
Special Article - Phytomedicine
J Plant Chem and Ecophysiol. 2017; 2(1): 1014.
In Vitro Evaluation of Free Radical Scavenging and Antioxidant Activities of Averrhoa bilimbi Fruit Extracts
Kurup SB and Mini S*
Department of Biochemistry, University of Kerala, India
*Corresponding author: Mini Saraswathy, Department of Biochemistry, University of Kerala, Kariavattom, Thiruvananthapuram - 695 581, Kerala, India
Received: July 10, 2017; Accepted: September 01, 2017; Published: September 11, 2017
Abstract
The phytochemicals are biologically active compounds and have therapeutic potential for free radical associated disorders. Free radicals are implicated in the development of various chronic and degenerative diseases including diabetes mellitus, coronary heart disease, arthritis, cancer, ageing etc. The present study evaluates the antioxidant potential of different solvent fractions of Averrhoa bilimbi Linn. (Oxalidaceae) fruits. Antioxidant activity was assessed by various in vitro methods such as 1, 1-Diphenyl-2-Picrylhydrazyl (DPPH) radical scavenging assay, superoxide radical scavenging assay, nitric oxide radical scavenging assay, total reducing power and total antioxidant activity. Total phenolic and flavonoid contents of different solvent fractions (petroleum ether, ethyl acetate, butanol and water) were also determined and expressed in gallic acid and quercetin equivalents respectively. The results of the study show that the ethyl acetate fraction (ABE) of the lyophilized aqueous extract of Averrhoa bilimbi fruits has superior antioxidant properties than the other fractions. IC50values of the ethyl acetate fraction for superoxide radical scavenging and nitric oxide radical scavenging activities were 72 and 61.5 μg/ml, respectively. Total phenolic content (31.26 ± 1.16 mg%) and flavonoid content (6.15 ± 0.23 mg%) of ABE was significantly higher than other fractions. The results of the present study indicate that Averrhoa bilimbi fruits are a rich source of natural antioxidants and might be utilized as a functional food/nutraceutical.
Keywords: Averrhoa bilimbi fruits; Phytomedicine; Free radicals; Antioxidant
Introduction
Plants serves as a viable source of drugs and several plant based drugs are extensively used in traditional medicine [1]. The rising incidence of life style disorders are alarming and becoming a serious public health problem. Many synthetic drugs afford protection against oxidative damage, but they have serious adverse effects. Plants and plant products have been traditionally used for the treatment of various diseases due to their free radical scavenging and antioxidant properties. Phytochemicals are reported to have antioxidant, antidiabetic, anti-inflammatory and anticancer activities. Antioxidants are important health promoting factors and have been reported to possess several biological properties [2]. Oxidative stress occurs as a result of the imbalance between Reactive Oxygen Species (ROS) production and antioxidant defenses. Oxidative stress is known to contribute to the onset of several life style diseases including atherosclerosis, hypertension, diabetes mellitus, heart disease, stroke, obesity, ischemic diseases and cancer [3]. ROS including superoxide radicals, hydroxyl radicals, singlet oxygen and hydrogen peroxide are often generated as byproducts of biological reactions or from exogenous factors [4]. Oxidative stress has been defined as harmful because free radicals attack several biological molecules like lipids, proteins, amino acids and DNA and have a useful role in physiologic adaptation and intracellular signal transduction. Together with increased generation of ROS, impaired formation of endogenous antioxidants, namely, superoxide dismutase, reduced glutathione and ascorbic acid and reduction in the antioxidant capacity ofuric acid and vitamin E, as well as the reduction in the activity of glutathione peroxidase, glutathione reductase and catalase, have been documented in several diseases. Auto-oxidation of glucose is the main source of ROS generation which results in protein fragmentation, oxidation of lipids and nucleic acids. Another important source of free radicals is the interaction of glucose with proteins which leads to the formation of an Amadori product and advanced glycation end products (AGEs) [5]. AGEs consist of heterogeneous group of macro protein derivatives, which are formed by non-enzymatic reaction between reducing sugars and amino groups of proteins, lipids and nucleic acids. Membrane lipids are mainly disposed to oxidation due to their high levels of Polyunsaturated Fatty Acids (PUFA) and are associated with enzymatic and non-enzymatic systems generating free radical species.
