
Research Article
J Plant Chem and Ecophysiol. 2016; 1(1): 1002.
Uptake and Metal Transfer from Biosolid-Amended Soil to Tomato (Solanum lycopersicum Mill L.) Plants
Carbonell G*, Torrijos M, Rodríguez JA and Ángel Porcel M
Department of the Environment, Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria (I.N.I.A), Spain
*Corresponding author: Gregoria Carbonell Martín, Department of the Environment, Laboratory for Ecotoxicology, Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria (I.N.I.A), Madrid, Spain
Received: February 19, 2016; Accepted: March 11, 2016; Published: March 14, 2016
Abstract
This study investigates the inputs of total and available metals from biosolid-amended soil, as well as their accumulation in tomato plants (Solanum lycopersicum Mill L.) and subsequent translocation. A greenhouse study with tomato seedlings grown in soil amended with two organic waste types (anaerobically digested thermal drying sludge and anaerobically digested municipal solid waste compost) was conducted. From an environmental viewpoint, the potential risk of metal uptake by crops must be considered. The results indicated significant increases in Cd, Cu and Zn in soil, even though their available fraction was not modified with Cu and Zn, and the Cd fraction significantly decreased. Roots showed the highest metal concentrations, but the differences between the plants grown in the biosolid-amended or control soils were not always significant. The root system acted as a barrier for Cr, Ni and Pb. No significant variations in the concentrations of the metals in the tomatoes grown in biosolid-amended or control soils were observed. Soil-to-plant metals transfer was in this order: Cu (1.14-2.85) > Cd (1.33-2.17) > Zn (1.4-1.48). The highest and lowest BAFs were observed in roots and tomatoes, respectively. According to the result obtained in the translocation and bioaccumulation factors, Cd was the heavy metal that indicated the greatest mo from soil. The canonical correlation analysis proved a highly significant relationship in the soil/ plant system, which was strongly and positively related with Cd, Cu and Zn. Biomass production was similar regardless of treatment, but some differences were found for aerial plant parts as regards metal accumulation, whereas the metal levels in tomatoes were negligible for all treatments.
Keywords: Biosolids; Soil contamination; Available metals; Plant uptake; Primary and dynamic factors; Tomato
Introduction
The tenth position of EU Member States in waste material production in 2012 has been report for Spain (463 kg per capita, below the European average of 487 kg per capita) [1]. This waste material can be deposited in landfills (63%), or can be recycled (17%), incinerated (9%) or managed as compost (10%) for agricultural purposes. Biosolids are the by-product of municipal wastewater treatment and also are known as sewage sludge. There are several biosolids management options, but spreading it on land has considerably increased and reached 70% from 2005 to 2010. European legislation considers that biosolids, containing nutrient-rich organic materials, may substantially benefit from climate change given their action on carbon sequestration by reducing CO2 and atmospheric pollutant emissions [2]. Lou and Nair (2009) [3] estimated that approximately 50 kg of C (183 kg of CO2) may be sequestered per ton of wet compost. Nonetheless, the ultimate benefits of recycling prove to be a more sustainable economy [4], not to mention the possibility of reducing chemical fertilizers [5]. Sewage sludge and Municipal Solid Waste (MSW) compost can be used beneficially on land as a soil conditioner and fertilizer. Due to contamination with pollutants, the application of sewage sludge requires having to know about trace element contents in soils. The main sources of metal input in agricultural soils include the use of sewage sludge composts [6], mineral fertilizers [7], MSW compost [8] and recycled waters for irrigation [9,10], which are of particular concern given the potential environmental risk they pose. The non-systematic use of biosolids to improve agricultural yields without considering possible negative effects might become a major concern. It should also be emphasized that high levels of metals in soils could pose a risk for consumers as a result of their toxicity, transfer and bioaccumulation through the food chain [11,12]. Distribution of metals in plants by considering primary factors for translocation and bioaccumulation, which involve the plant-soil interaction under similar environmental conditions, has been contemplated [13-18]. However, these primary factors express only the first-level comparison; i.e biogeochemical comparison of different media (plant and soil) is made in one place. So in order to integrate information about metal concentration into different media or plant parts, and to compare the process between control and treated samples, second-level factors (dynamic factors) are needed [19]. According to [20], this approach includes the influence of the environment on metal uptake (external factors) and translocation in crops grown at contaminated sites (internal factors).
Biosolid soil amendments on agricultural soils usually increase metals in plant tissues [21]. Many studies have been conducted about metal uptake in crops (wheat, tomatoes, strawberries, maize and squash) after biosolid amendments [10,16,22,23], but data on the translocation and bioaccumulation of metals on different tomato plant parts after being grown in sewage sludge- or MSW compostamended soils are relatively scarce. In the present work, a greenhouse experiment was conducted in loamy sand soil amended with different organic wastes, anaerobically digested thermal drying sludge and anaerobically digested municipal solid waste compost, with tomato (Solanun Lycospersicum L.) seedlings. Tomato was selected for this study with a view to stating its food safety implication for human consumption. This work focused on these objectives: 1) establishing the likelihood of total and available metals in soil; 2) determining the ability of metal uptake, translocation and distribution of metals from soil to other tomato plant parts (root, stem, leaf and fruit).
