
Research Article
Austin J In Vitro Fertili. 2023; 7(1): 1043.
Alloimmune Disorders in Recurrent Implantation Failures after IVF/ET Procedures
Hanna Motak-Pochrzest¹*; Andrzej Malinowski²
¹Department of Obstetrics and Gynecology, GMW Parens IVF Clinic, Opole & District Hospital Strzelce Opolskie, Poland
²Department of Surgical and Oncological Gynecology, Medical University of Lódz, Lódz, Poland
*Corresponding author: Hanna Motak-Pochrzest Department of Obstetrics and Gynecology, GMW Parens IVF Clinic, Opole & District Hospital Strzelce Opolskie, Poland. Email: hannamotak@gmail.com
Received: March 13, 2023 Accepted: April 18, 2023 Published: April 25, 2023
Introduction
Recurrent Implantation Failures (RIF) can be defined as the absence of implantation after 2 to 6 consecutive cycles of IVF, ICSI or frozen embryo replacement cycles where the cumulative numer of transferred high-quality embryos was no less than four for cleavage-stage embryos and no less than two for blastocytes. RIF can be considered with appropriate developemental stage with determination of implantation by an increasing quantitative Human Chorionic Gonadotropin (hCG) level and usually include also biochemical pregnancies [1].
Successful pregnancy implantation is related to adequate utero-placental circulation, absence of uterine anomalities and adequate endometrial receptivity. Immunological causes and thrombophilias play important roles in implantation failure through mechanisms similar to recurrent miscarriages [2]. Pregnancy is believed to appear a period of immunomodulation with greater pro-inflammatory activity at the beginning of pregnancy and change during the second and third trimester [3].
Figure 1: The number of cycles of IVF/ET among tested patients is presented.
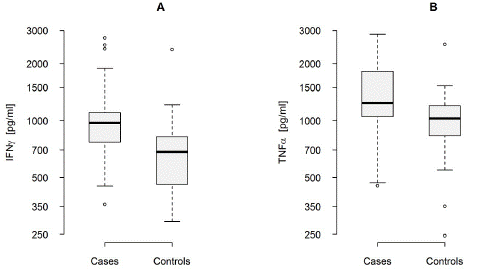
Figure 2: INFγ (A) and TNFa (B) levels in RIF and control patients.
The aim of our study was to find if a misbalance of Th1/Th2 cytokines in patients who underwent IVF/ET procedures may influence on the pregnancy outcome. Our study evaluated peripheral blood cytokines profile in patients after consecutive implantation failures following IVF/ET procedures in comparing to the patients after successfully pregnancy outcome following first attempt of IVF/ET procedures.
Material and Methods
One hundred fifty-eight patients with a history of two and more implantation failures after IVF/ET procedures were evaluated. All patients had a normal ovarian reserve as measured by the levels of Anti-Mullerian Hormone (AMH), Follicle-Stimulating Hormone (FSH) and oestradiol. The indications for IVF/ET procedures were unexplained infertility or male infertility.
The study was performed between January 2021 and January 2023. Patients were registered at the Department of Operative and Endoscopic Gynaecology at the Medical University of Lodz. The patients gave written consent for participation in the study, and the study was approved by the Ethics committee. The control group comprised seventy six women who had successfully given birth at the first attempt of ET. The indications for IVF procedures were the same as those for the study group.
The IVF/ET procedures were similar for all patients. Chromosomal abnormalities of the male and female were excluded after their karyotype analysis. Embryonic aneuploidy was evaluated using Fluorescense In Situ Hybridization (FISH) for chromosomes 13, 16, 18, 21, 22, X and Y.
All the women were investigated to exclude the known causes of implantation failures as:
Anatomical (congenital and aquired anatomic abnormalities of the uterus and andexa), endometriosis, infectious factors (Cytomegalovirus, Herpes simplex, Mycoplasma hominis, Ureaplasma urealyticum, Chlamydia trachomatis), inherited thrombophilias (mutations MTHFR, for homocysteine, factor V Leiden mutation, mutation 20210G for prothrombin and for antithrombin III, deficiences in protein S and protein C), endocrinological disturbances (luteal phase insufficiency, polycystic ovary syndrome, insulin resistance, diabetes mellitus, hyperprolactinaemia and hyperandrogenism).
Receptivity of the endometrium was estimated before first ET by endometrial biopsy during the cycle before the IVF cycle.
Women with medical conditions or treatment affecting their immunologic responses were excluded from the study. All studied RIF and control group women were qualified for immunological tests which consisted of PBL population profile and the PBL Th1/Th2 cytokine secretion upon mitogen stimulation. All examinations were performer in non-pregnant women in the midsecretory phase.
10ml of heparinized peripheral blood was drawn from tested women using a Vacutainer sampling system (Beckton-Dickinson, Eastern Europe Division, Heidelberg, Germany).