Natural antioxidants increase the antioxidant capacity of plasma and reduce the risk of several life style disorders [6]. Different parts of the plants are known to contain a substantial amount of phytoconstituents such as phenolics, flavonoids, phytosterols, tannins and alkaloids and have the ability to scavenge the free radicals [7]. Recently, interest has been increased noticeably in finding naturally occurring antioxidants for use in foods or medicinal resources to replace synthetic antioxidants, which are being limited due to their side effects such as carcinogenicity [8]. Medicinal plants having antioxidant activities may have good therapeutic potential in the treatment of several chronic and degenerative diseases. Antioxidant principles of natural resources possess multifacetedness in their multitude and magnitude of actions and provide enormous scope in correcting the imbalance [9].
Averrhoa bilimbi Linn. (Oxalidaceae), commonly known as cucumber tree or tree sorrel is a widely cultivated plant in India, Indonesia, Sri Lanka, Bangladesh, Myanmar, Malaysia, Central and South America. The whole plant is used for treating coughs, cold, itches, rheumatism, whooping cough, hypertension etc. [10,11]. Averrhoa bilimbi fruits are used as a culinary dish in the southern part of India. Traditionally A. bilimbi fruits are reported to have antioxidant, antimicrobial, antibacterial and antidiabetic properties [12,13]. In view of these, we evaluated the antioxidant activity of different fractions of Averrhoa bilimbi fruits through different in vitro test models so as to screen the antioxidant activity.
Materials and Methods
Chemicals
2-Diphenyl-2-Picrylhydrazyl (DPPH) was purchased from Sigma Chemicals Co. (St. Louis, MO, USA). Aluminium chloride was obtained from Merck (Germany). Nitro Blue Tetrazolium (NBT), Nicotinamide Adenine Dinucleotide (NADH), Phenazine Methosulphate (PMS), gallic acid, quercetin, Trichloroacetic Acid (TCA), Thiobarbituric Acid (TBA), Ethylene Diamine Tetra Acetic Acid (EDTA), deoxyribose, ascorbic acid and ferric chloride were purchased from Sisco Research Laboratories (India). All other chemicals, including the solvents used were of standard analytical grade.
Collection and taxonomical identification of plant material
Fresh fruits of Averrhoa bilimbi were obtained from Thiruvananthapuram, Kerala, India during the fruiting season, July- December. Authentication was done by Dr. Valsala Devi, Department of Botany, University of Kerala, India and a voucher specimen (Voucher No. KUBH 5865) has been deposited in the herbarium of Department of Botany, University of Kerala for further reference.
Extraction and fractionation of Averrhoa bilimbi fruits
Fruits (5 kg) were cut and shade dried at a temperature of 28ºC and stored at 4ºC. Shade dried fruits were ground in a blender to give 500 g of fine powder. The aqueous extract was prepared by cold maceration of 500 g powder in 1000 ml of distilled water and lyophilized (Thermo electron corporation, MODUL YOD-230). The lyophilized extract was then stored at -4ºC and the yield was 26%. 100g lyophilized A. bilimbi fruit extract (ABL) was partitioned successively using a separating funnel and with petroleum ether, ethyl acetate, butanol and water and the fractions were collected. The extraction continued till the solvent became colorless. The extracts were filtered through Whatman No. 1 filter paper to remove all unextractable matter, including cellular materials and other constituents that are insoluble in the extraction solvent. The organic fractions were concentrated in vacuum at temperatures below 60ºC in a rotary evaporator (Heidolph, Germany) while the aqueous fraction was concentrated in a freeze drier (Thermo electron corporation, MODUL YOD-230). The final dried samples were stored in labeled sterile bottles and kept at -4ºC until use. The percentage yield of different solvent fractions was petroleum ether -15%, ethyl acetate -5%, butanol -25% and aqueous -50%.
Preliminary phytochemical analysis
The qualitative phytochemical analysis of different solvent fractions of Averrhoa bilimbi fruits (AB) was carried out in order to determine the presence of its constituents using standard conventional protocols [14]. The percentage yield of each fraction was also calculated.
Test for carbohydrates
a) Fehling’s test: The extract was treated with 5 mL Fehling’s solution A and B and kept at boiling water bath for 5 minutes. Formation of yellow or red precipitate indicated the presence of reducing sugar.
b) Benedict’s test: To 1 mL of extract added 5 mL of Benedict’s solution and kept at boiling water bath for 5 minutes. Formation of red or green precipitate indicated the presence of reducing sugar.
