
Review Article
Austin J In Vitro Fertili. 2016; 3(1): 1027.
Cigarette Smoking and Structural, Biochemical, Functional Alterations of Spermatozoa and their Consequences for ART?
Mohamed Eid Hammadeh¹*, Houda Amor² and Montenarh M³
¹Department of Obstetrics and Gynecology, University of the Saarland, Germany
²Molecular Genetic and Reproduction Biology, Farhat Hached University Hospital, Tunisia
³Institute for Biochemistry and Molecular Biology, University of the Saarland, Germany
*Corresponding author: Mohamed Eid Hammadeh, Department of Obstetrics and Gynecology, IVF & Andrology Laboratory, University of the Saarland, D-66421, Homburg/Saar, Germany
Received: February 11, 2016; Accepted: April 28, 2016; Published: May 13, 2016
Abstract
Oxidative stress as a result of smoking is a condition that comes from overproduction of Reactive Oxygen Spezies (ROS) that exceeds the antioxidant capacity of the tissue. ROS causes cellular damage including destruction of all cellular components including lipids, proteins, nucleic acids, and sugars.
Reactive oxygen species include free radicals, as well as other oxygenrelated reactive compounds. ROS are formed during reduction of molecular oxygen to water in cellular respiration in the mitochondrial electron transport chain, by the cyclooxygenase pathway, and by cellular enzymes such cytochrome P450 oxidase and xanthine oxidase. The most common species that have potential implication for reproductive biology include superoxide anion (O2•-), Hydrogen peroxide (H2O2), peroxyl (ROO-) and hydroxyl (OH•-) radicals.
ROS may have good or harmful effects on sperm quality depending on the level of ROS, their nature, location and length of exposure. Under physiological conditions, low levels of ROS generated by spermatozoa are necessary for their acrosomal reaction and capacitation. There is a strong evidence for an association between oxidative stress and male sub fertility. The correlation between spermatozoa functions and oxidative stress have been identified by many studies. ROS negatively affect sperm function by contributing to the occurrence of Lipid Per Oxidation (LPO). Furthermore, severity of oxidative stress increased as the levels of antioxidants decreased. The effect of ROS on male fertility is determined by their concentration in seminal plasma but independent of their sources of production. Significant opposite correlation had been established between defective sperm chromatin structure and fertility.
This review will describe and discuss the current literature regarding cigarette smoking and ROS generation, oxidative stress and their physiology and pathology role and their consequences on Assisted Reproduction Technology (ART) outcome.
Keywords: Cigarette Smoking; Sperm; Nuclear sperm protein alteration; ART
Introduction
The impact of smoking on fertility
Infertility is a common reproductive disorder that affects approximately one of six couples worldwide and male factors account for 40-50% of all infertility cases [1]. It has been suggested that not only discrete genetic or environmental causes but also interactions between the two factors contribute to male infertility [2] (Figure 1).
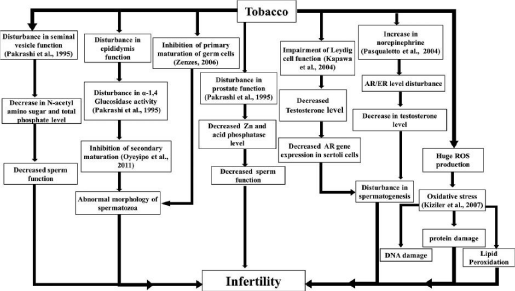
Figure 1: Pictorial depiction of tobacco smoking and chewing affecting male reproductive physiology in causing infertility [251].
Over the last decades, public concern over health effects related to environmental or industrial toxins, genetics, stress, smoking, etc, which are known to cause sperm DNA fragmentation and infertility has grown [3-6].
Recently studies correlating fertility to lifestyle and exposure to toxic environmental substances have received special attention. Moreover, men seem more likely to suffer these effects than women [7]. A specific concern is the possible effects of smoking on fertility and the health of offspring. Our understanding of how the lifestyle choices of adult men themselves might impact on their fertility remains uncertain and often contradictory [8]. Also, there are no definite agreements about the effects of cigarette smoking on these parameters [9]. Cigarette smoking is a common hazardous lifestyle with the highest prevalence of smoking observed in young adult males during their reproductive period (46% smokers between 20 and 39 years) [10]. Soares and Melo [11] demonstrated that cigarette smoking affects reproductive functions in many ways.
Various studies indicated that cigarette smoke is a complex milieu containing about 4000 hazardous materials; out of which 400 are toxic chemicals, about 40 are malignant, and more than 55 are carcinogens [12-14]. In addition, many of these chemicals are poisonous substances [15] such as nicotine and its metabolite cotinine.
A consistent number of studies have claimed the deleterious effects of cigarette smoking on male fertility due to its toxic constituents like nicotine, cotinine, hydroxycotinine, alkaloids, and nitrosamines. The concentration of substances in cigarette smoking such as cadmium, cotinine, lead, malondialdehyde and protein carbonyls are significantly higher in the seminal plasma of smokers [16], in some cases in proportion to the levels seen in serum [17].
Tobacco Specific Nitrosamines (TSNA) is derivatives of tobacco alkaloids which are formed by the action of nitrous acid on nicotine, nor nicotine, anabasine, and anatabine during the processing, fermentation and aging of tobacco. The TSNAs are among the major contributors to the carcinogenic activity of tobacco use [18]. Small amounts of these compounds, also called N’-methyl derivatives, are found in smoke and smokeless tobacco [18,19]. The daily exposure to tobacco-specific nitrosamines is estimated to about 20 μg in smokers and 68 μg in smokeless tobacco users, which may be due to the conversion of tobacco alkaloids into nitrosamines during the manufacturing and storage of smokeless tobacco products [20] (Figure 2).
The mechanisms by which cigarette smoking might affect semen quality could not exclude the direct involvement of toxic substances in cigarette smoke, such as nicotine, carbon monoxide, and recognized carcinogens and mutagens, such as radioactive polonium, cadmium, benzo(a) pyrene, and others. Most of these are known to affect male and female gametes and embryos [13,21]. Smoking is responsible for the generation of toxins that interact directly or indirectly with gametes affecting their function and viability [22].
Cadimum has been proven experimentally to disrupt spermatogenesis and decrease sperm concentration in smokers [23]. In addition, tobacco smoking was also associated with a significantly reduced level of zinc in seminal plasma, which is thought to be one of the important factors that affect sperm motility [24].
Nicotine, the primary alkaloid in tobacco, is a powerful oxidizing agent capable of causing damage to membranes and DNA fragmentation [25,26]. It is also used as an indicator of long term exposure to cigarette smoke since it has a half-life in sperm of about 5–7 days [27]. Cotinine is the major metabolite of nicotine, which is the major psychoactive substance found in cigarette smoke. Cotinine is detected in seminal plasma and is used as a smoking biomarker [28]. Chen and Kuo reported that cotinine decrease male fertility by declining sperm density, reducing motility, sperm counts, and elevating the level of morphologically abnormal spermatozoa [29].
Polycyclic aromatic hydrocarbons cause ultra structure abnormalities in the axoneme resulting in inhibition of ciliary movement [30]. Apart from the alterations in classical sperm parameters, tobacco compounds may affect sperm quality in many ways.
In addition to excessive smoking alcohol consumption and environmental factors such as radiation and toxins can contribute to the production of Reactive Oxygen Species (ROS) [31-33].
High levels of leukocytes [34] and reactive oxygen species [4,28] have also been found in seminal plasma of smokers in comparison to non- smokers. These conditions in seminal plasma cause oxidative stress and induce sperm DNA fragmentation, which reduces the fertilization potential of spermatozoa.
Many studies evaluated the influence of smoking on semen quality of fertile and infertile Men [35]. They showed a significant reduction in sperm density, number of spermatozoa, and motility in association with cigarette smoking [36].
Furthermore, a negative correlation was found between the volume of the ejaculate, spermatozoa count, and motility with the number of smoked cigarettes [37]. Further evidence showed an inverse correlation between cigarette smoking’s and Assisted Reproductive Technology (ART) outcomes [38]. The decline of semen parameters were inversely related to the number of smoked cigarettes and duration of cigarette smoking [39].
In contrast, some researcher failed to find a negative effect of cigarette smoking on conventional sperm parameters [10,40,41]. Thus, the impact of cigarette smoking on male fertility and sperm characteristics remains a highly controversial issue [42].
This review will describe the current literature regarding cigarette smoking and its influence on sperm parameters and their consequences on Assisted Reproduction Technology (ART) outcome (Figure 2).
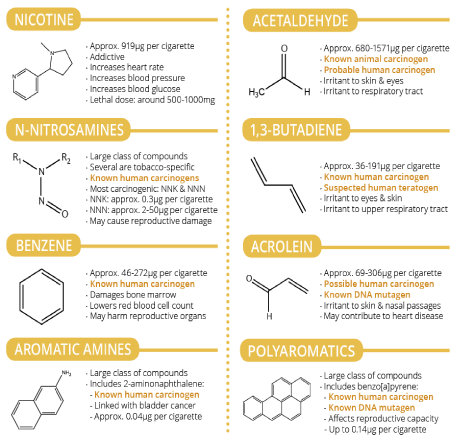
Figure 2: Chemical compound in cigarette smoke and their effects [252].
Reactive oxygen species (ROS) and oxidative stress
ROS represent a group of radicals like hydroxyl ion, superoxide ion, nitric oxide, peroxyl, lipid peroxyl and thiyl and non -radical molecules, such as hydrogen peroxide, hypochloric acid, lipid peroxide and ozone [43]. They form highly reactive molecules, due to the unpaired electron in the outer shell [44]. ROS are unstable substances and extremely reactive by-products of normal metabolism with harmful influences on numerous biomolecules [45]. Among other sources ROS are formed during reduction of molecular oxygen to water in the cellular respiration in the mitochondrial electron transport chain, by the cyclooxygenase pathway, and by cellular enzymes such cytochrome P450 oxidase and xanthine oxidase [46].
At the inner mitochondrial membrane electrons leaking from the electron transport chain are transferred to the oxygen molecule, resulting in oxygen with an extra unpaired electron [47,48].
Although, the biochemical mechanisms involved in the production of ROS are poorly understood, ROS production is believed to be a by product of metabolic processes. It seems to be induced by exogenous NADPH oxidase [49].
Studies have shown that cigarette smoking lead to increased seminal ROS by several mechanisms:
- Cigarette smoke itself contains high levels of ROS [50].
- Smoking metabolites may induce an inflammatory reaction in the male genital tract with a subsequent release of chemical mediation of inflammation that can recruit and active leukocytes.
- Activated leukocytes can generate high levels of ROS in semen and toxic metabolites of cigarette smoke may impair spermatogenesis, resulting in the production of abnormal spermatozoa [4].
Cigarette smoking has been associated with an up to a 48% increase in the level of leukocytes in semen, and 107% increase in seminal ROS levels in the body [41], which might be responsible for reduction of sperm production, increased oxidative stress in sperm, lipid peroxidation [51,52] and consequently decrease of the fertilization capacity and implantation rate of embryos [11]. Also, Zhang et al. showed that smoking cause a significant decline in semen quality and higher levels of leukocytes which may affect fertilization efficiency [34].
One of the effects of oxidative stress on the conventional seminal parameters is a reduction in sperm motility or the presence of asthenozoospermia [53]. Besides, high levels of free radicals may alter spermiogenesis resulting in production of abnormal spermatozoa [54].
The main sources of ROS in semen are leukocytes and abnormal/ dead spermatozoa [55,56]. Leukocytes found in male genital tract infections have the potential to produce ROS [57], which exceed the anti-oxidant defence system and could cause DNA damage. Moreover, leukocytes act as a source of oxidative stress by generating extracellular ROS in prostatic and seminal vesicle secretions [58,59].
In addition, leukocytospermia contributes to an increased release of pro-inflammatory mediators (cytokines) which alter the regulatory mechanisms of spermiogenesis and subsequently attribute to DNA aberrations [60]. Furthermore, many of the metabolites in cigarettes smoke trigger release of chemical inflammatory mediators such as interleukin-6 and interleukin-8 [22]. These mediators can recruit additional leukocytes, resulting in increased ROS formation in the semen. Several studies pointed out that short-term exposure to cigarette smoke in vivo is sufficient to increase in level of IL-1β and/ or TNF-a [61,62].
Cigarette smoke-induced oxidative stress leads to a reduction of the concentration of intracellular glutathione, decreased nitric oxide availability, and increased level of peroxynitrite. All of these factors activate NF-κB in leucocytes. Hans’s et al. demonstrated that human lymphocytes exposed to a mild intensity of cigarette smoke (−30 mmHg) showed significantly elevated NF-κB activation, suggesting that this cell signalling pathway may be also affected by passive smoke exposure [63] (Figure 3).
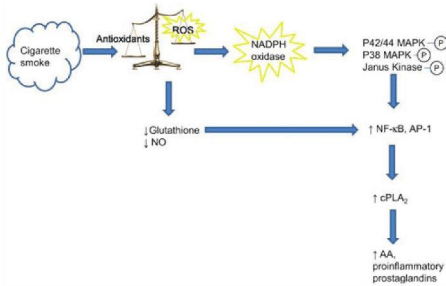
Figure 3: The link between cigarette smoke and oxidative stresses [253].