Immunologic assays were done in APC Medical Analyses laboratory that participates in the diagnostic immunologic division in the research institute.
Lymphocyte Populations
The percentage of B lymphocytes, T lymphocytes populations and NK cells was measured in the whole heparinized peripheral blood using Multitest 6-color TBNK reagent (Beckton-Dickinson and Co., BD Biosciences, San Jose, USA) with standard monoclonal antibodies: CD3FITC, CD19APC, CD16+, CD56PE, CD45PerCP-Cy5.5, CD4Pe-Cy7, CD8APC-Cy7 using Facs Canto cytometer (Beckton-Dickinson and Co., BD Biosciences, San Jose, USA) and BD FACSCanto Software. The reference value were as follows: Lymphocytes T (60-82.0%), Lymphocytes B (7-23.0%), lymphocytes helper CD3+/CD4+ (30-51%), lymphocytes supresor CD3+/CD8+ (19-39%), CD3-/CD16+/56+ (NK) (7-24%), CD5+/CD19+ (2-10%), CD 56+ (3-12.0%).
NK cells activity: was measured by NKTEST (Glycotope, Biotechnology, Germany), using cryopreserved K562 target cells and found by flow cytometric analysis. The reference value of the NK cytotoxic activity after incubation time of 2 hours determined as normal range was 13.7-33.5%.
PBL Th1/Th2 cytokine profile: PBLs were isolated from heparinized whole blood by gradient separation. A suspension of 1×106 cells was cultured in Roswell Park Memorial Institute (RPMI) 1640 medium (enriched with 2mM l-glutamine, 10% fetal calf serum, 100U/mL penicillin, and 10μg/mL streptomycin). The lymphocytes were stimulated with phytohemagglutinin (at a mitogenic dose of 5mg/mL), and then incubated for 24h in humid air enriched with 5% CO2 at 37°C. After centrifugation, the supernatant was collected for the cytokine assay. Cytokine levels were estimated using a BDTM Cytometric Bead Array Human Th1/Th2 Cytokine kit (Beckton-Dickinson and Co., BD Biosciences, San Jose, USA). The tests were performed on a FACSCanto™ cytometer (Beckton-Dickinson and Co., BD Biosciences). The concentrations of cytokines: Interferon [IFN]γ, Tumor Necrosis Factor [TNF]a, Interleukin [IL]-10, Interleukin [IL]-4, were determined and represented as pg/mL. The reference values, determined previously for fertile Polish women, were as follows: IFN-γ 209–1050pg/ml; TNFa 320–1380pg/ml; IL-10 1530–3830pg/ml; IL-4 20-120pg/ml.
Statistical Analysis
Median and two quartiles (first and third, Q1 and Q3, respectively) of PBL population’s percentage were used as summary statics. sn Statistic was computed as the measure of variability: sn= med{med|xi-xj |;j = 1..n} [1]. This is average distance between two randomly sampled observations among group. Higher sn level reflects higher variability. If it was necessary distributions of statistics were estimated numerically. Risk of failure of IVF/ET, was modeling for cases (Y = 1) and controls (Y = 0) groups with model h[P(Y=1|X)]=a+ΒT X, where X is matrix of immunological predictors and h is logit function. Results were adjusted to age of patients. Odds Ratio (OR) was used as a measure of the effect size as well as the confidence interval, CI 95%, for this statistic at 1 - a = 0.95 level [4].
Results
The mean age in the group of patients with RIF after IVF/ET was 36 years (Q1=33, Q3=39), in the control group was 34 years (Q1=31, Q3=37). The most dominant group (56 patients) consists of cases with 2 ET/IVF failures, the rest of tested patients underwent 3 to 6 and more cycles of ET/IVF procedures (from 18 to 36 patients).
PBL populations percentage in RIF and control patients were estimated using median and two quartiles (first and third, Q1 and Q3, respectively) the measure of variability, showed in table 1. In the tested RIF’s patients we found increased percentage of lymphocytes T, CD56+ and NK cells, comparing to the control group as well as enhanced NKcells activity. The percentage of lymphocytes B in RIF patients were decreased in significantly comparing to the control group. Compared to the reference values the percentage of NK cells was increased, however, insignificant in RIF’s patients.
Variable
Group
Q1
Median
Q3
Lymphocytes T
Cases
80.5
82
83.5
Controls
73
76
78.5
Lymphocytes B
Cases
6
6
7
Controls
10.5
13
14.5
Lymphocytes helper CD3+/CD4+
cases
38.5
39
44.5
controls
32.0
34
36.5
Lymphocytes supresor CD3+/CD8+
cases
28.5
31
36
controls
25
27
32
NK cells
Cases
16.7
20
24
Controls
11
14
19.7
Lymphocytes CD5+/CD19+
cases
3.5
4
5.5
controls
4
5
6
Lymphocytes CD 56+
cases
23
23
24.5
controls
9.5
11
13
Activity of NKcells
Cases
19.6
28.2
37.5
Controls
10
13.2
26.8
Q1, Q3 – first and third quartile
Table 1: Characteristics of cases and controls analyzed in this study according to PBL percentage and cytotoxic NK activity.