Materials and Methods
Experimental design
A greenhouse experiment, with tomato seedlings, was carried out from 28 May to 12 November 2012. It was performed following a randomised design with eight replications that involved three treatments, non-amended soil (control), thermally digested drying sludge (W-A)-amended soil (Treatment-A, [T-A]) and anaerobically digested municipal solid waste compost W-B (Treatment-B, [T-B]) Table 4, in flexible plastic pots (0.108 m², 26 L capacity) with loamy sand soil. Each pot was filled with 27 kg of control soil or with 27 kg of soil mixed with waste (W-A or W-B) in order to reach the selected application rate (150 kg N ha-1). The waste application rate was calculated by considering tomato plants’ N requirement, which never exceeded the levels set out in Directive 91/676/EEC on the contribution of nitrogen fertilizers. The application rate was covered by adding 192 g (W-A) or 629 g wet weight (W-B) to each pot with 27 kg of the control soil. The non-amended soil was fertilized with 33 g pot-1 of a commercial fertilizer (N: P: K, 15:15:15; Fertiberia, Spain). Previously, tomato seeds, kindly supplied by the Spanish office of Plant Varieties, were sown in May. After 2 weeks the obtained seedlings were placed in the 24 pots which constituted the experiment (3 seedlings / treatment / pot) in soil, which was previously watered for conditioning purposes. Flowering began late in July, the first fruits appeared in October, and fruits were harvested daily until mid- November. While the experiment lasted, all the pots were watered to maintain moisture close to 60% of the water-holding capacity. Finally in mid-November, all the unripe tomatoes were harvested. The tomato plants were divided into roots, stems, leaves and tomatoes. Soil samples and plants were collected for metal analyses.
S/R
Control
T- A
T- B
TFp > 1
Zn 1.04
Cd 1.43
Zn 1.48 > Cd 1.33
TFp < 1
Cd 0.73 > Cu 0.22
Z n 0.65 > Cu 0.46
Cu 0.58
TF dyn > 1
Cu 2.05 > Cd 1.96
Cu 2.60 > Cd 1.81 > Zn 1.42
TF dyn < 1
Zn 0.63
L/S
TFp > 1
Cu 2.85 > Cd 1.99
Cu 2.75 > Cd 2.17
Cu 2.19 > Cd 1.88
TFp < 1
Zn 0.90
Zn 0.62
Zn 0.75
TF dyn > 1
Cd 1.09
TF dyn < 1
Cu 0.96 > Zn 0.68
Cd 0.94 = Zn 0.83 > Cu 0.74
T/S
TFp > 1
Cu 1.84
Cu 1.46
Cu 1.14
TFp < 1
Zn 0.39 > Cd 0.34
Cd 0.42 > Zn 0.26
Cd 0.29 > Zn 0.27
TF dyn > 1
Zn 1.23
TF dyn < 1
Cu 0.79 > Zn 0.66
Cd 0.86 > Zn 0.70 > Cu 0.62
TFp: Primary Translocation Factor.
TFdyn: Dynamic Translocation Factor.
Table 4: Bioaccumulation Factors (BAFs) of metals in different plant parts of the tomato (Solanum Lycopersicun L.) plants grown in the control and amended soils. BAFs were calculated by considering the available metal fraction in soil, expressed by the mean (n=8). T-A: soil amended with anaerobically digested thermal drying sewage (W-A); T-B: soil amended with anaerobically digested municipal solid waste compost (W-B).
Metal concentrations in substrates and plant material
Soil samples were subjected to microwave acid digestion (ETHOS SEL Model Milestone, Monroe, CT, USA). Thus the digested samples were analysed for total metal contents (Cd, Cu, Cr, Ni, Pb and Zn) by U.S. EPA Method 3051 [24].This method involves very strong acid digestion that dissolves almost all the elements that could become “environmentally available” [25]. Atomic Absorption Spectrometry (Flame Atomic Absorption Spectroscopy [FAAS] or Graphite Furnace Atomic Absorption Spectroscopy [GF-AAS], equipped with Zeemaneffect background correction and an AS 800 auto-sampler [Perkin Elmer, Shelton, CT 06484-4794 USA]), was used to establish metal concentrations. The extractable metal contents (bioavailable) in the samples were analysed according to [26] using DTPA (diethylaminepenta- acetic acid) solution (0.005 M EDTA + 0.01 M CaCl+ 0.1 M TEA, pH 7.3). Mercury analyses were done in a direct Hg analyzer (DMA-80, atomic absorption spectrophotometer, Milestone, Wesleyan University Middletown, CT, USA) following EPA Method 7473 [27], validated for solid and liquid matrices. Collected plant samples (roots, stems, leaves and tomatoes) were washed with ethylene-diamine-tetra-acetic acid (0.02 M) and MQ water to remove soil particles. They were oven-dried (70ºC), weighed and finally lyophilized. For the metal analysis, samples (≈300 mg) were digested in 8 mL HNO3: water (1:1) in a laboratory microwave following the above-described procedure for soil samples; after cooling, the volume was made up to 25 ml. The total metal analysis was done by the atomic absorption spectrometry technique. Calcareous loam soil (BCR- 141-R) and olive leaves (Olea europea) (BCR-062), obtained from the European Commission Community Bureau of Reference, were used to check the accuracy and precision of the measurements, and to validate the applied methods for the metal analysis in soil and plants. For the soil and olive leaves samples, recoveries of metals ranged from 93% to 107% and from 100% to 103%, respectively.
Translocation and bioaccumulation of metals in plant parts
The ability of plants to translocate metals from roots to other plant parts is measured using primary translocation factors (TFp), defined as the ratio of the metal concentration in different plant parts (stems, leaves, tomatoes) to roots [14,16]. Dynamic translocation factors (TFdyn) were calculated following the equation proposed by [19]:
TFdyn = [(Citreated tissue) / (Citreated root)] x [(Cicontrol root) / (Cicontrol tissue)]
Where Citreated tissue is the concentration of metal (i) in the tissue plant grown in treated soil;
Citreated root is the concentration of metal (i) in the plant root grown in treated soil; Cicontrol root is the concentration of metal (i) in the plant root grown in the control soil; Cicontrol tissue is the concentration of metal (i) in the plant tissue grown in the control soil.