Over view of cigarette smoking-induced oxidative, inflammatory and genetic
mechanisms leading to psoriasis. ROS, reactive oxygen species; VEGF,
vascular endothelial growth factor.
Cigarette smoke contains 1015 free radicals per puff, tipping the antioxidant/
Reactive Oxygen Species (ROS) balance towards oxidative stress. ROS
activate NADPH oxidase, which phosphorylates p42/44 Mitogen-Activated
Protein Kinase (MAPK), p38 MAPK and Janus kinase. The resultant
increase in Nuclear Factor-κB (NF-κB) and Activator Protein-1 (AP-1) nuclear
transcription leads to enhanced gene expression of cytosolic Phospholipase
A2 (cPLA2) contributing to the production of Arachidonic Acid (AA) and
proinflammatory prostaglandins [253].
Other sources of ROS increased scrotal temperature due to an illness with fever [64,65,47], varicocele [66], and increased age [67- 69].
In contrast to the elevated levels of ROS and pathophysiological conditions ROS are produced in a controlled manner and play important roles as secondary messenger in many intracellular signalling pathways [70,71]. The controlled ROS production is known to play a role in physiological reproductive processes such as hormone signalling, oocyte maturation, folliculogenesis, tubal function, ovarian steroidogenesis, cyclical endometrial changes and germ cell function [72,73]. Therefore, physiological and fine controlled production of ROS is most important for sperm function and reactions, such as capacitation and acrosome reaction. The control of ROS production is also carried out by the synthesis of antioxidant substances, which have the ability to scavenge and neutralize free radicals [45,74]. Catalase and superoxide dismutase are important antioxidant enzymes quenching hydrogen peroxide and the excess of free superoxide, radicals, respectively.
Several studies show that antioxidative vitamins are lower in smokers resulting in systemic oxidative stress [75,76]. However, dietary antioxidant supplements provide only limited protection to smokers [77,78].
ROS in the cigarette gas-phase promote the destruction of endogenous antioxidants (vitamins and enzymatic antioxidants) reducing the vital role of cellular antioxidant defenses [79]. The concentrations of cadmium, lead, Reactive Oxygen Species (ROS) and others are significantly higher in smokers [16], and at the same time, the concentration of ascorbic acid and the activity of other components of the antioxidant defence are significantly reduced [80].
Moreover, increased ROS in the seminal plasma of infertile men may decrease the effective concentration of ascorbic acid, increasing the harmful effects of ROS to sperm cells that are associated with abnormal sperm parameters [81].
The results of Sobczak et al. indicated a statistically significant decrease in plasma tocopherol level (vitamin E) in passive and active smokers in comparison with the results obtained in the group of nonsmokers [82].
It has been shown by various researchers that smoking disrupts the dynamic balance between oxidation and antioxidation reactions and clearly exacerbates oxidative stress [83]. Therefore, abnormalities in oxidant-antioxidant balance may be one of the mechanisms leading to testicular damage following chronic inhalation of cigarette smoke [84]. As low levels of natural antioxidants production or excessive ROS production result in oxidative stress which may cause oxidative damage to tissues [85,86]. The severity of oxidative stress increase when the levels of antioxidants decrease [4,87].
Sperm DNA fragmentation induced by oxidative attacks like the hydroxyl radical and ionizing radiation results in the formation of 8-OH-guanine and 8-OH-20-deoxyguanosine (8-OHdG) at a first stage and single-stranded DNA fragmentation thereafter. Hydroxyl radical formation may lead to the induction of doublestranded sperm DNA damage through the activation of sperm caspases and endonucleases [88]. 8- Hydroxy-2- deoxyguanosine and two ethenonucleosides (1, N6-ethenoadenosine and 1,N6- ethenoguanosine) are the two major DNA adducts found in human sperm DNA, both of which have been considered key biomarkers of DNA damage caused by oxidative stress [89,90]. Recently, 8-Hydroxy-20- deoxyguanosine (8-OHdG), a type of oxidized DNA base adducts resulting from remodelled chromatin after ROS attack, has been recommended as a reliable indicator evaluating oxidative stress on spermatozoa [91].
In addition, protein carbonyl, another biomarker of oxidative stress, is one of the oxidative stress by-products which form during ROS interactions with proteins. This interaction modifies biomolecules which in turn changes their functions eventually leading to irretrievable cellular damage [92].
Other factors capable of causing oxidative stress in the germ line include pesticides, herbicides, alcohol, vinclozolin (an antiandrogenic fungicide), cobalt and the common environmental toxicant bisphenol A [93,94].
Cigarette smoking and sperm abnormalities
One of the effects of oxidative stress on the conventional seminal parameters is a reduction in sperm motility or the presence of asthenozoospermia [53].
Reactive oxygen species induced DNA damage may accelerate the process of germ cell apoptosis, leading to the decline in sperm counts and correlate with the poor fertility outcomes seen in the assisted reproductive technology setting [95].
Cigarette smoking and conventional semen parameters: Cigarette smoking and developed oxidative stress is a reason for many hazardous effects on spermatozoa. All cellular components including lipids, proteins, nucleic acids, and sugars are potential targets of oxidative stress [96]. Multiple studies indicate a reduction in the semen volume, sperm concentration, motility, and normal morphology, especially of the headpiece [16,28,37,97] and a decrease in the fertilizing capacity of the spermatozoa [38]. Mehrannia analysed the semen quality of infertile men in a Pakistani an population and found that the quality of spermatozoa obtained from smokers were much lower than from non-smokers (p<0.01) and the sperm concentration, viability and forward progression were negatively correlated with cigarette smoking (P<0.01) [98]. Similar, Colagar et al. showed a reverse effect of smoking on sperm quality [99].
Pasqualotto et al. evaluated sperm concentration, motility, motion variables and hormonal levels of 889 fertile men (522 non-smokers, 143 mild smokers (<10 cigarettes/day), 154 moderate smokers (11-20/ day), and 70 heavy smokers (>20/day) [41]. They found no significant differences in sperm concentration or motility among these groups. However, semen volume was the only semen variable which tended to decrease with the number of smoked cigarettes. Ramlau-Hansen et al. studied the association between cigarette smoking and classical sperm parameters in a large cross-sectional study with 2, 542 healthy males [37]. They showed that with increasing smoking, a 20%–30% reduction in sperm count, volume and motile spermatozoa was observed. A meta-analysis found that smoking was associated with a 13-17% decrease in sperm concentration [100]. It was also demonstrated a negative correlation of smoking with sperm motility [26,10].
In a retrospective study, Collodel et al. evaluated the effects of cigarette consumption on semen parameters in a group of men with idiopathic infertility [101]. The semen quality of 2 groups of men with idiopathic infertility, smokers (n = 118) and non-smokers (n = 153), were compared. Infertile smokers and non-smoker showed similar sperm parameters, although sperm motility and sperm morphology analysed by Transmission Electron Microscopy (TEM) parameters in both groups were significantly impaired compared with controls. Smokers were classified as mild (>or=1 and <or=10 cigarettes/d), moderate (>10 and <20 cigarettes/day), or heavy smokers (>or=20 cigarettes/d). Sperm concentration was significantly (P < 0.05) different among the 3 considered smoker classes. Sperm concentration in heavy smokers was significantly lower (p < 0.05) than that observed for mild smoker and non-smokers [102].
Smokers’ ejaculates contain high levels of morphologically abnormal spermatozoa and sperm and with cytoplasmic droplets and high levels of seminal leukocytes [99,103,104]. Heavy smoking is linked to decrease sperm counts whereas alcohol drinking is linked to high rates of morphologically abnormal sperm [105]. Nadeem et al. demonstrated that cigarette smoking negatively affects male fertility by decreasing the sperm motility and morphologically normal spermatozoa, this deterioration of sperm morphology are also correlated with the number of cigarettes smoked per day [106].
Zhang et al. analysed the effects of cigarette smoking on sperm parameters and detection of presence of leukocytes within the semen of idiopathic infertile men from North-eastern China [34]. They found that in comparison to non-smokers, smokers had a significant decrease in semen volumes (p=0.006), rapid progressive motility (p=0.002) and sperm viability (p=0.019); moreover, smokers had a significant elevated level of immotile sperms (p=0.005) and semen leukocytes (p=0.002); whereas pH and sperm concentration were not statistically significant. Sperm motion parameters were all lower in the smokers except for Beat-Cross Frequency (Hz) (BCF). Further, the percentage of morphologically normal spermatozoa was significantly lower in smokers (p=0.003). Correa-Perez showed that the exposure of nonsmoker spermatozoa to seminal plasma from smokers significantly reduced sperm motility and membrane functional integrity [107]. The negative effects on sperm viability increased during short- and long-term incubation in smoker’s seminal plasma, incubation of smoker’s spermatozoa with seminal plasma from non-smokers improved viability. According to a study conducted by Belcheva et al. spermatozoa cultured in a medium enriched with cotinene have a significant reduction of hyperactivation and impaired membrane function evaluated by the hypoosmotic swelling test and the capacity of penetrating zona-free hamster oocytes [108]. Davar et al. found that semen parameters (morphology, motility, concentration and leukocyte count) were lower in smokers compared to non-smokers [109,110].
Taha et al. reported that smoking has a negative effect on sperm progressive motility, sperm membrane integrity as evaluated by Hypo Osmotic cell test (HOS-test), DNA integrity and seminal zinc concentration [111]. On the contrary, other studies showed that cigarette smoking and alcohol consumption do not appear to significantly affect sperm parameters, such as volume, sperm count, motility and morphology or pregnancy outcome [112].
Cigarette smoking and DNA integrity: Conventional semen analysis is a predictor of reproductive outcome. In 2010, the World Health Organization established new reference values for human semen characterizations, which are lower than those previously reported [113]. For this reason, it has been suggested that sperm function tests, such as sperm DNA integrity, are better indicators of male fertility and should, therefore, be included in the semen evaluations [114].
Many authors [115-117] proposed DNA integrity as an additional parameter of semen quality and a potential fertility predictor. Normal sperm nuclear composition is essential to maintain sperm DNA integrity [118] and any disruption will likely have profound effects on the integrity of the sperm DNA and its capacity to participate in reproduction. Chromatin remodelling in human spermatogenesis occurs during the replacement of histones by transition proteins followed by protamines in spermatids from step 3 to step 5 [119].
Evidence from the literature indicates that DNA in the sperm of infertile men is less well-protected and could suffer from more damage in vitro than the sperm of fertile men [120]. It has been suggested that abnormal DNA integrity may adversely affect fecundity in couples having natural sexual intercourse and in those treated by IUI, IVF and ICSI [121,122].
It was found that the etiology of sperm DNA damage appeared to be multi-factorial and might be due to either intrinsic or extrinsic factors. Various mechanisms such as abnormal chromatin packing, ROS and apoptosis are the most important etiological factors affecting DNA integrity [123].
Abnormalities in protamine packaging of DNA may cause aberrant gene expression resulting in either hyper transcription or transcriptional arrest, leading to a failure in spermatogenesis [124]. Consequently, disturbances in nuclear condensation or changes in the expression of Protamins (P1) and (P2) could increase DNA damage in spermatozoa and have been related to human male infertility [125].
P1 and P2 are normally expressed in a 1:1 ratio in human sperm, and provide a tight packaging of the sperm DNA, resulting in a compaction of the nucleus and cessation of gene expression [126,127]. Incomplete protamination makes the sperm chromatin more vulnerable to an attack by mutagenic compounds disulphide bond attacking agents [128] or ROS [129].
A significant correlation was found for protamine deficiency, poor fertilizing ability of sperm and consequently a poor quality of embryos [126,130]. In addition, alterations in the P1, P2 family or histone expression identified through proteomic approaches were connected to male infertility [125,131]. Simon et al. and Garcia- Peiro et al demonstrated that abnormal high and low P1/P2 ratios are associated with increased sperm DNA fragmentation, lower fertilization, poor embryo quality and reduced pregnancy rates [132,133].
Also, De Mateo reported that the protamine protein ratio is associated with pregnancy rates and proportion of embryos obtained by IVF, but not ICSI, whereas, Rogenhofer et al. did not observe any significant correlation between the protamine mRNA ratio and pregnancy Rate [134,135].
Recently, Hammadeh et al. and Hamad et al. found a significant negative effect for smoking and sperm parameters, protamines and histone ratios [28,136].