The mean age in the group of RIF patients was 36 years (Q1=33, Q3=39), in the control group was 34 years (Q1=31, Q3=37). The difference of 2 years is insignificant comparing the both groups. Taking into consideration the PBL percentage in patients with similar age we estimated the risk of RIF, presented in table 2.
Variable
OR
CI 95%
p-value
Lymphocytes T
increase at 1% point1.077
1.010
1.149
0.01
Lymphocytes B
increase at 1% point0.472
0.298
0.748
0.01
Lymphocytes CD56 +
increase at 1% point1.119
1.027
1.219
0.01
NK cells activity
increases at 1% point1.144
1.060
1.236
0.01
OR: Odd Ratio; CI: Confidence interval
Table 2: Risk of RIF after IV/ETF in dependence of the PBL percentage.
Parameter
RIF median (Q1-Q3)
Sn
Control median (Q1-Q3)
Sn
IFNγ
975 (770-1104.5)
230
683 (468.5-822)
290
TNFa
1240 (1050-1821.5)
349
1020 (798.5-1185)
309
IL10
957 (600-1690)
655
967 (828.5-1595)
210
IFNγ/IL10
0.97 (0.55-1.36)
0.54
0.71 (0.52-0.82)
0.26
TNFa/IL10
1.29 (0.72-2.06)
0.76
0.87 (0.52-1.36)
0.51
Q1, Q3 – first and third quartile
Table 3: Th1/Th2 cytokine profile in patients with RIF versus successful pregnancy outcome after IVF/ET.
In the tested group we observed that increase only of 1% of PBL T may influence on the risk of implantation failures almost for 8% (OR=1.077). Similarly we noted that increase of percentage of lymphocytes CD56+ in peripheral blood is associated with the rise of the risk of RIF as 12% (OD=1.119). Activity of NK cells over reference values is connected with enlarged risk of RIF as 15% (OR=1.144). Moreover, decrease of 1% of PBL B is supposed to be connected with twice decline of the risk of RIF in tested women (OR=0.472).
Mean concentrations of IFNγ, TNFa, IL-10, IL-4 cytokines produced by mitogen-stimulated PBL and Th1/Th2 ratio (IFNγ/IL10, TNFa/IL10) was measured in RIF and control patients.
Sn – measure of variability: higher Sn → higher variability
IFNγ- interferon γ, TNFa-tumor necrosis a, IL-10- interleukin 10,
The expression of IFNγ and TNFa in PBL were found higher in patients with RIF than in the control group. The average concentration of IFNγ among cases was 975pg/ml comparing to the controls with the average concentration 683pg/ml.
The levels of IL-10 didn’t differ significantly between the two studied groups with the similar average of the concentration about 960pg/ml but 3-fold differentiation of the concentration of IL-10 was noted in the tested women with RIF. The average difference of concentration of IL-10 among patients with RIF was estimates as Sn=655pg/ml, in the controls Sn=210pg/ml. High expression of TNFa and IFNγ in the tested group with the similar levels of IL-10 suggest abnormal ratios of IFNγ/IL-10 amount 0.97 comparing to 0.71 among controls. Similar ratios of TNF a /1IL-10 were elevated in women with RIF 1.29 comparing to the controls 0.87 for TNFa. This difference was not statistically significant.
Comparing the Th1/Th2 cytokines profile in patients with same age we found increasing risk of RIF with increasing values of Th1 cytokines produced by PBL (Table 4).
Variable
OR
CI 95
p-value
IFNγ
increases at 10%1.13
0.998
1.28
0.03
TNF a
increases at 10%1.11
0.968
1.29
0.06
Table 4: The risk of RIF after IVF/ERT in dependence of the cytokines levels adjusted to the same patients’ age.
In patients with same age we found that the risk of implantaion failure increased as 11% (OR=1.11) with rising of expression of TNFa for 10%. Similar high expression of IFNγ with 10% extension implicated over 2 fold increased risk of RIF (OR=1.28).
We did’nt noted that the change in levels of cytokine IL-10 and IL-4 is correlated with the successful implantation after IVF/ET (p=0.03).