The primary bioaccumulation factor (BAFp) is defined as the ratio between the metal tissue concentration and the metal concentration in soil. To gain a better understanding of the environmental and physiological parameters involved in metal bioaccumulation, [19] suggested using dynamic BAFs (BAFsdyn) following this equation:
BAFdyn = [(Ciplant treated) / (Cisoil treated)] x [(Cisoil control) / (Ciplant control)]
where Ciplant treated is the concentration of metal (i) in the whole plant (roots + stems + leaves + tomatoes) grown in the treated soil; Cisoil treated is the concentration of metal (i) in the treated soil; Cisoil control is metal concentration (i) in the control soil; Ci plant control is metal concentration (i) in the whole plant grown in the control soil.
Statistical analyses
The significance of the differences in the metal concentrations in the various tomato plant parts, and in the soil total metal concentrations, for the different soil treatments, was evaluated. Differences between groups were determined by a one-way analysis of variance (ANOVA). A non-parametric analysis (Kruskal-Wallis) was done to evaluate the differences between the metal concentrations in the different tomato plants parts grown in the control and treated soils. Multivariate linear equations were established to describe the total amount of the various heavy metals in tomato by the forced removal method in a regression analysis. The regression analysis is used to predict the value of the heavy metals in tomatoes from a set of predictors (total and bioavailable heavy metal in soil, and root and stem heavy metal content). It can also be used to describe associations within data, and to estimate the linear association between predictors and responses. Acanonical Correlation Analysis (CCorA) is one of the many methods that allow the relationship between two sets of variables to be studied. We used CCorA [28] to study the correlation among all the heavy metals in tomato (fruit, stem and roots) and the heavy metal content in soil (total and bioavailable). There are two tables in the CCorA, and the ultimate intention is to maximize the covariance between two sets of variables and to minimize their respective variance [29]. Let Y1 and Y2 be (tomato contents), and the response variables (Y2) based on the heavy metal contents in soil and different tomato plants parts, and with variables p and q, respectively, we obtain:
The CCorA (used considerably in ecology, [30] provides two vectors, a(i) and b(i), that are maximized. Constraints must be introduced, so the solution for a(i) and b(i) is unique. The purpose is to maximize the covariance between Y1a(i) and Y2b(i) and to minimise their respective variance. All the statistical analyses were carried out by XLSTAT (Addinsoft Version 2012.2.02) and Statgraphics plus 5.1
Results
Biomass production
Total biomass production (stems, leaves and tomatoes), as well as the individual weights and percentages of ripe and unripe tomatoes, are shown in Table 1. A significant (p<0.05) increase in biomass (stem and leaves) was observed in the plants grown in the treated (T-A) soil compared to that in the control soil. The percentages of ripe tomatoes (30-32%) and unripe tomatoes (67-70%) were similar regardless of treatment; the fresh weight of the ripe tomatoes grown in the treated (T-B) soil significantly (p<0.05) dropped compared to those grown in the control or treated (T-A) soil, while the unripe tomatoes obtained similar weights for all treatments.
Parameter
Control soil
W-A
W-B
pH
7.50
7.84
8.76
EC (1:10) at 25 ºC (dSm-1)
1.90
2.23
2.8
Organic C (%)
3
33.21
11.55
N-NO3- (mg kg-1)
230
158
440
N-NH4+ (mg kg-1)
9.7
3025
1214
N Kjeldahl (%)
0.11
5.86
1.19
Extractable P (%)
0.01
2.99
0.84
Extractable K (%)
0.11
0.36
0.85
Mg (%)
0.04
0.6
0.69
CaCO3, equiv. (%)
<3
33.21
11.55
Ca (%)
0.24
4.32
14.13
Sand-0.05<D<2 mm) (%)
82.2
Silt-0.02<D<0.05) (%)
3.8
Silt-0.002<D<0.02) (%)
5.6
Clay-0.002 mm (%)
8.4
Soil Type (USDA)
Loamy sand
Cd (mg kg-1)
0.05
0.67
1.05
Cr (mg kg-1)
24.86
149.64
25.67
Cu (mg kg-1)
8.85
374
188.9
Ni (mg kg-1)
14.27
80.82
21.06
Pb (mg kg-1)
16.36
57.24
54.66
Zn (mg kg-1)
38
1770
234
Hg (mg kg-1)
0.03
0.91
0.36
Table 1: Physico-chemical characterization of control soil. Metal concentrations in soil and biosolids: the anaerobically digested thermal drying sludge (W-A) and anaerobically digested municipal solid waste compost (W-B) used in this study.
Soil and biosolids characterization
The physico-chemical characterization of the control soil and biosolids [anaerobically digested thermal drying sludge (W-A) and anaerobically digested municipal solid waste compost (W-B)] used in this study are shown in Table 2. The C/N ratio in the solid phase of both wastes, lower than 20 (6.70 and 15.90 for W-A and W-B, respectively), can only be considered a mandatory, but insufficient, condition for compost maturity. However, other parameters, such as organic matter content (60% for W-A and 25% for W-B) and absence of toxicity observed in tomato plants during the experiment, may help explain the maturity of biosolids [31]. Total metals were found in the following order: Zn > Cr > Ni = Pb > Cu > Cd > Hg. Table 3 shows the heavy metal concentrations in the control and treated soils at the end of the experiment. The percentages of the bioavailable (DTPAextractable) metals in soils were in this order: Cd [28-47%] > Cu = Zn [20-30%] > Pb [14-20%] > Ni [2-4%] > Cr [0.02-0.03%].