DNA in sperm is not homogeneously packed. The peripheral portion of nuclear DNA (15%) is associated with histones whereas the inner core of DNA is a highly compact crystalline structure bound to protamines [137]. It was estimated that approximately 15% of histones remain in the pericentric region after protamines are incorporated [138]. The remaining core histone fraction is enriched with histone H2B, with a selective distribution of H2B variants in sperm nuclei [139]. These histones may play a crucial role in male pronucleus formation and marking genes for early embryonic gene expression [140,141]. Histone retention and protamine deficiency in sperm are hallmarks of certain forms of idiopathic infertility [142-144], but the genetic and mechanistic causes underlying this defect remain enigmatic. Various studies suggest that DNA fragmentation could be used as a marker of semen quality and a predictor for the outcome of assisted reproduction technology [145]. Sperm DNA damage may be initiated by a wide range of effectors such as drugs, chemotherapy, radiation therapy, cigarette smoking and environmental toxins, genital tract inflammation, testicular hyperthermia, varicoceles and hormonal factors [125]. Sperm DNA fragmentation can be induced by various internal and external factors, which include improper or deficiencies in DNA recombination, packaging and ligation during spermatogenesis and sperm maturation [146]. DNA fragmentation induced by endogenous endonucleases [147], apoptosis and abortive apoptosis [148] which occur mainly during spermatogenesis [120,148]. DNA Double-Strand Breaks (DSBs) in spermatozoa may also occur through an abortive apoptosis pathway [149] or posttesticular oxidative stress [150,151], lifestyle and environmental factors [22,152] among others.
Also, sperm DNA damage may result from various physiological and pathological conditions, environmental factors can also be involved such as smoking and air pollution [28,153].
The exposure to environmental or industrial toxins, oxidative stress and smoking are known to cause sperm DNA fragmentation and infertility [3-6]. Generally, the impact of cigarette smoking on sperm DNA integrity is somewhat conflicting (Table 1).
Authors
Years
Study population
Assay used
Effect
Shen et al. [254]
1999
60
8-OHdG
Positive correlation between 8-OHdG & DNA integrity
Sun et al. [255]
1997
113
TUNEL
Positive correlation between TUNE & DNA integrity
Potts et al. [3]
1999
52
TUNEL
Positive correlation between TUNEL& DNA integrity
Sergerie et al. [256]
2000
97
TUNEL
No correlation between smoking and DNA strand break
Saleh et al. [4]
2002
65
SCSA
Higher ROS but no DNA strand breaks
Storgaard et al.[257]
2003
265
SCSA
Prenatal exposure to CS lower sperm counts and no effect on DNA integrity
Horak et al. [258]
2003
179
32-postlabeling method
DNA adducts
Loft et al. [259]
2003
225
8-OHdG
No correlation
between Smoking and DNA integrity
Trisini et al.[260]
2004
COMET neutral
No correlation
between and DNA integrity
Belcheva et al.[261]
2004
40
COMET
No correlation
between and DNA integrity
Robbins et al.[270]
2008
63
FISH
Smoking and sperm aneuploidy
Sepaniak et al. [22]
2006
108
TUNEL
Positive correlation between TUNEL & DNA integrity
Viloria et al.[268]
2009
55
OxyDNA assay
Low level of antioxidative enzyme, but no DNA damage
Hammadeh et al. [28]
2010
53 CS
63 non CS
P1 and P2
gel electrophoresis
Inverse effect on the protamination process by disrupting P2.
Hamad et al. [136]
2014
35 CS
19 NCS
H2B & P1& P2 using acid-urea polyacrylamide gel electrophoresis
Patients who smoke possess a higher proportion of spermatozoa with an alteration of the histone to protamine ratio than patients who do not smoke
Table 1: The Impact of cigarette smoking on sperm DNA integrity.
The most recent studies on the origin of sperm DNA damage suggested that there might be a cascade of changes that progress from the induction of oxidative stress and oxidized DNA base formation to DNA fragmentation and cell death [129]. Cigarette smoke, for example, increases oxidative DNA damage in human sperm cells [154]. Alkaloids, nitrosamines, and cotinine are harmful substances found in cigarette smoke producing ROS. These compounds attack the integrity of DNA in the sperm nucleus causing base modification, DNA strand breaks, and chromatin packing failure. The result is DNA fragmentation that can increase abnormal forms of sperm in smokers [155]. Increased percentage of spermatozoa with fragmented DNA in male smokers compared with non-smokers has been estimated to be 4.7% vs. 1.1% in one study [156] and 32% vs. 25.9% in another one [157].
Two additional cigarette metabolites, vinyl chloride and benzopyrene, can also lead to an increased attachment of DNA adducts [158]. These adducts contribute to mismatched pairs, improper DNA replication, and incorrect protein synthesis. Consistent evidence shows that sperm DNA damage adversely affects male fertility, contributing to poorer embryo development and lower pregnancy rates among partners of men undergoing assisted reproductive treatments [159-161]. Moreover, such DNA damage does not dramatically disrupt fertility, however; it does have a profound impact upon the health and development of their children, resulting in a significant increase in the incidence of childhood cancer [162]. Moreover, Fuentes et al. found that male smoking is associated with a significant decrease of live birth rates [163].
Cigarette smoking mitochondrial DNA: The maturation and differentiation of male germ cells during spermatogenesis are regulated by hormones [164]. This process is also physiologically regulated by a fine-tuned apoptotic mechanism having the objective to eliminate abnormal cells and minimizing the negative consequences for the fertility of a man and on the health of his progeny [165]. Ramos et al. showed that sperm apoptosis is related to both incomplete remodelling and protamine over oxidation [166]. Apoptosis controls the overproduction of male gametes and restricts normal proliferation levels so that they do not surpass the supportive capacity of sertoli cells [167]. Fischer et al. stated that when the apoptosis process is defect, sperm cells might appear in the ejaculate [168]. These sperm cells usually contain damaged DNA which will contribute to poor sperm quality [97]. Indications for an apoptosis-like process in sperm are numerous, ranging from mitochondrial membrane and caspase determinations to the discovery of endonucleases activity in the sperm nucleus [169,170].
During spermatogenesis apoptosis does not eliminate malfunctioning spermatozoa, but apoptosis seems to be involved in cytoplasmic remodelling during the final stages of sperm maturation [171]. Increased apoptosis has been found to occur during maturation arrest, hypo spermatogenesis, and sertoli cell only syndrome [172,173].
Oxidative stress may induce sperm DNA damage, possibly through an apoptotic mechanism [174]. Moreover, the exposure of mitochondria to ROS leads to the release of Apoptosis -Inducing Factor (AIF), which directly interacts with the DNA and induces DNA fragmentation [175].
The mitochondrial membrane potential, responsible for ATP production, is indispensable for the flagellar beat and sperm motility [176]. High level of ROS can disrupt the mitochondrial membranes, which results in the release of cytochrome c from mitochondria and activating caspases and subsequently apoptosis. Apoptosis in spermatozoa may also include the activation of caspases 1, 3, 8 and 9 and the mitochondrial generation of ROS [175]. Furthermore, the elevated number of damaged mitochondria in spermatozoa may increase the production of ROS which in turn disrupt the function of the spermatozoa [177].
Therefore, not only is nuclear DNA analysis useful, the mitochondrial genome of sperm has been shown to be an even more sensitive marker of sperm health [178].
Calogero et al. evaluated the effects of Cigarettes Smoke Extract (CSE) on motility, Mitochondrial Membrane Potential (MMP), chromatin integrity and apoptosis in spermatozoa obtained from healthy non-smokers with normal sperm parameters, by flow cytometry [179].
They found that CSE suppressed sperm motility in a concentration- and time-dependent manner and increased the number of spermatozoa with low MMP, the main source of energy for sperm motility.
A reduction in the incidence of sperm DNA fragmentation by oral antioxidant treatment with scavengers such as vitamin C and E has been also reported [180]. Dietary antioxidants may have a positive impact on semen quality [181]. A positive association was found for an antioxidant therapy and some semen parameters [182-184].
A recent report from the Practice Committee of the American Society for Reproductive Medicine in 2008 suggested that although the antioxidant supplementation reduces the sperm DNA damage, no definitive effect has been observed on the routine sperm parameters and reproductive outcomes [185]. The report also found a lack of sufficient evidence to support the use of any treatment for abnormal sperm DNA integrity.
Cigarette smoking and genetic alteration: Despite normal semen analysis, a high proportion of couples undergoing ART still fail to achieve a pregnancy. Additional parameters should be used for the evaluation of sperm quality. The quality of DNA within the sperm has been recognized as an additional factor for infertility [186,116].
Recently, Rogenhofer et al. demonstrated that sperm mRNAs coding for protamines are useful biomarkers for predicting male infertility [135].
Among the putative functions ascribed to protamine are those related to imprinting of the paternal genome during spermatogenesis and the control of transcription factors to allow its reprogramming by the oocyte [125]. Consequently, changes in the expression of P1 and P2 could increase DNA damage in spermatozoa and have been related to human male infertility (for review see: Oliva, (2006)) [125].
Sperm haplo insufficiency for the P1 and P2 results in aberrant sperm chromatin condensation and the aberrant P1/P2 ratio play an important role in male infertility [187]. It has been postulated that protamine deficiency is related to DNA damage in human sperm. P1deficiency has also been identified in a population of subfertile males [130].
Simon et al. demonstrated a relationship between alteration of protamine ratio (both low and high) and DNA fragmentation as well as fertilization rate [132]. Male germ cells package their chromatin into a small size in order to ensure that the transmission of paternal DNA to the oocyte is as efficient and safe as possible [188]. The unique and exact sperm chromatin architecture implies a specific gene-expression schedule after fertilization [189].
It is becoming increasingly apparent that environmental factors alter epigenetic profiles, such as DNA methylation, chromatin modifications, and non-coding RNAs, thereby changing chromatin structure and gene expression [190]. Epigenetic modifications to the male germ line during gametogenesis are designed to silence unwanted paternally imprinted genes in preparation for future embryonic development and also to regulate the post- meiotic expression of genes during late spermatogenesis [191]. Genetic and epigenetic sperm abnormalities such as abnormal nuclear packaging, chromosome aneuploidy, sperm DNA damage, genomic imprinting errors and centrosome malformations might contribute to poor embryogenesis [192]. Epigenetic variations of DNA, particularly the attachment of methyl groups to cytosine bases neigh bored by guanine (CpG sites), are an important source for variation and regulation in the genome [193,194]. Aberrant pattern of DNA methylation in the sperm of some men correlates with known pregnancy failures in their partners [195]. Smoking has been associated with altered global methylation [196,197] and differential methylation extent in several cancer-related genes [198,199]. Alterations in DNA methylation are one possible mechanism potentially mediating the harmful effects of tobacco smoking.
A large number of studies have considered gene–environment interactions between tobacco smoking and genetic polymorphisms, including DNA repair genes and genes involved in carcinogen metabolism [200,201]. In addition, oxidative stress is one of the key mediators with the potential to attack both the DNA and the epigenetic role of sperm RNA [202]. DNA damages within promoter of genes caused down regulation of susceptible genes during aging and oxidative stress [203].
Recent work has suggested that some of the constituents of cigarette smoke can induce harmful phenotypes in the descendants of exposed individuals [204-206]. Benzo[a] pyrene and nicotine in cigarette smoke have recently been shown to induce harmful alterations of sperm DNA that can be transmitted through the germ line to future generations [207,204].
Furthermore, smokers have been shown to exhibit altered spermatozoal mRNA profiles compared with non-smokers, and miRNA changes have been observed in the placenta of smokers [208,209].
Marczylo et al. showed that cigarette smoke induces specific differences in the spermatozoal microRNA content and that these altered microRNAs appear to predominantly mediate pathways vital for healthy sperm and normal embryo development, particularly cell death and apoptosis [210]. A meta-analysis addressing this question found a significant correlation between paternal smoking and the incidence of childhood cancer [162]. Specifically, Acrolein (Acr) appears to be a potential carcinogen associated with smoking-related lung cancer [211].
In one study the acetylation status was found to modulate the association of bladder cancer and cigarette smoking through smoking intensity and not smoking duration [212].
Spira et al. analysed global gene expression in bronchial epithelial cells and found that the expression levels of metabolizing and antioxidant genes had reverted to control levels after two years of smoking cessation [213].
In smoking-associated lung cancer, elevated levels of Cyclooxygenase-2 (COX-2) and Prostaglandin (PGE2) indicate apoptosis resistance, proliferation, immunosuppression, angiogenesis, invasion, and epithelial-mesenchymal transition [214].
Cigarette smoking and membrane integrity of spermatozoa: The plasma membrane is responsible for the preservation of cellular homeostasis; in this way the plasma membrane integrity exerts a vital role on sperm survival in the female reproductive tract and on the preservation of its fertility capability [176]. The sperm head is considered normal when the plasma membrane, nuclear envelope, acrosome and chromatin are all of normal appearance; in addition, normal axonemal components, mitochondria and plasma membrane are required for the tail to be considered normal [215]. Although, human sperms are particularly sensitive to ROS-induced damage because of a high concentration of polyunsaturated fatty acids in their plasma and mitochondrial membranes, generating lipid peroxidation, which in turn causes damage to cell membranes and alterations in sperm functional aspects, DNA integrity and mitochondrial activity [216]. ROS-induced damage leads to decreased sperm motility by a rapid loss of intracellular ATP which results in axonemal damage, decreased sperm viability, increased midpiece morphology defects, deleterious effects on sperm capacitation and acrosome reaction [217]. In other studies, it was reported that peroxidation reaction affects membrane structure and fluidity and causes damage to axonemal proteins leading to a permanent impairment in sperm motility [218-220].