Discussion
Several studies have showed modelled inflammation across pregnancy with immunomodulation of Th1/Th2 activity [3,5]. Maternal systemic inflammatory response could stimulate maternal-fetal interface unit to adverse implantation. Abnormal immune responses are significantly increased in women suffering from recurrent miscarriages [6,7]. Implantation failure after IVF procedures is still controversial with different definition of rising in the quantitative hCG level after embryo transfer [8]. Despite increasing number of gene mutations in the gene coding for the proteins responsible for thrombophilias, endometrial remodeling events indispensable to endometrial decidualization and the good quality of transferred embryos, peripheral blood immunological balance is independent required factor for the successful pregnancy outcome [1,9,10].
Our study revealed increased levels of Th1 cytokine TNFa and IFNγ produced by PBL in women with RIF after IVF/ET procedures comparing to the controls. Strong correlation was observed in the elevated level of TNFa, similar as Zhang et al.[11] found in RM patients. Increased level of 10% level of TNFa with normal levels of IFNγ can in the same patients rised the risk of implantation failure as 11% (OR=1.11). The elevated ratio of TNFa/IL-10 and IFNγ/1IL-10 also significantly influence on the risk of implantation failure (p<0.05).
The average concentration of IL-10 didn’t differ significantlty between tested and controls but very wide range of concentration of IL-10 among sera of women with RIF indicates heterogeneity of peripheral blood immunological profile.
In women undergoing IVF/ET treatment, an increased count of activated not only uNK but PB NK cells (CD56 dim CD16+ CD69+) was reported to be associated with reduced rate of embryo implantation in IVF treatment [12]. The result of our study show the incidence of higher percentage of CD56+ and elevated activity of NK cells in RIF patients comparing to the control group. The increase of CD56+ levels elevates the risk of the unsuccessful implantation as 12% (OR=1.119).
Increased activity of NK cells found in RIF patients was observed as a factor provoking the risk of implantation failure estimated as 15% (OR=1.144).
Different immunological models found in patients suffering from RIF after IVF/ET procedures allow to use the treatment methods appropriate for identified disorders which gives the opportunity to increase the percentage of success [13].
Conclusions
Our study revealed different alloimmune disorders found in the sera of patients with RIF after IVF/ET, in particular those concerning cytokine balance, lymphocyte profile and NK cells activity in peripheral blood.
The rate of immunological disorders found among patients undergoing the IVF/ET procedures reveals the necessity of performing certain immunological tests particularly among patients with at least 2 failures.
References
- Mekinian A, Cohen J, Alijotas-Reig J, Carbillon L, Nicaise-Roland P, et al. Unexplained recurrent miscarriage and recurrent implantation failure: is there a place for immunomodulation? Am J Reprod Immunol. 2016; 76: 8-28.
- Simon A, Laufer N. Assessment and treatment of repeated implantation failure (RIF). J Assist Reprod Genet. 2012; 29: 1227-1239.
- Mor G, Cardenas I, Abrahams V, Guller S. Inflammation and pregnancy: the role of the immune system at the implantation site. Anna N Y Acad Sci. 2011; 1221: 80-87.
- Rousseeuw PJ, Croux. Alternatives to the median absolute deviation JASA. 1993; 424: 1273-1283.
- Kraus TA, Sperling RS, Engel SM, Lo Y, Kellerman L, et al. Peripheral blood cytokine profiling during pregnancy and post-partum periods. Am J Reprod Immunol. 2010; 64: 411-426.
- Coughlan C, Ledger W, Wang Q, Liu F, Demirol A, et al. Reprod Biomed Online. 2014; 28: 14-38.
- Kwak-Kim J, Yang KM, Gilman-Sachs A. Recurrent pregnancy loss: a disease of inflammation and coagulation. J Obstet Gynaecol. 2009; 35: 609–622.
- Rinehart J. Recurrent implantation failure: definitione J Assist Reprod Genet. 2007; 24: 284-287.
- Chernyshov VP, Dons’koi BV, Sudoma IO, Goncharova YO. Multiple immune deviations predictive for IVF failure as possible markers for IVIG therapy. Immunol Lett. 2016; 176: 44-50.
- Franasiak JM, Alecsandru D, Forman EJ, Gemmell LC, Goldberg JM, et al. A review of the pathophysiology of recurrent implantatiomn failure. Fertil Steril. 2021; 116: 1436-1448.
- Zhang C, Deng X, Zhang X, Pan Z, Zhao W et al. Association between serum TNF alfa levels and recurrent spontaneous miscarriages: a meta-analysis. Am J Reprod Immunol. 2015; 75: 86-93.
- Kwak-Kim J, Bao S, Lee SK. Kim JW, Gilman-Sachs A, et al. Immunological modes of pregnancy loss: inflammation, immune effectors and stress. Am J Reprod Immunol. 2014; 72: 129-140.
- Turocy J, Williams S. Novel therapeutic options for treatment of recurrent implantation failure. Fertil Steril. 2021; 116: 1449-1454.