Treatment
Cd
Cu
Cr
Ni
Pb
Zn
Hg
Control (mg kg-1)
0.05 ± 0.003a
8.85 ± 0.66a
24.86 ± 3.59a
14.27 ± 3.05a
16.36 ± 1.82a
38.03 ± 3.66a
0.03 ± 0.005a
Control (% bioavailable metal)
47
27
0,03
2
20
29
nd
T-A (mg kg-1)
0.06 ± 0.026a
12.11 ± 0.84b
26.26 ± 8.84a
19.66 ± 12.57a
18.85 ± 4.52a
54.22 ± 8.93b
0.04 ± 0.005a
T-A (% bioavailable metal)
45
30
0,03
4
16
28
nd
T-B (mg kg-1)
0.09 ± 0.010b
14.38 ± 0.97c
28.21 ± 10.66a
14.22 ± 6.14a
19.82 ± 4.42a
44.45 ± 4.27c
0.04 ± 0.005a
T-B (% bioavailable metal)
28
20
0,02
2
14
22
Nd
Extractable metals: DTPA (Lindsay and Norvell, 1978).
Table 2: Metal concentrations (mg kg-1d.w.) in the control and treated soils at the end of the experiment. (T-A): soil amended with anaerobically digested thermal drying sludge; (T-B) soil amended with anaerobically digested municipal solid waste. The bioavailable metal fraction corresponds to a single value.
Control
T-A
T-B
Root/Soil
BAFp > 1
Cu 8.30 > Ni 6.56 > Cd 5.48 >Zn 5.09
Zn 8.98 > Cu 3.30 > Cd 1.29 >Ni 1.06
Zn 5.98 > Cu 4.80 > Ni 2.58 > Cd 2.03
BAFp < 1
Cr 0.33 = Pb 0.32
Pb 0.25 > Cr 0.16
Pb 0.25 = Cr 0.21
BAFdyn>1
Zn 1.28
BAF dyn<1
Pb 0.81 > Cr 0.54 > Cu 0.26 >Cd 0.16 > Ni 0.07
Zn 0.86 > Pb 0.81 > Cr 0.73 > Cu 0.39 >Cd 0.25 > Ni 0.16
Stem/Soil
BAFp > 1
Zn 5.28 > Cd 4.02 > Cu 1.86
Zn 5.83 > Cd 1.84 > Cu 1.52
Zn 8.84 > Cu 2.84 > Cd 2.69
BAFp < 1
BAFdyn>1
Zn 1.22 > Cu 1.00
BAFdyn <1
Zn 0.80 > Cu 2.54 > Cd 0.47
Cd 0.69
Leaves/Soil
BAFp > 1
Cd 8.01 >Cu 5.31 > Zn 4.78
Cu 4.18 >Cd 4.01 > Zn 3.60
Zn 6.65 > Cu 5.96 > Cd 5.07
BAFp < 1
BAFdyn >1
Zn 1.01
BAFdyn <1
Zn 0.55 = Cu 0.52 > Cd 0.34
Cu 0.74 > Cd 0.44
Tomato/Soil
BAFp > 1
Cu 3.43 > Zn 2.04 > Cd 1.36
Cu 2.21 > Zn 1.49
Cu 3.23 > Zn 2.38
BAFp < 1
Cd 0.77
Cd 0.78
BAFdyn>1
Zn 1.28 > Cu 1.20
BAFdyn <1
Zn 0.53 > Cu 0.43 > Cd 0.39
Cd 0.64
BAFp: Primary Bioaccumulation Factor; BAFdyn: Dynamic Bioaccumulation Factor.
Table 3: Translocation Factors (TFs) from the Root to the Stem (S/R), and from the Stem to Leaves (L/S) and Tomato (T/S) in the tomato plants (Solanum Lycopersicum L.) grown in the control and treated soils. T-A: soil amended with anaerobically digested thermal drying sewage (W-A); T-B: soil amended with anaerobically digested municipal solid waste compost (W-B). TFs values are expressed by the mean (n=8).
Heavy metals in tomato plants
Figure 1 summarizes the total metal concentrations in the various tomato plant parts in the harvesting stage; the figures correspond to the mean and standard deviation of the mean (n=8). Metals followed two different patterns: (i) for Cd, Cu, and Zn, distribution took place from roots to different plant parts; (ii) Cr, Ni, Pb and Hg were strongly retained in the root system. The range of concentrations (mg/kg-1 d.w.) for each metal was in the following order: Zn [23-103] > Cu [4.40 -16.74] > Cr [1.03-1.78] = Ni [0.79-1.27] > Pb [0.58-0.70] > Cd [0.02-0.18] > Hg [0.01-0.07]. The highest levels in leaves were Cd (followed by stems> roots>> tomato) and Cu (followed by roots> tomato > stems). Stems had the highest Zn concentrations (followed by roots> leaves > tomato). On the contrary, Cr, Ni and Pb were detected only in the root system. Mercury concentrations in roots were negligible (<0.02 mg kg-1) for all treatments. Tomatoes showed the lowest metal concentrations for Cd, Cu and Zn, regardless of treatment. Cr, Ni, Pb and Hg were never detected.