Also ROS causes sperm damage by inducing Lipid Peroxidation (LPO) of the high levels of polyunsaturated fatty acids in the sperm membrane [47]. This will affect sperm plasma membrane fluidity following with loss of the ability for oocyte fusion and fertilization [221].
ROS in tobacco smoke also induce lipid peroxidation of the plasma membrane of sperm, which is considered to be the key mechanism for inducing sperm damage that leads to decreased sperm viability, sperm concentration, sperm motility, and increased morphology defects [53,222].
ROS attack the double bonds associated with unsaturated fatty acids, initiating a lipid peroxidation that will lead to a loss of membrane fluidity and integrity, which is important for sperm motility, and a subsequent loss of sperm and fertilization capacity [223].
In addition, overexposure of spermatozoa to ROS was proposed to result in excess lipid peroxidation, which will subsequently not only lead to loss of plasma membrane fluidity and function, but also to mutagenic and cytotoxic effects [224]. In addition, the sperm cell membrane is easily attacked by ROS with further detrimental effects on nuclear membranes as well as on sperm DNA [225].
Moreover, these substances in seminal plasma of smokers can react negatively upon sperm membranes, probably by disrupting the equilibrium between antioxidants and prooxidants. They can also act upon genomic integrity and chromatin structure of the spermatozoon, damaging sperm cell DNA [226].
Severe pathologic changes in the testicular tissue are associated with a high level of lipid peroxidation which suggests that overproduction of ROS may play a role in the mechanism of testicular degeneration associated with infertility [227]. Nabil et al. showed that lipid peroxidation plays a significant role in disrupting sperm functions and especially motility and morphology and may account for some cases of male infertility [228]. Taha et al. reported that smoking has a negative effect on sperm progressive motility, sperm membrane integrity as evaluated by Hypo Osmotic Swell test (HOStest), DNA integrity and seminal zinc concentration [111].
Malondialdehyde (MDA) is a lipid peroxidation product of polyunsaturated fatty acids that is used as diagnostic tool for lipid peroxidation and male infertility [229]. Oxidative stress in sperm and its effect on membrane lipids, was measured by the formation of Malondialdehyde (MDA) [121]. A high MDA levels concentration in seminal plasma correlates negatively with other sperm parameters such as concentration and motility [219,230,231]. Nevertheless, higher MDA levels have been found in patients with varicocele when compared to controls, indicating increased peroxidation activity [232].
Cigarette Smoking and ART Outcome
Sperm quality is a crucial determinant for successful fertilization and pregnancy achieved by ART [233]. Fertilizing capacity is reduced due to failure to extrude residual spermatozoon cytoplasm, diminished membrane maturation and acrosin activity and an increased incidence of structural abnormalities in sperm tail [234].
Further evidence for the damage of spermatozoa by cigarette smoking can be inferred by the low success rate in assisted reproductive programmes when the male partner smokes [38]. A similar effect has been observed in other studies which found that cigarette smoking adversely affects male reproductive health [235].
Studies of natural conception in couples with a smoking male partner demonstrate a significant reduction in fecundity, with an increased time-to-pregnancy, only when tobacco consumption is above 15 cigarettes a day [236].
Seminal ROS levels correlate with success rates of Intrauterine Insemination (IUI), In Vitro Fertilization (IVF), and ICSI [237]. Also, male infertility is connected to increased ROS and reduction of whole antioxidant activity in the seminal plasma.
Moreover, fertilization by sperm with fragmented DNA results in poor embryonic development, decreased implantation, lower pregnancy rates, and recurrent pregnancy losses [238-240].
A study conducted by Hammadeh et al. demonstrated that metaphase oocytes incubated with DNA damaged spermatozoa during IVF were associated with high rate of failed fertilization, defective embryo development, implantation failure and early abortion [241].
Depending on the degree of DNA damage, embryo development can be affected and thus result in embryonic death [240]. Embryo formed with damaged DNA can also develop to full term [242]. These negative effects are seen whenever the extent of sperm DNA damage exceeds the repair capabilities of the oocyte [145].
Early onset paternal effects on zygote development and early cleavage186 have also been described. In human reproduction, a link between a paternal factor and poor embryo quality resulting in reduced pregnancy rates, has been observed [243]. In addition, DNA damage is a contributory factor for apoptosis, poor fertilization rate, high frequency of miscarriage, and morbidity of off springs [244]. In the last decade, various studies have highlighted the importance of the ‘paternal factor‘, including male age or paternal exposure to toxins for spontaneous miscarriages [245,246].
A number of studies have shown that DNA damage in sperm, can be passed to the offspring following ICSI/IVF treatment. In addition, this has been reported to lead to an increase in incidence of childhood cancers [247].
Abnormal sperm nuclear chromatin condensation, apart from fertilization, has been shown to affect embryo quality and pregnancy rates following IVF. Specifically, the increased DNA breaks due to faulty replacement of histones by protamines have been associated with decreased embryo morphology at early cleavage stages, whereas the improper protamine ratios have been proposed to be responsible for poor preimplantation stage embryo morphologies [130]. These defects may be attributed to increased oxidative stress and insufficient scavenging by antioxidant enzymes in the seminal fluid. Therefore, it is advisable for men to quit smoking who will undergo infertility therapy.
Much of the reduced fecundity associated with smoking may be reversed within 1 year of smoking abstinence. Whenever a definite link between paternal smoking and infertility is found, effective interventions helping patients to quit smoking should be addressed for the benefit of general health and fertility [248].
Several in vivo and in vitro studies showed that antioxidants have positive effects on oxiodative –induced sperm DNA damage and so, this agent can manage male infertility and sub fertility [160]. Male infertility which are suggested to be a results of existing sperm DNA damage can be given oral antioxidant during two months before an ICSI attempt. The subsequent ICSI cycle led to a significant increase in implantation rates and clinical pregnancy in comparison to the pre-treatment ICSI outcomes despite of the absence of differences in fertilization and cleavage rates on in embryo quality [180] (Table 2).
Authors
Year
Study population
Antioxidant measured
Outcome
Silver et al. [263]
2005
Non-smoking, healthy ,on history of infertility (n=89)
Beta-carotene, Vit. C and E
No correlation between increate antioxidant intake and improbe sperm chromatin
Eskinazi et al. [264]
2005
Non-smoking, healthy ,on history of infertility (n=87)
Zinc, Folate, beta-carotene Vit C and E
High antioxidant intake association with improved sperm parameters,no dose relationship
Song et al. [265]
2006
Infertile men (n=75)
Seminal levels of Vet. C
Low levels of seminal vitamin C correlate with increased sperm damage
Tremellen et al.
2007
Server male factor infertility, randomized double blind placebo controlled trail (n=60)
Menevit anti oxidant (3 months) prior to IVF cycle
The antioxidant improved in pregnancy rate.
No significant changes in oocyte fertilization rate or embryo quality
Menezo et al. [158]
2007
Healthy men (n=58)
Antioxidant vitamins associated with zinc and selenium (90 days)
Antioxidant treatment lead to a decrease in sperm DNA fragmentation
Young et al.
2008
Healthy, non-smoking men,no history of infertility (n= 89)
Zinc, Folate, beta-carotine, Vit Cand E
High folate intake associated with low sperm aneuploidy, no correlation with others
Piomboni et al. [266]
2008
Infertile men (n= 51)
Papaya, Lactoferrin Vit. C and E
Increased dietary intake associated with improved semen parameters and decrease chromatin
Mendiola et al. [273]
2009
Healthy men(31) poor sperm quality (n= 30)
Fat rich food, fruits and vegetable
Maintained or improved semen quality
Minguez et al. [267]
2012
Healthy young men
(n= 215)
Dietary intake (Vitamine C, lycopene & ß-Carotine
Semen quality improved by lycopene & ß-Carotine
Semen volume increased by Vitamine C.
Zareba et al. [268]
2013
Healthy young men
(n=180)
Carotenoide & Lycopene
Higher sperm motility
Better sperm morpholgy
Chavarro et al. [269]
2014
Healthy young men
(n=209)
Dietary tra-fatty acid and high cholesterol
Negative effect on semen quality
Table 2: Supplementation with individual antioxidant may lead to semen quality.
Moreover, treatment of infections has been shown to decrease DNA damage and improve reproductive outcome [249]. Severe varicocele treatment through varicocelectomy has been proven to results in a beneficial effect in sperm quality [249,250].
In conclusion, a strong body of evidence demonstrates that exposure to tobacco affects sperm function and integrity. Some of the drawbacks identified seem to be overcome by ICSI, but genetic damage would still be a problem in semen from smokers, regardless of the ART technique used.
References
- Brugh VM, Lipshultz LI. Male factor infertility: evaluation and management. Med Clin North Am. 2004; 88: 367-385.
- Cummings AM, Kavlock RJ. Gene-environment interactions: a review of effects on reproduction and development. Crit Rev Toxicol. 2004; 34: 461-485.
- Potts RJ, Newbury CJ, Smith G, Notarianni LJ, Jefferies TM. Sperm chromatin damage associated with male smoking. Mutat Res. 1999; 423: 103-111.
- Saleh RA, Agarwal A. Oxidative stress and male infertility: from research bench to clinical practice. J Androl. 2002; 23: 737-752.
- Wang X, Sharma RK, Gupta A, George V, Thomas AJ, Falcone T, et al. Alterations in mitochondria membrane potential and oxidative stress in infertile men: a prospective observational study. Fertil Steril. 2003; 80: 844-850.
- Bain C, Feskanich D, Speizer FE, Thun M, Hertzmark E, Rosner BA, et al. Lung cancer rates in men and women with comparable histories of smoking. J Natl Cancer Inst. 2004; 96: 826-834.
- Skakkebaek NE, Jorgensen N, Main KM, Rajpert-De Meyts E, Leffers H, Andersson AM, et al. Is human fecundity declining? Int J Androl. 2006; 29: 2-11.
- Sharpe RM. Environmental/lifestyle effects on spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010; 365: 1697-1712.
- Anderson K, Nisenblat V, Norman R. Lifestyle factors in people seeking infertility treatment - A review. Aust N Z J Obstet Gynaecol. 2010; 50: 8-20.
- Trummer H, Habermann H, Haas J, Pummer K. The impact of cigarette smoking on human semen parameters and hormones. Hum Reprod. 2002; 17: 1554-1559.
- Soares SR, Melo MA. Cigarette smoking and reproductive function. Curr Opin Obstet Gynecol. 2008; 20: 281-291.
- Bernhard D, Rossmann A, Wick G. Metals in cigarette smoke. IUBMB Life. 2005; 57: 805-809.
- Kumosani TA, Elshal MF, Al-Jonaid AA, Abduljabar HS. The influence of smoking on semen quality, seminal microelements and Ca2+-ATPase activity among infertile and fertile men. Clin Biochem. 2008; 41: 1199-1203.
- Kumar PS, Matthews CR, Joshi V, de Jager M, Aspiras M. Tobacco smoking affects bacterial acquisition and colonization in oral biofilms. Infect Immun. 2011; 79: 4730-4738.
- Järup L, Hellstrom L, Alfven T, Carlsson MD, Grubb A, Persson B, et al. Low level exposure to cadmium and early kidney damage: the OSCAR study. Occup Environ Med. 2000; 57: 668-672.
- Kiziler AR, Aydemir B, Onaran I, Alici B, Ozkara H, Gulyasar T, et al. High levels of cadmium and lead in seminal fluid and blood of smoking men are associated with high oxidative stress and damage in infertile subjects. Biol Trace Elem Res. 2007; 120: 82-91.
- Wong WY, Thomas CM, Merkus HM, Zielhuis GA, Doesburg WH, Steegers-Theunissen RP. Cigarette smoking and the risk of male factor subfertility: minor association between cotinine in seminal plasma and semen morphology. Fertil Steril. 2000; 74: 930-935.
- Hoffmann D, Djordjevic MV. Chemical composition and carcinogenicity of smokeless tobacco. Adv Dent Res. 1997; 11: 322-329.
- Hukkanen J, Jacob P, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005; 57: 79-115.
- Jacob P, Yu L, Shulgin AT, Benowitz NL. Minor tobacco alkaloids as biomarkers for tobacco use: comparison of users of cigarettes, smokeless tobacco, cigars, and pipes. Am J Public Health. 1999; 89: 731-736.
- Thompson J, Bannigan J. Cadmium: toxic effects on the reproductive system and the embryo. Reprod Toxicol. 2008; 25: 304-315.
- Sepaniak S, Forges T, Gerard H, Foliguet B, Bene MC, Monnier-Barbarino P. The influence of cigarette smoking on human sperm quality and DNA fragmentation. Toxicology. 2006; 223: 54-60.
- Martelli A, Rousselet E, Dycke C, Bouron A, Moulis JM. Cadmium toxicity in animal cells by interference with essential metals. Biochimie. 2006; 88: 1807-1814.
