Hauer C, Brucker C, Penumatsa K, et al. Autoantigens in ovarian autoimmunity associated with unexplained infertility and premature ovarian failure. Fertil Steril. 2010; 94: 2636-2641.
- Novosad JA, Sophia NK, Zhi-Bin T, Nelson LM. Ovarian antibodies as detected by indirect immunofluorescence are unreliable in the diagnosis of autoimmune premature ovarian failure: a controlled evaluation. BMC Women’s Health. 2003; 3: 2.
- De Fried PE, Blanco L, Lancuba S, Asch RH. Improvement of clinical pregnancy rate and implantation rate of in-vitro fertilization-embryo transfer patients by using methylprednisone. Hum Reprod. 1993; 8: 393–395.
- Forges T, Monnier-Barbarino P, Guillet-May F, Faure GC, Béné MC. Corticosteroids in patients with antiovarian antibodies undergoing in vitro fertilization: a prospective pilot study. Eur J Clin Pharmacol. 2006; 62: 699-705.
- Narayanan M, Murthy PS, Munaf SA, Shah LC, Kini MD. Antiovarian antibodies and their effect on the outcome of assisted reproduction. J Assist Reprod Genet. 1995; 12: 599-605.
- Cowchock S. Prevention of fetal death in the antiphospholipid antibody syndrome. Lupus. 1996; 5: 467-472.
- Würfel W, Krüsmann G, Rothenaicher M, Hirsch P, Krüsmann W Sr. [Pregnancy following in vitro fertilization and intratubal embryo transfer]. Geburtshilfe Frauenheilkd. 1988; 48: 179-181.
- Hoek A, Schoemaker J, Drexhage HA. Premature ovarian failure and ovarian autoimmunity. Endocr Rev. 1997; 18: 107-134.
- Corenblum B, Rowe T, Taylor PJ. High-dose, short-term glucocorticoids for the treatment of infertility resulting from premature ovarian failure. Fertil Steril. 1993; 59: 988-991.
- Kalantaridou SN, Braddock DT, Patronas NJ, Nelson LM. Treatment of autoimmune premature ovarian failure. Hum Reprod. 1999; 14: 1777-1782.
- Barbarino-Monnier P, Gobert B, Guillet-May F, Béné MC, Barbarino A, Foliguet B, et al. Ovarian autoimmunity and corticotherapy in an in-vitro fertilization attempt. Hum Reprod. 1995; 10: 2006-2007.
- Zou SH, Zhang P, Song DP, Li B, Wu RY. Impact of antiovarian antibodies (AOA) on ovarian responsiveness in vitro fertilization and embryo transfer. Neuro Endocrinol Lett. 2008; 29: 949-952.
- Pires ES, Parikh FR, Mande PV, Uttamchandani SA, Savkar S, Khole VV. Can anti-ovarian antibody testing be useful in an IVF-ET clinic? J Assist Reprod Genet. 2011; 28: 55-64.
- Pires ES, Khole VV. Anti- ovarian antibodies: specificity, prevalence, multiple antigenicity and significance in human ovarian autoimmunity. Current Paradigm of Reproductive Immunology. 2009: 159-190.