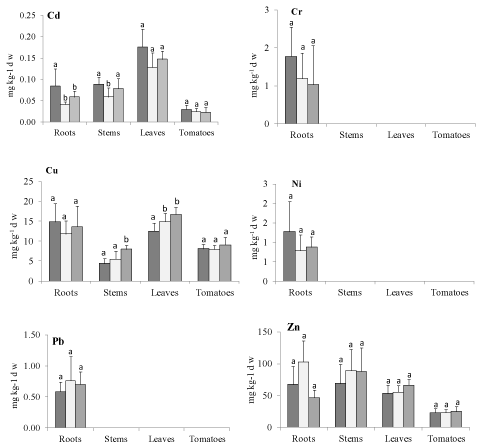
Figure 1: Metals concentrations (mg kg-1d.w.) in different plant parts of the tomato plants (Solanum Lycospersicum Mill L.) grown in control (black bars) or amended
soil with anaerobically digested thermal drying sludge (grey [T-A]) and anaerobically digested municipal solid waste (dark grey [T-B]). Different letters indicate a
significant difference at p<0.05.
Translocation and bioaccumulation factors in tomato plants
The primary and dynamic translocation factors (TFp and TFdyn) of metals from roots to stems, and from stems to leaves and tomatoes are shown in Table 3. As Cr, Ni and Pb were lacking in stems, leaves and tomatoes, it was not possible to calculate the corresponding TFs. The TFp stem/root for Zn and Cd >1 indicated that the metal concentration in stems was higher than in roots as a result of the mobilisation of these metals. When dynamic factors were considered, the mobilisation of Cu with TFdyn stem/root> 1 was observed. From stems to leaves (TFp leaves/stem), the mobilisation of Cd and Cu took place regardless of treatment. However for Cd, TFdyn leaves/stem>1 showed mobilisation of Cd for the plants grown in the treated (T-A) soil. Finally from stems to tomatoes (TFp tomato/stem), only Cu underwent mobilisation, and showed a primary translocation >1 regardless of treatment. When the dynamic translocation factor was considered, only Zn in the treated (T-A) soil showed TFdyn tomato/stem >1.
Table 5 offers the primary and dynamic bioaccumulation factors (BAFp and BAFdyn) in the different plant parts in the harvesting stage. The data that expressed the bioaccumulation of metals in plants were obtained after only considering the bioavailable fraction of metals in soil. In spite of treatment, roots showed BAFp root/soil> 1 for most metals (Cd, Cu, Ni and Zn). Conversely, BAFp root/soil was always < 1 for Cr and Pb. Nevertheless, information was enhanced when BAFdyn was calculated. Then BAFdyn root/soil> 1 was observed only for Zn (T-A soil). BAFpstem/soil and BAFpleaves/soil were always > 1 for Zn, Cd and Cu regardless of treatment. BAFdynstem/soil> 1 was observed only for Zn and Cu in the treated (T-B) soil, while BAFdynleaves/soil>1 was found for Zn in the treated (T-B) soil. BAFptomato/soil> 1 was observed for Cu, Zn and Cd in the control soil, while BAFptomato/soil> 1 was found for Cu and Zn, but not for Cd, in the treated (T-A and T-B) soils. BAFdyntomato/soil> 1 was presented in the treated (T-B) soil for Zn and Cu.
Tomato
Multiple linear regresion equation
R2
F
P-value
Cd
0.0179 Soil bioavailable + 0.1131 Root + 0.1261 Stem
0.9372
85.27
0
Cu
0.4227 Total soil + 0.0906 Root + 0.2446 Stem
0.9843
357.5
0
Zn
1.1004 Soil bioavailable + 0.1049 Stem
0.9789
348.7
0
Table 5: Multivariate linear equations describe associations with in Cd, Cu and Zn in the tomato plants (Solanum Lycopersicum L.) contents.
Heavy metal relationship in soil and tomato plant
Multivariate linear equations were established to describe the total amount of Cd, Cu and Zn in tomatoes by the forced removal method in a regression analysis. All the statistical equations (Table 6) were highly significant (p <0.01). Heavy metals in tomato can be predicted from the coexisting heavymetals in soil (total and bioavailable), roots and stems. In particular, according to the result in the translocation and bioaccumulation factors, Cd was the heavy metal that displayed the greatest mobilisation from soil.
Parameter
Control
T-A- amended soil
T-B- amended soil
Stem (g w.w.)
1.84 ± 3.23a
21,80 ± 3.14b
19.54 ± 1.76ab
Leaves (g w.w.)
3.18 ± 5.47a
36.39 ± 3.58b
33.78 ± 3.48ab
Total Number of tomatoes
104
123
123
Total biomass (g w.w.)
2312
2264
2246
Number of ripe tomatoes
34
37
39
Biomass of ripe tomatoes (g w.w.)
1015
816
685
Mean weight of ripe tomatoes
29.86 ± 25.77a
22.06 ± 16.08ab
17.58 ± 12.6b
Percentage of ripe tomatoes
33
30
32
Number of un-ripe tomatoes
70
86
84
Biomass of un- ripe tomatoes (g w.w.)
1297
1448
1561
Mean weight of un-ripe tomatoes
18.53 ± 16.82a
16.95 ± 15.67a
18.58 ± 15.74a
Percentage of un-ripe tomatoes
67
70
68
aData followed by different letters in a row differed significantly at p<0.5.
Table 6: Effects of anaerobically digested thermal drying sewage (W-A) and anaerobically digested municipal solid waste compost (W-B) on tomato plants (Solanum Lycopersicum). T-A: soil amended with W-A and T-B: soil amended with W-B. Concentrations are expressed by the mean±SD (n=8).
The CCorA analysis (Figure 2) provided the relationships between these heavy metal contents in soil (total and bioavailable), roots or stems, and the heavy metal contents in tomato. The analysis proved highly significant where Factors 1 and 2 represented 85.08% of total variance. As expected, Cd in tomato correlated strongly and positively with the Cd bioavailable content in soil and Cd in roots, but was not associated with total Cd in soil and Cd in tomato stems. Zn in tomato was associated with Zn bioavailable content in soil, but did not show any statistical significance with the total Zn content in soil. This behaviour differed from than observed with the Cu levels in tomato. Cuin tomato was associated with total Cu in soil. This result is in accordance with other studies that have examined heavy metal effects on maize plants [16].