- Chia SE, Lim ST, Tay SK, Lim ST. Factors associated with male infertility: a case-control study of 218 infertile and 240 fertile men. BJOG. 2000; 107: 55-61.
- Benoff S, Gilbert BR. Varicocele and male infertility: part I. Preface. Hum Reprod Update. 2001; 7: 47-54.
- Arabi M. Nicotinic infertility: assessing DNA and plasma membrane integrity of human spermatozoa. Andrologia. 2004; 36: 305-310.
- Jarvis MJ, Russell MA, Benowitz NL, Feyerabend C. Elimination of cotinine from body fluids: implications for noninvasive measurement of tobacco smoke exposure. Am J Public Health. 1988; 78: 696-698.
- Hammadeh ME, Hamad MF, Montenarh M, Fischer-Hammadeh C. Protamine contents and P1/P2 ratio in human spermatozoa from smokers and non-smokers. Hum Reprod. 2010; 25: 2708-2720.
- Chen HW, Kuo CT. Cotinine characterization and quality effect of sperm for smoking and nonsmoking students. Bull Environ Contam Toxicol. 2007; 79: 11-14.
- Zavos PM, Correa JR, Antypas S, Zarmakoupis-Zavos PN, Zarmakoupis CN. Effects of seminal plasma from cigarette smokers on sperm viability and longevity. Fertil Steril. 1998; 69: 425-429.
- Esteves SC. Effect of cigarette smoking on levels of seminal oxidative stress in infertile men: a prospective study. Int Braz J Urol. 2002; 28: 484-485.
- Choudhary R, Chawala VK, Soni ND, Kumar J, Vyas RK. Oxidative stress and role of antioxidants in male infertility. Pak J Physiol. 2010; 6: 54-59.
- Gharagozloo P, Aitken RJ. The role of sperm oxidative stress in male infertility and the significance of oral antioxidant therapy. Hum Reprod. 2011; 26: 1628-1640.
- Zhang ZH, Zhu HB, Li LL, Yu Y, Zhang HG, Liu RZ. Decline of semen quality and increase of leukocytes with cigarette smoking in infertile men. Iran J Reprod Med. 2013; 11: 589-596.
- Aryanpur M, Heydari GH, Tarahomi M, Akhondi MA, Zeraati H, Masjedi M. Prevalence of tobacco Smoking among infertile couples in Tehran. Reproduction and infertility Journal. 2009; 9: 342-349.
- Kunzle R, Mueller MD, Hanggi W, Birkhauser MH, Drescher H, Bersinger NA. Semen quality of male smokers and nonsmokers in infertile couples. Fertil Steril. 2003; 79: 287-291.
- Ramlau-Hansen CH, Thulstrup AM, Storgaard L, Toft G, Olsen J, Bonde JP. Is prenatal exposure to tobacco smoking a cause of poor semen quality? A follow-up study. Am J Epidemiol. 2007; 165: 1372-1379.
- Zitzmann M, Rolf C, Nordhoff V, Schrader G, Rickert-Fohring M, Gassner P, et al. Male smokers have a decreased success rate for in vitro fertilization and intracytoplasmic sperm injection. Fertil Steril. 2003; 79: 1550-1554.
- Zhang JP, Meng QY, Zhang LJ, Mao YL, Sun ZX. [Study of correlation and effect of smoking on semen quality of men]. Zhonghua Nan Ke Xue. 2002; 8: 35-37.
- Taszarek-H G, Depa-Martynow M, Derwich K, Pawelczyk L, Jedrzejczak P. [The influence of cigarette smoking on sperm quality of male smokers and nonsmokers in infertile couples]. Przegl Lek. 2005; 62: 978-981.
- Pasqualotto FF, Sobreiro BP, Hallak J, Pasqualotto EB, Lucon AM. Cigarette smoking is related to a decrease in semen volume in a population of fertile men. BJU Int. 2006; 97: 324-326.
- Arabi M, Moshtaghi H. Influence of cigarette smoking on spermatozoa via seminal plasma. Andrologia. 2005; 37: 119-124.
- Bansal AK, Bilaspuri GS. Impacts of oxidative stress and antioxidants on semen functions. Vet Med Int. 2010.
- Miranda-Vilela AL, Alves PCZ, Akimoto AK, Lordelo SG, Gonçalves CA, Cesar K Grisolia CK, et al. Gene polymorphisms against DNA damage induced by hydrogen peroxide in leukocytes of healthy humans through comet assay: a quasi-experimental study. Environmental Health. 2010; 9: 21-30.
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000; 408: 239-247.
- Janssen YM, Van Houten B, Borm PJ, Mossman BT. Cell and tissue responses to oxidative damage. Lab Invest. 1993; 69: 261-274.
- Agarwal A, Said TM. Oxidative stress, DNA damage and apoptosis in male infertility: a clinical approach. BJU Int. 2005; 95: 503-507.
- Stowe DF, Camara AK. Mitochondrial reactive oxygen species production in excitable cells: modulators of mitochondrial and cell function. Antioxid Redox Signal. 2009; 11: 1373-1414.
- Aitken RJ, Jones KT, Robertson SA. Reactive oxygen species and sperm function--in sickness and in health. J Androl. 2012; 33: 1096-1106.
- Hamatani T, Falco G, Carter MG, Akutsu H, Stagg CA, Sharov AA, et al. Age-associated alteration of gene expression patterns in mouse oocytes. Hum Mol Genet. 2004; 13: 2263-2278.
- Linschooten JO, Laubenthal J, Cemeli E, Baumgartner A, Anderson D, Sipinen VE, et al. Incomplete protection of genetic integrity of mature spermatozoa against oxidative stress. Reprod Toxicol. 2011; 32: 106-111.
- Fariello RM, Pariz JR, Spaine DM, Gozzo FC, Pilau EJ, Fraietta R, et al. Effect of smoking on the functional aspects of sperm and seminal plasma protein profiles in patients with varicocele. Hum Reprod. 2012; 27: 3140-3149.
- Kao SH, Chao HT, Chen HW, Hwang TI, Liao TL, Wei YH. Increase of oxidative stress in human sperm with lower motility. Fertil Steril. 2008; 89: 1183-1190.
- Sanocka D, Kurpisz M. Reactive oxygen species and sperm cells. Reprod Biol Endocrinol. 2004; 2: 12.
- Bungum M, Humaidan P, Axmon A, Spano M, Bungum L, Erenpreiss J, et al. Sperm DNA integrity assessment in prediction of assisted reproduction technology outcome. Hum Reprod. 2007; 22: 174-179.
- Zini A, Zhang X, San Gabriel M. Sperm nuclear histone H2B: correlation with sperm DNA denaturation and DNA stainability. Asian J Androl. 2008; 10: 865-871.
- Wolff H. The biologic significance of white blood cells in semen. Fertil Steril. 1995; 63: 1143-1157.
- Jozwik M, Jozwik M, Kuczynski W, Szamatowicz M. Nonenzymatic antioxidant activity of human seminal plasma. Fertil Steril. 1997; 68: 154-157.
- Tarozzi N, Bizzaro D, Flamigni C, Borini A. Clinical relevance of sperm DNA damage in assisted reproduction. Reprod Biomed Online. 2007; 14: 746-757.
- Kasturi SS, Osterberg C, Tannir J, Brannigan RE. The effect of genital tract infection and inflammation on male infertility. In: Lipshultz L, Howard S, Niederberger C, Eds. Infertility in the male, 4th ed. UK: Cambridge University Press. 2009; 295-300.
- Barbieri SS, Weksler BB. Tobacco smoke cooperates with interleukin-1ß to alter ß -catenin trafficking in vascular endothelium resulting in increased permeability and induction of cyclooxygenase-2 expression in vitro and in vivo. FASEB J. 2007; 21: 1831–1843.
- Castro P, Legora-Machado A, Cardilo-Reis L, Valença S, Porto LC, Walker C, et al. Inhibition of interleukin-1beta reduces mouse lung inflammation induced by exposure to cigarette smoke. Eur J Pharmacol. 2004; 498: 279-286.
- Hasnis E, Bar-Shai M, Burbea Z, Reznick AZ. Cigarette smokeinduced NF-kappaB activation in human lymphocytes: the effect of low and high exposure to gas phase of cigarette smoke. J Physiol Pharmacol. 2007; 58: 263–274.
- Virro MR, Larson-Cook KL, Evenson DP. Sperm chromatin structure assay (SCSA) parameters are related to fertilization, blastocyst development, and ongoing pregnancy in in vitro fertilization and intracytoplasmic sperm injection cycles. Fertil Steril. 2004; 81: 1289-1295.
- Younglai EV, Holloway AC, Foster WG. Environmental and occupational factors affecting fertility and IVF success. Hum Reprod Update. 2005; 11: 43-57.
- Zini A, Defreitas G, Freeman M, Hechter S, Jarvi K. Varicocele is associated with abnormal retention of cytoplasmic droplets by human spermatozoa. Fertil Steril. 2000; 74: 461-464.
- Siciliano G, Mancuso M, Ceravolo R, Lombardi V, Iudice A, Bonuccelli U. Mitochondrial DNA rearrangements in young onset parkinsonism: two case reports. J Neurol Neurosurg Psychiatry. 2001; 71: 685-687.
- Labbe C, Martoriati A, Devaux A, Maisse G. Effect of sperm cryopreservation on sperm DNA stability and progeny development in rainbow trout. Mol Reprod Dev. 2001; 60: 397-404.
- Linfor JJ, Meyers SA. Detection of DNA damage in response to cooling injury in equine spermatozoa using single-cell gel electrophoresis. J Androl. 2002; 23: 107-113.
- Thomas DD, Ridnour LA, Isenberg JS, Flores-Santana W, Switzer CH, Donzelli S, et al. The chemical biology of nitric oxide: implications in cellular signaling. Free Radic Biol Med. 2008; 45: 18-31.
- Elahi MM, Kong YX, Matata BM. Oxidative stress as a mediator of cardiovascular disease. Oxid Med Cell Longev. 2009; 2: 259-269.
- Du Plessis SS Makker K, Desai NR, Agarwal A. Impact of oxidative stress on IVF. Expert Rev Obstet Gynecol. 2008; 3: 539-554.
- Gupta S, Sekhon L, KimY, Agarwal A. The Role of Oxidative Stress and Antioxidants in Assisted Reproduction. urrent Women’s Health Reviews. 2010; 6: 227-238.
- Hammadeh ME, Filippos A, Hamad MF. Reactive oxygen species and antioxidants in seminal plasma and their impact on male fertility. Int J Fertil Steril. 2009; 3: 87-110.
- Panda K, Chattopadhyay R, Chattopadhyay DJ, Chatterjee IB. Vitamin C prevents cigarette smoke-induced oxidative damage in vivo. Free Radic Biol Med. 2000; 29:115–124.
- Traber MG, van der Vliet A, Reznick AZ, Cross CE. Tobacco-related diseases. Is there a role for antioxidant micronutrient supplementation? Clin Chest Med. 2000; 21: 173-187.
- Hennekens CH, Buring JE, Manson JE, Stampfer M, Rosner B, Cook NR, et al. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med. 1996; 334: 1145-1149.
- Prieme H, Loft S, Nyyssonen K, Salonen JT, Poulsen HE. No effect of supplementation with vitamin E, ascorbic acid, or coenzyme Q10 on oxidative DNA damage estimated by 8-oxo-7,8-dihydro-2'-deoxyguanosine excretion in smokers. Am J Clin Nutr. 1997; 65: 503-507.
- Cross CE, Traber M, Eiserich J, van der Vliet A. Micronutrient antioxidants and smoking. Br Med Bull. 1999; 55: 691-704.
- Mostafa T, Tawadrous G, Roaia MM, Amer MK, Kader RA, Aziz A. Effect of smoking on seminal plasma ascorbic acid in infertile and fertile males. Andrologia. 2006; 38: 221-224.
- Aitken RJ, Baker MA. Oxidative stress, sperm survival and fertility control. Mol Cell Endocrinol. 2006; 250: 66-69.
- Sobczak A, Golka D, Szoltysek-Boldys I. The effects of tobacco smoke on plasma alpha- and gamma-tocopherol levels in passive and active cigarette smokers. Toxicol Lett. 2004; 151: 429-437.
- Orosz Z, Csiszar A, Labinskyy N, Smith K, Kaminski PM, Ferdinandy P, et al. Cigarette smoke-induced proinflammatory alterations in the endothelial phenotype: role of NAD(P)H oxidase activation. Am J Physiol Heart Circ Physiol. 2007; 292: 130-139.
- Li Y, Wang H. “In utero exposure to tobacco and alcohol modifies neurobehavioral development in mice offspring: consideration a role of oxidative stress,” Pharmacological Research. 2004; 9: 467–473.
- Balercia G, Armeni T, Mantero F, Principato G, Regoli F. Total oxyradical scavenging capacity toward different reactive oxygen species in seminal plasma and sperm cells. Clin Chem Lab Med. 2003; 41: 13-19.
- Aitken RJ. Founders' Lecture. Human spermatozoa: fruits of creation, seeds of doubt. Reprod Fertil Dev. 2004; 16: 655-664.
- Hammadeh ME, Radwan M, Al-Hasani S, Micu R, Rosenbaum P, Lorenz M, et al. Comparison of reactive oxygen species concentration in seminal plasma and semen parameters in partners of pregnant and non-pregnant patients after IVF/ICSI. Reprod Biomed Online. 2006; 13: 696-706.
- Badouard C, Menezo Y, Panteix G, Ravanat JL, Douki T, Cadet J, et al. Determination of new types of DNA lesions in human sperm. Zygote. 2008; 16: 9-13.
- Gonzalez-Marin C, Gosalvez J, Roy R. Types, causes, detection and repair of DNA fragmentation in animal and human sperm cells. Int J Mol Sci. 2012; 13: 14026-14052.
- Valavanidis A, Vlachogianni T, Fiotakis C. 8-hydroxy-2' -deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009; 27: 120-139.
- Aitken RJ, Koppers AJ. Apoptosis and DNA damage in human spermatozoa. Asian J Androl. 2011; 13: 36-42.
- Bains M, Hall ED. Antioxidant therapies in traumatic brain and spinal cord injury. Biochim Biophys Acta. 2012; 1822: 675-684.
- Reinardy HC, Syrett JR, Jeffree RA, Henry TB, Jha AN. Cobalt-induced genotoxicity in male zebrafish (Danio rerio), with implications for reproduction and expression of DNA repair genes. Aquat Toxicol. 2013; 126: 224-230.
- Wu S, Deng F, Liu Y, Shima M, Niu J, Huang Q, et al. Temperature, traffic-related air pollution, and heart rate variability in a panel of healthy adults. Environ Res. 2013; 120: 82-89.
- Agarwal A, Sharma RK, Nallella KP, Thomas AJ, Alvarez JG, Sikka SC. Reactive oxygen species as an independent marker of male factor infertility. Fertil Steril. 2006; 86: 878-885.
- Shiota K, Yamada S. Intrauterine environment -genom interaction and child’s development (3) assisted conception technology and development disorders. J Toxicol Sci. 2009; 34: 286-291.
- Moustafa MH, Sharma RK, Thornton J, Mascha E, Abdel-Hafez MA, Thomas AJ, et al. Relationship between ROS production, apoptosis and DNA denaturation in spermatozoa from patients examined for infertility. Hum Reprod. 2004; 19: 129-138.
- Mehrannia T. The effect of cigarette smoking on semen quality of infertile men. Pak J Med Sci. 2007; 23; 717-719.
- Colagar AH, Jorsaraee GA, Marzony ET. Cigarette smoking and the risk of male infertility. Pak J Biol Sci. 2007; 10: 3870-3874.
- Vine MF, Margolin BH, Morrison HI, Hulka BS. Cigarette smoking and sperm density: a meta-analysis. Fertil Steril. 1994; 61: 35-43.
- Gaur DS, Talekar M, Pathak VP. Effect of cigarette smoking on semen quality of infertile men. Singapore Med J. 2007; 48: 119-123.
- Collodel G, Capitani S, Pammolli A, Giannerini V, Geminiani M, Moretti E. Semen quality of male idiopathic infertile smokers and nonsmokers: an ultrastructural study. J Androl. 2010; 31: 108-113.
- Hassan A, Abo-Azma SM, Fayed SM, Mostafa T. Seinal plasma cotinine and insulin-like growth factor-I in idiopathic oligoasthenoteratozoospermic smokers. Bull Journal Urology International. 2009; 103: 108-111.
- Mostafa T. Cigarett smoking and male infertility. Journal of Advanced Research. 2010; 179-186.
- Joo KJ, Kwon YW, Myung SC, Kim TH. The effects of smoking and alcohol intake on sperm quality: light and transmission electron microscopy findings. J Int Med Res. 2012; 40: 2327-2335.
- Nadeem F, Fahim A, Bugti S. Effect of cigarette smoking on male fertility: Turkish Journal of Medical Science. 2012; 42: 1400-1405.
- Correa-Perez JR. Smoking and sperm viability--a never-ending story. Fertil Steril. 2003; 79: 1469.
- Belcheva A, Ivanova-Kicheva M, Tzvetkova P, Marinov M. Effects of cigarette smoking on sperm plasma membrane integrity and DNA fragmentation. Int J Androl. 2004; 27: 296-300.
- Davar R, Sekhavat L, Naserzadeh N. Semen parameters of non-infertile smoker and non-smoker men. J Med Life. 2012; 5: 465-468.
- Meri ZB, Irshid IB, Migdadi M, Irshid AB, Mhanna SA. Does cigarette smoking affect seminal fluid parameters? A comparative study. Oman Med J. 2013; 28: 12-15.
- Taha E, Ezz-Addin A, Sayed S, Ghandour N, Mostafa T. Smoking influence on sperm vitality, DNA fragmentation, reactive oxygen species and zinc in ligoasthenoteratozoospermic men with varicocele. Andrologia. 2014; 80: 822-825.
- de Jong AM, Menkveld R, Lens JW, Nienhuis SE, Rhemrev JP. Effect of alcohol intake and cigarette smoking on sperm parameters and pregnancy. Andrologia. 2014; 46: 112-117.
- World Health Organization, WHO Laboratory Manual for the Examination and Processing of Human Semen, WHO, Geneva, Switzerland, 5th edition. 2010.
- Zini A, Bielecki R, Phang D, Zenzes MT. Correlations between two markers of sperm DNA integrity, DNA denaturation and DNA fragmentation, in fertile and infertile men. Fertil Steril. 2001; 75: 674-677.
- Zini A, Sigman M. Are tests of sperm DNA damage clinically useful? Pros and cons. J Androl. 2009; 30: 219-229.
- Aitken RJ, De Iuliis GN, McLachlan RI. Biological and clinical significance of DNA damage in the male germ line. Int J Androl. 2009; 32: 46-56.
- Barratt CL, De Jonge CJ. Clinical relevance of sperm DNA assessment: an update. Fertil Steril. 2010; 94: 1958-1959.
- Cho C, Jung-Ha H, Willis WD, Goulding EH, Stein P, Xu Z, et al. Protamine 2 deficiency leads to sperm DNA damage and embryo death in mice. Biol Reprod. 2003; 69: 211-217.
- Kimmins S, Sassone-Corsi P. Chromatin remodelling and epigenetic features of germ cells. Nature. 2005; 434: 583-589.
- Shen H, Ong C. Detection of oxidative DNA damage in human sperm and its association with sperm function and male infertility. Free Radic Biol Med. 2000; 28: 529-536.
- Li Z, Wang L, Cai J, Huang H. Correlation of sperm DNA damage with IVF and ICSI outcomes: a systematic review and meta-analysis. J Assist Reprod Genet. 2006; 23: 367-376.
- Practice Committee of the American Society for Reproductive Medicine. The clinical utility of sperm DNA integrity testing: a guideline. Fertil Steril. 2013; 99: 673-677.
- Cohen-Bacrie P, Belloc S, Menezo YJ, Clement P, Hamidi J, Benkhalifa M. Correlation between DNA damage and sperm parameters: a prospective study of 1,633 patients. Fertil Steril. 2009; 91: 1801-1805.
- Kleene KC. Patterns, mechanisms, and functions of translation regulation in mammalian spermatogenic cells. Cytogenet Genome Res. 2003; 103: 217-224.
- Oliva R. Protamines and male infertility. Hum Reprod Update. 2006; 12: 417-435.
- Carrell DT, Liu L. Altered protamine 2 expression is uncommon in donors of known fertility, but common among men with poor fertilizing capacity, and may reflect other abnormalities of spermiogenesis. J Androl. 2001; 22: 604-610.
- Corzett M, Mazrimas J, Balhorn R. Protamine 1: protamine 2 stoichiometry in the sperm of eutherian mammals. Mol Reprod Dev. 2002; 61: 519-527.
- Szczygiel MA, Ward WS. Combination of dithiothreitol and detergent treatment of spermatozoa causes paternal chromosomal damage. Biol Reprod. 2002; 67: 1532-1537.
- Aitken RJ, De Iuliis GN, Finnie JM, Hedges A, McLachlan RI. Analysis of the relationships between oxidative stresse, DNA damage and sperm vitality in a patient population: development of diagnostic criteria. Hum Reprod. 2010; 25: 2415-2426.
- Aoki VW, Liu L, Carrell DT. Identification and evaluation of a novel sperm protamine abnormality in a population of infertile males. Hum Reprod. 2005; 20: 1298-1306.
- Carrell DT, Emery BR, Hammoud S. Altered protamine expression and diminished spermatogenesis: what is the link? Hum Reprod Update. 2007; 13: 313-327.
- Simon L, Castillo J Oliver R, Lewis. Relationship between human sperm protamines, DNA damage and assisted reproduction outcome. Reprod Biomed Online. 2011; 23: 724-734.
- Garcia-Peiro A, Martinez-Heredia J, Oliver-Bonet M, Abad C, Amengual MJ, Navarro J, et al. Protamine 1 to protamine 2 ratio correlated with dynamics aspects of DNA fragmentation in human sperm. Fertil Steril. 2011; 95: 105-109.
- de Mateo S, Gazquez C, Guimera M, Balasch J, Meistrich ML, Ballesca JL, et al. Protamine 2 precursors (Pre-P2), protamine 1 to protamine 2 ratio (P1/P2), and assisted reproduction outcome. Fertil Steril. 2009; 91: 715-722.
- Rogenhofer N, Dansranjavin T, Schorsch M, Spiess A, Wang H, von Schonfeldt V, et al. The sperm protamine mRNA ratio as a clinical parameter to estimate the fertilizing potential of men taking part in an ART programme. Hum Reprod. 2013; 28: 969-978.
- Hamad MF, Shelko N, Kartarius S, Montenarh M, Hammadeh ME. Impact of cigarette smoking on histone (H2B) to protamine ratio in human spermatozoa and its relation to sperm parameters. Andrology. 2014; 2: 666-677.
- Rousseaux S, Caron C, Govin J, Lestrat C, Faure AK, Khochbin S. Establishment of male-specific epigenetic information. Gene. 2005; 345: 139-153.
- Govin J, Escoffier E, Rousseaux S, Kuhn I, Ferro M, Thevenon R, et al. Pericentric heterochromatin reprogramming by new histone variants during mouse spermiogenesis. J Cell Biol. 2007; 176: 283-294.
- Zalensky AO, Siino JS, Gineitis AA, Zalenskaya IA, Tomilin NV, Yau P, et al. Human testis/sperm-specific histone H2B (hTSH2B). Molecular cloning and characterization. J Biol Chem. 2002; 277: 43474-43480.
- Gineitis AA, Zalenskaya IA, Yau PM, Bradbury EM, Zalensky AO. Human sperm telomere-binding complex involves histone H2B and secures telomere membrane attachment. J Cell Biol. 2000; 151: 1591-1598.
- Govin J, Caron C, Lestrat C, Rousseaux S, Khochbin S. The role of histones in chromatin remodelling during mammalian spermiogenesis. Eur J Biochem. 2004; 271: 3459-3469.
- Zhang X, San Gabriel M, Zini A. Sperm nuclear histone to protamine ratio in fertile and infertile men: evidence of heterogeneous subpopulations of spermatozoa in the ejaculate. J Androl. 2006; 27: 414-420.
- Singleton S, Zalensky A, Doncel GF, Morshedi M, Zalenskaya IA. Testis/sperm-specific histone 2B in the sperm of donors and subfertile patients: variability and relation to chromatin packaging. Hum Reprod. 2007; 22: 743-750.
- Zini A, Gabriel MS, Zhang X. The histone to protamine ratio in human spermatozoa: comparative study of whole and processed semen. Fertil Steril. 2007; 87: 217-219.
- Agarwal A, Said TM. Role of sperm chromatin abnormalities and DNA damage in male infertility. Hum Reprod Update. 2003; 9: 331-345.
- McPherson S, Longo FJ. Chromatin structure –function alteration during mammalian spermatogenesis: DNA nicking and repair in elongation spermatids. EUJ Histochem. 1993; 37: 170-181.
- Alvarez JG. The predictive value of sperm chromatin structure assay. Hum Reprod. 2005; 20: 2365-2367.
- Sakkas D, Moffatt O, Manicardi GC, Mariethoz E, Tarozzi N, Bizzaro D. Nature of DNA damage in ejaculated human spermatozoa and the possible involvement of apoptosis. Biol Reprod. 2002; 66: 1061-1067.
- Cisternas P, Moreno RD. Comparative analysis of apoptotic pathways in rat, mouse, and hamster spermatozoa. Mol Reprod Dev. 2006; 73: 1318-1325.
- Agarwal A, Saleh RA. Role of oxidants in male infertility: rationale, significance, and treatment. Urol Clin North Am. 2002; 29: 817-827.
- Henkel R, Kierspel E, Stalf T, Mehnert C, Menkveld R, Tinneberg HR, et al. Effect of reactive oxygen species produced by spermatozoa and leukocytes on sperm functions in non-leukocytospermic patients. Fertil Steril. 2005; 83: 635-642.