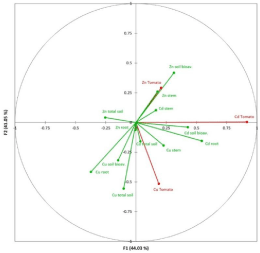
Figure 2: Ordination diagram based on Canonical Correlation Analysis
(CCorA). The represented variables were metals (Cd, Cu and Zn) in soil (total
and available), and total metal concentrations (Cd, Cu and Zn) in different
plant parts of the tomato (Solanum Lycospersicum Mill L.) plants.
Discussion
Metals in soil
A single biosolid-soil application in the loamy sand soil used herein increased the total concentrations of Cd, Cu and Zn. A positive and significant increase in the total Cu and Zn [32,33] and Cd [34] concentrations was detected as a result of MSW compost amendments in soil. In the present study, which was conducted in slightly alkaline soil (7.50), the application of biosolids in soil provoked a significant increase in the total Zn concentration in soil, although the available Zn fraction fell within a similar range (22-29%) for all treatments. Numerous studies have shown that soil pH affects Zn availability [35]. [19] Reported that organic waste amendments in soil modify the soil medium by adding organic substances. Consequently, changes in metal binding and mobility can be expected. The extractable fractions in the treated soils constituted [28-47%] of total Cd, [20- 30%] of total Cu and Zn, [14-20%] of total Pb, [2-4%] of total Ni and [0.02-0.03%] of total Cr. As seen, the available fraction of most metals in the digested thermal drying sludge-amended soil (T-A) was higher than in the anaerobically digested municipal solid waste compost-amended soil (T-B). This can be understood as revealing evidence for the reduced availability of metals from composted biosolids compared to other sewage sludge types [36]. In this study, the available metal fraction of Cd, Cu and Pb lowered in the soils that received the anaerobically digested municipal waste (T-B), while Ni and Cu increased in the soil treated with anaerobically digested thermal drying sludge (T-A). Different patterns for metal availability in amended soils have been documented; [34] reported an increased Cd concentration, while [37] observed no changes in Ni, Cd and Cr concentrations after applying MSW compost to soil. In the present study, no differences were found for the Cr, Ni and Pb concentrations in soil for any treatment. The available metal content was probably more significant than the total concentration because the former can predict the risk of metal uptake by plants and their mobility in the system [16,38].Only soluble, exchangeable and chelated metal species in soil are available for plants [39]. In short, the relationships between the total metal concentrations in the biosolid-amended and control soils (0.99-1.8) < 2 for all the metals indicated that, according to [40], soils were not polluted under our experimental conditions.
Accumulation, translocation and bioaccumulation of heavy metals in tomato plants
After a single biosolid-soil amendment, the Zn, Cr and Pb concentrations in the plants did not improve, but statistically lowered for Cd and Ni, while the Cu concentration increased, in the various plant parts. [41] suggested that MSW compost amendments may cause Zn immobilisation in soil, and could result in reduced availability to uptake by plants. [33] found that MSW compost improved Zn accumulation in the root system of ryegrass or red clover. In our study, the highest Zn concentrations were detected in stems>roots> leaves > tomato, and no differences were found in either the control or treated soils. The Cd (0.02-0.03 mg kg-1), Cu (8.12-9.09 mg kg-1) and Zn (22- 25 mg kg-1) concentrations detected in tomatoes were lower than the maximum levels established in plant material and fruit for human consumption (European Regulation 466/2001 and EC 1881/2006 regulation). Cd, Cu and Zn mobility has been variously associated with tomato in the CCorA and accumulation, translocation and bioaccumulation analyses. The ability of Cd, Cu and Zn mobilisation from soil to plants is revealed according to plant metabolism. Cu and Zn are essential micronutrients for plants. However, Cd is no essential element for plants and is highly labile throughout soil [42]. [13] Understood that Cu and Zn had high translocation factors as they are essential nutrients for plants. Cr, Ni and Pb were detected exclusively in roots. Roots are the most essential organ for nutrient uptake, and their structure and architecture can alter the nutrient uptake rate, especially for those elements that are not essential for plants. We observed no differences in the Cr and Pb concentrations for any treatment. However, Ni in the roots of the plants grown in biosolids-amended soil statistically lowered compared with those grown in the control soil.
Ratios >1 in the primary and dynamic translocation factors from roots to stems (TFstem/roots), and from stems to leaves (TFleaves/ stem) or tomatoes (TFtomatoes/stem), is understood as metal mobilization occurring. TFp stem/root >1 suggested that Zn could effectively be translocated from roots to stems in the plants grown in the control and treated (T-B) soils, and the same was found for Zn and Cd in both the treated (T-A and T-B) soils; TFdyn also showed that Cu was mobilised from roots to stem of the plants grown in both the biosolidamended soils. A similar pattern of distribution was observed for TFp leaves/stem with a clear mobilisation of Cd and Cu regardless of treatment. However, TFdyn revealed that only Cd was mobilised from stems to leaves in the plants grown in treated (T-A) soil. Singh et al. (2010) also revealed translocation factors > 1 for Cr and Pb in tomato (Solanumlycopersicum L.) plants from fly ash-contaminated areas. The synthesis Cd, Cu and Zn was translocated from roots to stems of the plants grown in both biosolid-amended soils, as revealed by TFdyn >1; However, only Cd and Zn from the stems to leaves and stems from tomatoes, respectively, were mobilised in the plants grown in treated (T-A) soil.