- Meeker JD, Rossano MG, Protas B, Diamond MP, Puscheck E, Daly D, et al. Cadmium, lead, and other metals in relation to semen quality: human evidence for molybdenum as a male reproductive toxicant. Environ Health Perspect. 2008; 116: 1473-1479.
- Said TM, Agarwal A, Sharma RK, Thomas AJ, Sikka SC. Impact of sperm morphology on DNA damage caused by oxidative stress induced by beta-nicotinamide adenine dinucleotide phosphate. Fertil Steril. 2005; 83: 95-103.
- Ahmadi A, Ng SC. Developmental capacity of damaged spermatozoa. Hum Reprod. 1999; 14: 2279-2285.
- Elshal MF, Ibrahim H, El-Sayed M, El saied S, El-Asry T, Kumosani A. Sperm head defects and disturbances in spermatozoal chromatin and DNA integrities in idiopathic infertile subjects: Association with cigarette smoking. Clinical Bioch. 2009; 42: 589–594.
- Sun JG, Jurisicova A, Casper RF. Detection of deoxyribonucleic acid fragmentation in human sperm: correlation with fertilization in vitro. Biol Reprod. 1997; 56: 602-607.
- Sepaniak S, Forges T, Fontaine B, Gerard H, Foliguet B, Guillet-May F, et al. [Negative impact of cigarette smoking on male fertility: from spermatozoa to the offspring]. J Gynecol Obstet Biol Reprod (Paris). 2004; 33: 384-390.
- Menezo Y, Dale B, Cohen M. DNA damage and repair in human oocytes and embryos: a review. Zygote. 2010; 18: 357-365.
- Duran EH, Morshedi M, Taylor S, Oehninger S. Sperm DNA quality predicts intrauterine insemination outcome: a prospective cohort study. Hum Reprod. 2002; 17: 3122-3128.
- Agarwal A, Allamaneni SS. The effect of sperm DNA damage on assisted reproduction outcomes. A review. Minerva Ginecol. 2004; 56: 235-245.
- Borini A, Tarozzi N, Bizzaro D, Bonu MA, Fava L, Flamigni C, et al. Sperm DNA fragmentation: paternal effect on early post-implantation embryo development in ART. Hum Reprod. 2006; 21: 2876-2881.
- Lee KM, Ward MH, Han S, Ahn HS, Kang HJ, Choi HS, et al. Paternal smoking, genetic polymorphisms in CYP1A1 and childhood leukemia risk. Leuk Res. 2009; 33: 250-258.
- Fuentes A, Munoz A, Barnhart K, Arguello B, Diaz M, Pommer R. Recent cigarette smoking and assisted reproductive technologies outcome. Fertil Steril. 2010; 93: 89-95.
- Liu PY, Baker HW, Jayadev V, Zacharin M, Conway AJ, Handelsman DJ. Induction of spermatogenesis and fertility during gonadotropin treatment of gonadotropin-deficient infertile men: predictors of fertility outcome. J Clin Endocrinol Metab. 2009; 94: 801-808.
- Oldereid NB, Angelis PD, Wiger R, Clausen OP. Expression of Bcl-2 family proteins and spontaneous apoptosis in normal human testis. Mol Hum Reprod. 2001; 7: 403-408.
- Ramos L, van der Heijden GW, Derijck A, Berden JH, Kremer JA, van der Vlag J, et al. Incomplete nuclear transformation of human spermatozoa in oligo-astheno-teratospermia: characterization by indirect immunofluorescence of chromatin and thiol status. Human Reproduction. 2008; 23: 259–270.
- Kocak I, Dundar M, Hekimgil M, Okyay P. Assessment of germ cell apoptosis in cryptorchid rats. Asian J Androl. 2002; 4: 183-186.
- Fischer MA, Willis J, Zini A. Human sperm DNA integrity: correlation with sperm cytoplasmic droplets. Urology. 2003; 61: 207-211.
- Ward MA, Ward WS. A model for the function of sperm DNA degradation. Reprod Fertil Dev. 2004; 16: 547-554.
- Sotolongo B, Huang TT, Isenberger E, Ward WS. An endogenous nuclease in hamster, mouse, and human spermatozoa cleaves DNA into loop-sized fragments. J Androl. 2005; 26: 272-280.
- El-Domyati MM, Al-Din AB, Barakat MT, El-Fakahany HM, Xu J, Sakkas D. Deoxyribonucleic acid repair and apoptosis in testicular germ cells of aging fertile men: the role of the poly(adenosine diphosphate-ribosyl)ation pathway. Fertil Steril. 2009; 91: 2221-2229.
- Tesarik J, Greco E, Mendoza C. Late, but not early, paternal effect on human embryo development is related to sperm DNA fragmentation. Hum Reprod. 2004; 19: 611-615.
- Bozec A, Amara S, Guarmit B, Selva J, Albert M, Rollet J, et al. Status of the executioner step of apoptosis in human with normal spermatogenesis and azoospermia. Fertil Steril. 2008; 90: 1723-1731.
- Blasco MA. Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet. 2005; 6: 611-622.
- Paasch U, Grunewald S, Agarwal A, Glandera HJ. Activation pattern of caspases in human spermatozoa. Fertil Steril. 2004; 81: 802-809.
- Flesch FM, Gadella BM. Dynamics of the mammalian sperm plasma membrane in the process of fertilization. Biochim Biophys Acta. 2000; 1469: 197-235.
- Evenson DP, Darzynkiewicz Z, Melamed MR. Simultaneous measurement by flow cytometry of sperm cell viability and mitochondrial membrane potential related to cell motility. J Histochem Cytochem. 1982; 30: 279-280.
- Bennetts LE, Aitken RJ. A comparative study of oxidative DNA damage in mammalian spermatozoa. Mol Reprod Dev. 2005; 71: 77-87.
- Calogero AE, Polosa R, Perdichizzi A, Guarino F, La Vignera S, Scarfia A, et al. Cigarette smoke extract immobilizes human spermatozoa and induces sperm apoptosis. Reprod Biomed Online. 2009; 19: 564–571.
- Greco E, Iacobelli M, Rienzi L, Ubaldi F, Ferrero S, Tesarik J. Reduction of the incidence of sperm DNA fragmentation by oral antioxidant treatment. J Androl. 2005; 26: 349-353.
- Zareba P, Colaci DS, Afeiche M, Gaskins AJ, Jørgensen N, Mendiola J, et al. Semen quality in relation to antioxidant intake in a healthy male population. Fertil Steril. 2013; 100: 1572-1579.
- Chi HJ, Kim JH, Ryu CS, Lee JY, Park JS, Chung DY, et al. Protective effect of antioxidant supplementation in sperm-preparation medium against oxidative stress in human spermatozoa. Hum Reprod. 2008; 23: 1023–1028.
- Agarwal A, Sekhon LH. Oxidative stress and antioxidants for idiopathic oligoasthenoteratospermia: Is it justified? Indian J Urol. 2011; 27: 74-85.
- Showell MG, Brown J, Yazdani A, Stankiewicz MT, Hart RJ. Antioxidants for male subfertility. Cochrane Database Syst Rev. 2011; CD007411.
- Practice Committee of American Society for Reproductive Medicine. The clinical utility of sperm DNA integrity testing. Fertil Steril. 2008; 90: 178-180.
- Lewis SE, Aitken RJ. DNA damage to spermatozoa has impacts on fertilization and pregnancy. Cell Tissue Res. 2005; 322: 33-41.
- Steger K, Failing K, Klonisch T, Behre HM, Manning M, Weidner W, et al. Round spermatids from infertile men exhibit decreased protamine-1 and -2 mRNA. Hum Reprod. 2001; 16: 709-716.
- Miller D, Brinkworth M, Iles D. Paternal DNA packaging in spermatozoa: more than the sum of its parts? DNA, histones, protamines and epigenetics. Reproduction. 2010; 139: 287-301.
- Wykes SM, Krawetz SA. The structural organization of sperm chromatin. J Biol Chem. 2003; 278: 29471-29477.
- Feil R, Fraga MF. Epigenetics and the environment: emerging patterns and implications. Nat Rev Genet. 2012; 13: 97-109.
- Sin HS, Barski A, Zhang F, Kartashov AV, Nussenzweig A, Chen J, et al. RNF8 regulates active epigenetic modifications and escape gene activation from inactive sex chromosomes in post-meiotic spermatids. Genes Dev 2012; 26: 2737-2748.
- Emery BR, Carrell DT. The effect of epigenetic sperm abnormalities on early embryogenesis. Asian J Androl. 2006; 8: 131-142.
- Feinberg AP. Genome-scale approaches to the epigenetics of common human disease. Virchows Arch. 2010; 456: 13-21.
- Lister R, Pelizzola M, Dowen RH, Hawkins RD, Hon G, Tonti-Filippini J, et al. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature. 2009; 462: 315-322.
- Benchaib M, Braun V, Ressnikof D, Lornage J, Durand P, Niveleau A, et al. Influence of global sperm DNA methylation on IVF results. Hum Reprod. 2005; 20: 768-773.
- Smith IM, Mydlarz WK, Mithani SK, Califano JA. DNA global hypomethylation in squamous cell head and neck cancer associated with smoking, alcohol consumption and stage. Int J Cancer. 2007; 121: 1724-1728.
- Furniss CS, Marsit CJ, Houseman EA, Eddy K, Kelsey KT. Line region hypomethylation is associated with lifestyle and differs by human papillomavirus status in head and neck squamous cell carcinomas. Cancer Epidemiol. Biomarkers Prev. 2008; 17: 966–971.
- Marsit CJ, Houseman EA, Schned AR, Karagas MR, Kelsey KT. Promoter hypermethylation is associated with current smoking, age, gender and survival in bladder cancer. Carcinogenesis. 2007; 28: 1745-1751.
- Wu GJ, Chang FW, Lee SS, Cheng YY, Chen CH, Chen IC. Apoptosis-related phenotype of ejaculated spermatozoa in patients with varicocele. Fertil Steril. 2009; 91: 831-837.
- Dong LM, Potter JD, White E, Ulrich CM, Cardon LR, Peters U. Genetic susceptibility to cancer: the role of polymorphisms in candidate genes. JAMA. 2008; 299: 2423-2436.
- Malats N. Genetic epidemiology of bladder cancer: scaling up in the identification of low-penetrance genetic markers of bladder cancer risk and progression. Scand J Urol Nephrol Suppl. 2008; 131-140.
- Aitken RJ, Curry BJ. Redox regulation of human sperm function: from the physiological control of sperm capacitation to the etiology of infertility and DNA damage in the germ line. Antioxid Redox Signal. 2011; 14: 367-381.
- Valacchi G, Fortino V, Bocci V. The dual action of ozone on the skin. Br J Dermatol. 2005; 153: 1096-1100.
- Holloway AC, Cuu DQ, Morrison KM, Gerstein HC, Tarnopolsky MA. Transgenerational effects of fetal and neonatal exposure to nicotine. Endocrine. 2007; 31: 254-259.
- Franklin TB, Russig H, Weiss IC, Graff J, Linder N, Michalon A, et al. Epigenetic transmission of the impact of early stress across generations. Biol Psychiatry. 2010; 68: 408-415.
- Burdge GC, Hoile SP, Uller T, Thomas NA, Gluckman PD, Hanson MA, et al. Progressive, transgenerational changes in offspring phenotype and epigenotype following nutritional transition. PLoS One. 2011; 6: 28282.
- Mohamed el-SA, Song WH, Oh SA, Park YJ, You YA, Lee S, et al. The transgenerational impact of benzo(a)pyrene on murine male fertility. Hum Reprod. 2010; 25: 2427-2433.
- Linschooten JO, Van Schooten FJ, Baumgartner A, Cemeli E, Van Delft J, Anderson D, et al. Use of spermatozoal mRNA profiles to study gene-environment interactions in human germ cells. Mutat Res. 2009; 667: 70-76.
- Maccani MA, Avissar-Whiting M, Banister CE, McGonnigal B, Padbury JF, Marsit CJ. Maternal cigarette smoking during pregnancy is associated with downregulation of miR-16, miR-21, and miR-146a in the placenta. Epigenetics. 2010; 5: 583-589.
- Marczylo EL, Amoako AA, Konje JC, Gant TW, Marczylo TH. Smoking induces differential miRNA expression in human spermatozoa. A potential transgenerational epigenetic concern? Epigenetics. 2012; 7: 432-439.
- Hecht SS. Smoking and lung cancer--a new role for an old toxicant? Proc Natl Acad Sci USA. 2006; 103: 15725-15726.
- Lubin JH, Kogevinas M, Silverman D, Malats N, Garcia-Closas M, Tardon A, et al. Evidence for an intensity-dependent interaction of NAT2 acetylation genotype and cigarette smoking in the Spanish Bladder Cancer Study. Int J Epidemiol. 2007; 36: 236-241.
- Spira A, Beane J, Shah V, Liu G, Schembri F, Yang X, et al. Effects of cigarette smoke on the human airway epithelial cell transcriptome. Proc Natl Acad Sci USA. 2004; 101: 10143-10148.