When considering BAF, the metals distribution pattern was comparable in roots regardless of treatment, with BAFp> 1 for Cu, Cd, Ni and Zn. For all the other aerial parts, stems, leaves and tomatoes, BAF >1 was observed for Cu, Cd and Zn. In this last case the exception was Cd, which showed BAF <1 in the tomatoes from the plants grown in biosolid-amended soils. When BAFdyn was measured, Zn accumulation was found only in the roots of the plants grown in treated (T-A) soil, with BAFdyn> 1. Cu and Zn accumulation was observed in the stems and leaves of the plants grown in both the treated (T-A and T-B) soils. The highest and the lowest BAFs were obtained in roots and tomatoes, respectively, which indicate good metal accumulation ability in roots, and scarce translocation from roots to fruits. The main differences between BAFs in roots and other plant parts may result from the metal-binding capacity to roots because metals strongly bind to the compost matrix and organic matter, which thus limits their solubility and potential bioavailability in soil [43]. After considering the metal available fraction in soil, BFAs provided much more information than the total metal concentration [16]. So it would be better to offer available metal concentrations in soil in order to gain a better understanding of plants’ real metal uptake ability. There were many discrepancies in the BAFs when compared with the data obtained from other studies, which could be due to differences in available metals in soil, interferences of physico-chemical parameters, and the chemical characterization of sludge in soils [14]. Plant nutrition is a difficult subject to completely understand partly because of the variation between different plants, and even between different species or individuals of a given clone.
Conclusion
Biosolids increase Cu and Zn concentrations in amended soil. Metal uptake by plants differs depending on the metal and plant parts in question: Cu increases significantly in the leaves of the plants grown in both the biosolids-amended soils; Cd, Cu and Zn present a translocation ability from roots to different plant parts; fruits and roots always present the lowest and highest metal concentrations, respectively. The root system acts as a barrier for Cr, Ni, Pb and Hg, so metal uptake is poor and causes very low concentrations of these metals in aerial plant parts.
The CCorA proved a highly significant relationship in the soil/ plant system that is strongly and positively related with Cd, Cu and Zn. Although the BAFs calculated on the basis of the available metals in soil provide much more information, the total metal concentrations in soil should be complemented with the available metal concentrations. Generally, leaves present the highest concentration for Cd and Cu, while tomatoes have the lowest metal concentrations in all cases. The concentrations of Cd (0.02-0.03 mg kg-1), Cu (8.12-9.09 mg kg-1) and Zn (22-25 mg kg-1) detected in fruits were lower than the maximum levels established in plant materials and fruits for human consumption (European Regulation nº 466/2001 and EC 1881/2006 regulation).
Acknowledgment
Support for this work has been provided by Spanish Projects CTM2013-44986-R and CTM2014-52338-R.
References
- Eurostat. Sewage sludge production and disposal from urban wastewater. 2015.
- European Communities, Waste Management Options and Climate Change. 2001.
- Lou XF, Nair J. The impact of landfilling and composting on greenhouse gas emissions--a review. Bioresour Technol. 2009; 100: 3792-3798.
- USEPA. Solid Waste and Emergency Response (5306P). EPA-530-F-009-021 November. 2009.
- Favoino E, Hogg D. The potential role of compost in reducing greenhouse gases. Waste Manag Res. 2008; 26: 61-69.
- Carbonell G, Pro J, Gómez N, Babín MM, Fernández C, Alonso E, et al. Sewage sludge applied to agricultural soil: Ecotoxicological effects on representative soil organisms. Ecotoxicol Environ Saf. 2009; 72: 1309-1319.
- Nziguheba G, Smolders E. Inputs of trace elements in agricultural soils via phosphate fertilizers in European countries. Sci Total Environ. 2008; 390: 53-57.
- Lakhdar A, Iannelli MA, Debez A, Massacci A, Jedidi N, Abdelly C. Effect of municipal solid waste compost and sewage sludge use on wheat (Triticum aestivum): growth, heavy metal accumulation and antioxidant activity. J. Sci. Food Agric. 2010; 90: 965-971.
- Jia L, Wang W, Li Y, Yang L. Heavy metals in soil and crops of an intensively farmed area: a case study in Yucheng City, Shandong Province, China. Int J Environ Res Public Health. 2010; 7: 395-412.
- Khan S, Cao Q, Zheng YM, Huang YZ, Zhu YG. Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in Beijing, China. Environ Pollut. 2008; 152: 686-692.
- Jolly YN, Islam A, Akbar S. Transfer of metals from soil to vegetables and possible health risk assessment. Springerplus. 2013; 2: 385.
- Stasinos S, Nasopoulou C, Tsikrika C, Zabetakis I. The bioaccumulation and physiological effects of heavy metals in carrots, onions, and potatoes and dietary implications for Cr and Ni: a review. J Food Sci. 2014; 79: R765-780.
- Yoon J, Cao X, Zhou Q, Ma LQ. Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci Total Environ. 2006; 368: 456-464.
- Bose S, Bhattacharyya AK. Heavy metal accumulation in wheat plant grown in soil amended with industrial sludge. Chemosphere. 2008; 70: 1264-1272.
- Singh R, Singh DP, Kumar N, Bhargava SK, Barman SC. Accumulation and translocation of heavy metals in soil and plants from fly ash contaminated area. J Environ Biol. 2010; 31: 421-430.
- Carbonell G, de Imperial RM, Torrijos M, Delgado M, Rodriguez JA. Effects of municipal solid waste compost and mineral fertilizer amendments on soil properties and heavy metals distribution in maize plants (Zea mays L.). Chemosphere. 2011; 85: 1614-1623.
- Rezvani M, Zaefarian F. Bioaccumulation and translocation factors of cadmium and lead in Aeluropus littoralis. Australian J. Agr. Eng. 2011; 2: 114-119.
- Murtaza G, Javed W, Hussain A, Wahid A, Murtaza B, Owens G. Metal uptake via phosphate fertilizer and city sewage in cereal and legume crops in Pakistan. Environ Sci Pollut Res Int. 2015; 22: 9136-9147.
- Baltrèinaité E, Lietuvninkas A, Baltrènas P. Use of dynamic factors to assess metal uptake and transfer in plants- Example of trees. Water Air Soil Poll. 2012; 223: 4297-4306.
- Kumar A, Maiti SK. Translocation and bioaccumulation of metals in Oryza sativa and Zea mays growing in chromite-asbestos contaminated agricultural fields, Jharkhand, India. Bull Environ Contam Toxicol. 2014; 93: 434-441.
- Gigliotti G, Businelli D, Giusquiani PL. Trace metals uptake and distribution in corn plants grown on a 6-year urban-waste compost amended soil. Agric. Ecosyst. Environ. 1996; 58: 199-206.
- Bahemuka TE, Mubofu EB. Heavy metals in edible green vegetables grown along the sites of the Sinza and Msimbazi Rivers in Dar es Salaam, Tanzania. Food Chem. 1999; 66: 63-66.
- Hargreaves JC, Adl MS, Warman PR. A review of the use of composted municipal solid waste in agriculture. Agric. Ecosyst. Environ. 2008; 123: 1-14.
- USEPA. Method 3051: Microwave assisted acid digestion of sediments, sludge, soils and oils. In: Test Methods for Evaluating Solid Waste, Physical/ Chemical Methods, Office of Solid Waste and Emergency Response, SW-846. 1994.
- Carbonell G, Bravo JC, López-Mancisidor P, Pro J, Fernández C, Tarazona JV. Distribution, fractionation and mobility assessment of heavy metals in a spiked soil using a multi-species-soil system. Span. J. Agric. Res. 2009b; 79: 629-637.
- Lindsay WL, Norvell WA. Development of a DTPA soil test for zinc, iron, manganese and copper. Soil Sci. Soc. Am. J. 1978; 42: 421-428.
- USEPA. Mercury in soils and solutions by thermal decomposition amalgamation and atomic spectrophotometry. Method. 1998; 7473.
- Hotelling H. Relations between two sets of variates. Biometrika. 1936; 28: 321-377.
- Kianifard F. Applied Multivariate Data Analysis, Volume II: Categorical and Multivariate Methods. Technometrics. 1993; 35: 326-327.
- Rodríguez Martín JA, Gutiérrez C, Escuer M, García-González MT, Campos-Herrera R, Aguila N. Effect of mine tailing on the spatial variability of soil nematodes from lead pollution in La Union (Spain). Sci Total Environ. 2014; 473-474: 518-29.
- CCQC. California Compost Quality Council. Compost maturity index. 2001.
- Zheljazkov VD, Warman PR. Phytoavailability and fractionation of copper, manganese, and zinc in soil following application of two composts to four crops. Environ Pollut. 2004; 131: 187-195.
- Guerra P, Ahumada I, Carrasco A. Effect of biosolid incorporation to mollisol soils on Cr, Cu, Ni, Pb, and Zn fractionation, and relationship with their bioavailability. Chemosphere. 2007; 68: 2021-2027.
- Mantovi P, Baldoni G, Toderi G. Reuse of liquid, dewatered, and composted sewage sludge on agricultural land: effects of long-term application on soil and crop. Water Res. 2005; 39: 289-296.
- Zeng F, Ali S, Zhang H, Ouyang Y, Qiu B, Wu F, et al. The influence of pH and organic matter content in paddy soil on heavy metal availability and their uptake by rice plants. Environ Pollut. 2011; 159: 84-91.
- Smith SR. A critical review of the bioavailability and impacts of heavy metals in municipal solid waste composts compared to sewage sludge. Environ Int. 2009; 35: 142-156.
- Zhang M, Heaney D, Henriquez B, Solberg E, Bittner E. A four-year study on the influence of biosolids/MSW compost application in less productive soils in Alberta: nutrient dynamics. Compost Sci. Util. 2006; 14: 68-80.
- Bell FP, James BR, Chaney RL. Heavy metal extractability in long term sewage sludge and metal salt amended soils. J. Environ. Qual. 1991; 20: 481-486.
- Kabata-Pendias A. Behavioural properties of trace metals in soils. Appl. Geochem. 1993; 2: 3-9.
- Al-Hwaiti M, Al-Khashman O. Health risk assessment of heavy metals contamination in tomato and green pepper plants grown in soils amended with phosphogypsum waste materials. Environ Geochem Health. 2014.
- Hernando A, Lobo M, Polo A. Effects of the application of a municipal refuse compost on the physical and chemical properties of soil. Sci. Total. Environ. 1989; 81/82: 589-596.
- Beesley L, Moreno-Jiménez E, Clemente R, Lepp N, Dickinson N. Mobility of arsenic, cadmium and zinc in a multi-element contaminated soil profile assessed by in-situ soil pore water sampling, column leaching and sequential extraction. Environ Poll. 2010; 158: 155-160.
- Singh RP, Agrawal M. Effects of sewage sludge amendment on heavy metal accumulation and consequent responses of Beta vulgaris plants. Chemosphere. 2007; 67: 2229-2240.