- Walser T, Cui X, Yanagawa J, Lee JM, Heinrich E, Lee G, et al. Smoking and lung cancer: the role of inflammation. Proc Am Thorac Soc. 2008; 5: 811-815.
- Skowronek MF, Alciaturi J, Casanova G, Capurro A, Montes JM, Sapiro R. Value of quantitative ultramorphological sperm analysis in infertile men. Reprod Biol. 2010; 10: 125-139.
- Li K, Shang X, Chen Y. High-performance liquid chromatographic detection of lipid peroxidation in human seminal plasma and its application to male infertility. Clin Chim Acta. 2004; 346: 199-203.
- Sikka SC. Oxidative stress and role of antioxidants in normal and abnormal sperm function. Front Biosci. 1996; 1: 78-86.
- Baker MA, Aitken RJ. Reactive oxygen species in spermatozoa: methods for monitoring and significance for the origins of genetic disease and infertility. Reprod Biol Endocrinol. 2005; 3: 67.
- Tavilani H, Doosti M, Saeidi H. Malondialdehyde levels in sperm and seminal plasma of asthenozoospermic and its relationship with semen parameters. Clin Chim Acta. 2005; 356: 199-203.
- Kothari S, Thompson A, Agarwal A, du Plessis SS. Free radicals: their beneficial and detrimental effects on sperm function. Indian J Exp Biol. 2010; 48: 425-435.
- Mammoto A, Masumoto N, Tahara M, Ikebuchi Y, Ohmichi M, Tasaka K, et al. Reactive oxygen species block sperm-egg fusion via oxidation of sperm sulfhydryl proteins in mice. Biol Reprod. 1996; 55: 1063-1068.
- Arabi M, Moshtaghi H. Influence of cigarette smoking on spermatozoa via seminal plasma. Andrologia. 2005; 37: 119-124.
- Velando A, Torres R, Alonso-Alvarez C. Avoiding bad genes: oxidatively damaged DNA in germ line and mate choice. Bioessays. 2008; 30: 1212-1219.
- Henkel RR. Leukocytes and oxidative stress: dilemma for sperm function and male fertility. Asian J Androl. 2011; 13: 43-52.
- Chatterjee R, Haines GA, Perera DM, Goldstone A, Morris ID. Testicular and sperm DNA damage after treatment with fludarabine for chronic lymphocytic leukaemia. Hum Reprod. 2000; 15: 762-766.
- Abbasi M, Alizadeh R, Abolhassani F, Amidi F, Ragerdi KI, Fazelipour S, et al. Effect of aminoguanidine in sperm DNA fragmentation in varicocelized rats: role of nitric oxide. Reprod Sci. 2011; 18: 545-550.
- Koksal IT, Usta M, Orhan I, Abbasoglu S, Kadioglu A. Potential role of reactive oxygen species on testicular pathology associated with infertility. Asian J Androl. 2003; 5: 95-99.
- Nabil H, Moemen LA, Elela MHA. Studying the levels of malondialdehyde and antioxidant parameters in normal and abnormal human seminal plasma. Australian Journal of Basic and Applied Science. 2008; 2: 773-778.
- Laudat A, Lecourbe K, Guéchot J, Palluel AM. Values of sperm thiobarbituric acid-reactive substance in fertile men. Clin Chim Acta. 2002; 325: 113-115.
- Hsieh YY, Chang CC, Lin CS. Seminal malondialdehyde concentration but not glutathione peroxidase activity is negatively correlated with seminal concentration and motility. Int J Biol Sci. 2006; 2: 23-29.
- Ben Abdallah F, Dammak I, Attia H, Hentati B, Ammar-Keskes L. Lipid peroxidation and antioxidant enzyme activities in infertile men: correlation with semen parameter. J Clin Lab Anal. 2009; 23: 99-104.
- Abd-Elmoaty MA, Saleh R, Sharma R, Agarwal A. Increased levels of oxidants and reduced antioxidants in semen of infertile men with varicocele. Fertil Steril. 2010; 94: 1531-1534.
- Tarozzi N, Nadalini M, Stronati A, Bizzaro D, Dal Prato L, Coticchio G, et al. Anomalies in sperm chromatin packaging: implications for assisted reproduction techniques. Reprod Biomed Online. 2009; 18: 486-495.
- Zalata AA, Ahmed AH, Allamaneni SS, Comhaire FH, Agarwal A. Relationship between acrosin activity of human spermatozoa and oxidative stress. Asian J Androl. 2004; 6: 313-318.
- Li Y, Lin H, Li Y, Cao J. Association between socio-psycho-behavioral factors and male semen quality: systematic review and meta-analyses. Fertil Steril. 2011; 95: 116-123.
- Hassan MA, Killick SR. Negative lifestyle is associated with a significant reduction in fecundity. Fertil Steril. 2004; 81: 384-392.
- Agarwal A, Makker K, Sharma R. Clinical relevance of oxidative stress in male factor infertility: an update. Am J Reprod Immunol. 2008; 59: 2-11.
- Henkel R, Hajimohammad M, Stalf T, Hoogendijk C, Mehnert C, Menkveld R, et al. Influence of deoxyribonucleic acid damage on fertilization and pregnancy. Fertil Steril. 2004; 81: 965-972.
- Saleh RA, Agarwal A, Sharma RK, Said TM, Sikka SC, Thomas AJ. Evaluation of nuclear DNA damage in spermatozoa from infertile men with varicocele. Fertil Steril. 2003; 80: 1431-1436.
- Seli E, Gardner DK, Schoolcraft WB, Moffatt O, Sakkas D. Extent of nuclear DNA damage in ejaculated spermatozoa impacts on blastocyst development after in vitro fertilization. Fertil Steril. 2004; 82: 378-383.
- Hammadeh ME, Radwan M, Al-Hasani S, Micu R, Rosenbaum P, Lorenz M, et al. Comparison of reactive oxygen species concentration in seminal plasma and semen parameters in partners of pregnant and non-pregnant patients after IVF/ICSI. Reprod Biomed Online. 2006; 13: 696-706.
- Gandini L, Lombardo F, Paoli D, Caruso F, Eleuteri P, Leter G, et al. Full-term pregnancies achieved with ICSI despite high levels of sperm chromatin damage. Hum Reprod. 2004; 19: 1409-1417.
- Loutradi KE, Tarlatzis BC, Goulis DG, Zepiridis L, Pagou T, Chatziioannou E, et al. The effects of sperm quality on embryo development after intracytoplasmic sperm injection. J Assist Reprod Genet. 2006; 23: 69-74.
- Chen X, Zhang W, Luo Y, Long X, Sun X. Predictive value of semen parameters in in vitro fertilisation pregnancy outcome. Andrologia. 2009; 41: 111-117.
- Carrell DT, Liu L, Peterson CM, Jones KP, Hatasaka HH, Erickson L, et al. Sperm DNA fragmentation is increased in couples with unexplained recurrent pregnancy loss. Arch Androl. 2003; 49: 49-55.
- Hjollund NH, Bonde JP, Ernst E, Lindenberg S, Andersen AN, Olsen J. Spontaneous abortion in IVF couples--a role of male welding exposure. Hum Reprod. 2005; 20: 1793-1797.
- Ji BT, Shu XO, Linet MS, Zheng W, Wacholder S, Gao YT, et al. Paternal cigarette smoking and the risk of childhood cancer among offspring of nonsmoking mothers. J Natl Cancer Inst. 1997; 89: 238-244.
- Bassiony MM. Smoking in Saudi Arabia. Saudi Med J. 2009; 30: 876-881.
- Moskovtsev SI, Willis J, White J, Mullen JB. Sperm DNA damage: correlation to severity of semen abnormalities. Urology. 2009; 74: 789-793.
- Zini A, Meriano J, Kader K, Jarvi K, Laskin CA, Cadesky K. Potential adverse effect of sperm DNA damage on embryo quality after ICSI. Hum Reprod. 2005; 20: 3476-3480.
- Fowlers J, Bates M, Noiton D. Modified from New Zeland Ministry of Health. 2000.
- Armstrong AW, Armstrong EJ, Fuller EN, Sockolov ME, Voyles SV. Smoking and pathogenesis of psoriasis: a review of oxidative, inflammatory and genetic mechanisms. Br J Dermatol. 2011; 165: 1162-1168.
- Sunanda P, Panda B, Dash C, Ray PK, Padhy RN, Routray P. Prevalence of abnormal spermatozoa in tobacco chewing sub-fertile males. J Hum Reprod Sci. 2014; 7: 136-142.
- Shen HM, Chia SE, Ong CN. Evaluation of oxidative DNA damage in human sperm and its association with male infertility. J Androl. 1999; 20: 718-723.
- Sun JG, Jurisicova A, Casper RF. Detection of deoxyribonucleic acid fragmentation in human sperm: correlation with fertilization in vitro. Biol Reprod. 1997; 56: 602-607.
- Sergerie M, Ouhilal S, Bissonnette F, Brodeur J, Bleau G. Lack of association between smoking and DNA fragmentation in the spermatozoa of normal men. Hum Reprod. 2000; 15: 1314-1321.
- Storgaard L, Bonde JP, Ernst E, Spano M, Andersen CY, Frydenberg M, et al. Does smoking during pregnancy affect sons' sperm counts? Epidemiology. 2003; 14: 278-286.
- Horak S, Polanska J, Widlak P. Bulky DNA adducts in human sperm: relationship with fertility, semen quality, smoking, and environmental factors. Mutat Res. 2003; 537: 53-65.
- Loft S, Kold-Jensen T, Hjollund NH, Giwercman A, Gyllemborg J, Ernst E, et al. Oxidative DNA damage in human sperm influences time to pregnancy. Hum Reprod. 2003; 18: 1265-1272.
- Trisini AT, Singh NP, Duty SM, Hauser R. Relationship between human semen parameters and deoxyribonucleic acid damage assessed by the neutral comet assay. Fertil Steril. 2004; 82: 1623-1632.
- Belcheva A, Ivanova-Kicheva M, Tzvetkova P, Marinov M. Effects of cigarette smoking on sperm plasma membrane integrity and DNA fragmentation. Int J Androl. 2004; 27: 296-300.
- Viloria T, Meseguer M, Martínez-Conejero JA, O'Connor JE, Antonio P, Remohi J, et al. Cigarette smoking affects specific sperm oxidative defenses but does not cause oxidative DNA damage in infertile men. Fertility Sterility. 2009; 94: 631-637.
- Silver EW, Eskenazi B, Evenson DP, Block G, Young S, Wyrobek AJ. Effect of antioxidant intake on sperm chromatin stability in healthy nonsmoking men. J Androl. 2005; 26: 550-556.
- Eskenazi B, Kidd SA, Marks AR, Sloter E, Block G, Wyrobek AJ. Antioxidant intake is associated with semen quality in healthy men. Hum Reprod. 2005; 20: 1006-1012.
- Song G, Kochman L, Andolina E, Herko RC, Brewer KJ, Lewis V. Beneficial effects of dietary intake of plant phytoestrogens on semen parameters and sperm DNA integrity in infertile men. 62nd American Society of Reproductive Medicine Annual Meeting, New Orleans, LA, October 21–25. Fertil Steril. 2006; 86: 49.
- Piomboni P, Gambera L, Serafini F, Campanella G, Morgante G, De Leo V. Sperm quality improvement after natural anti-oxidant treatment of asthenoteratospermic men with leukocytospermia. Asian J Androl. 2008; 10: 201-206.
- Minguez-Alarcon L, Mendiola J, Lopez-Espin JJ, Sarabia-Cos L, Vivero-Salmeron G, Vioque J, et al. Dietary intake of antioxidant nutrients is associated with semen quality in young university students. Hum Reprod. 2012; 27: 2807-2814.
- Zareba P, Colaci DS, Afeiche M, Gaskins AJ, Jorgensen N, Mendiola J, et al. Semen quality in relation to antioxidant intake in a healthy male population. Fertil Steril. 2013; 100: 1572-1579.
- Chavarro JE, Minguez-Alarcon L, Mendiola J, Cutillas-Tolin A, Lopez-Espin JJ, Torres-Cantero AM. Trans fatty acid intake is inversely related to total sperm count in young healthy men. Hum Reprod. 2014; 29: 429-440.
- Robbins WA, Xun L, Jia J, Kennedy N, Elashoff DA, Ping L. Chronic boron exposure and human semen parameters. Reprod Toxicol. 2010; 29: 184-190.
- Tremellen K, Miari G, Froiland D, Thompson J. A randomised control trial examining the effect of an antioxidant (Menevit) on pregnancy outcome during IVF-ICSI treatment. Aust N Z J Obstet Gynaecol. 2007; 47: 216-221.
- Young SS, Eskenazi B, Marchetti FM, Block G, Wyrobek AJ. The association of folate, zinc and antioxidant intake with sperm aneuploidy in healthy non-smoking men. Hum Reprod. 2008; 23: 1014-1022.
- Mendiola J, Alberto M, Torres-Cantero D, Jose M, Moreno-Grau J, Manuela R, et al. Fertility and Sterility. 2009.