Test for tannins and phenolic compounds
a) Ferric chloride test: 1 mL of the extract was treated with few mL of 5% neutral ferric chloride. Formation of dark blue or bluish black product indicated the presence of tannins.
b) Lead acetate test: 1 mL of the extract was treated with few mL of lead tetra acetate solution. Formation of precipitate indicated the presence of tannins and phenolic compounds.
Test for flavonoids
Alkaline reagent test: 1 mL of the extract was treated with 1 mL of NaOH solution and observes for the intensity of yellow color which would become colourless on the addition of few drops of dilute HCl that indicated the presence of flavonoids.
Test for alkaloids
Dragendroff’s test: About 2 mL aliquot of the extract was treated with Dragendroff’s reagent and observed for red precipitate that indicated the presence of alkaloids.
Test for glycosides
Keller-Kiliani test: To the solution of 1 mL extract in glacial acetic acid, few drops of FeCl3 and Conc. H2SO4 were added and observed for reddish brown coloration at the junction of two layers. Bluish green color in the upper layer indicated the presence of glycosides.
Test for saponins
Frothing test: About 1 mL of alcoholic extract was diluted separately with 20 mL of distilled water and shaken in a graduated cylinder for 15 minutes. Formation of 1 cm layer of foam indicated the presence of saponins.
Test for terpenoids
Libermann test: To 1 mL of extract, 3 mL of acetic acid and few drops of Conc. H2SO4 were added. The color change from red to blue indicated the presence of terpenoids.
Test for steroids
Sulphuric acid test: To 2 mL of extract, 1 mL of Conc. H2SO4 was added carefully along the sides of the test tube and observed for the formation of a red color layer which indicated the presence of steroids.
Total phenolic content
The Total Phenolic Content (TPC) was determined according to the method described by Singleton and Rossi [15]. Different concentrations of Averrhoa bilimbi fruit extracts (lyophilized aqueous extract, petroleum ether, ethyl acetate, butanol and aqueous extracts) were mixed with distilled water (final volume of 3.5 mL) and added 0.5 mL of Folin-Ciocalteu reagent. 1 mL of 20% sodium carbonate solution was added after 5 minutes and incubated at ambient temperature (25-27ºC) for 90 minutes. The color developed was read at 760 nm using UV visible spectrophotometer (Shimadzu UV-Vis Spectrophotometer, Model 1240). Gallic acid was used as the reference standard. The content of phenolic compounds was expressed as milligram percentage on dry weight basis.
Total flavonoid content
The Total Flavonoid Content (TFC) was determined by aluminium chloride colorimetric assay [16]. Different concentrations of Averrhoa bilimbi fruit extracts were added to 0.3 mL of 5% (w/v) sodium nitrite. After 5 minutes, 0.3 Ml of 10% (w/v) aluminum chloride and 2 mL 1M sodium hydroxide was added. The absorbance was read against a blank at 510 nm. Quercetin was used as the reference standard. The TFC was expressed as milligram percentage on dry weight basis.
DPPH radical scavenging assay
The free radical scavenging effect was assessed by the method described by Hollman Peter [17]. 2.8 mL of 0.1mM 2,2-Diphenyl-1- Picrylhydrazyl (DPPH) solution was added to different concentrations of Averrhoa bilimbi fruit extracts (lyophilized aqueous extract, petroleum ether, ethyl acetate, butanol and aqueous extracts). In control, methanol was used in place of the sample. When DPPH reacts with an antioxidant compound that can donate hydrogen, it gets reduced and the resulting decrease in absorbance was recorded at 517 nm after 30 minutes using Jasco V-630 UV-VIS spectrophotometer, Easton, MD, USA. Gallic acid was used as a reference free radical scavenger and the percentage inhibition calculated by using the formula;
Inhibition (%) = (control - test) / control x 100
Superoxide radical scavenging assay
Superoxide radical scavenging activity was measured by the method of Robak and Gryglewski [18]. Superoxide anions were generated in a non-enzymatic PMS-NADH system through the reaction of PMS, NADH and oxygen and it was assayed by the reduction of NBT. The reaction mixture contained 1mL NBT (156μM), 1mL NADH (468μM), 100μL PMS (60μM) and different concentrations of Averrhoa bilimbi fruit extracts (lyophilized aqueous extract, petroleum ether, ethyl acetate, butanol and aqueous extracts). Incubated the mixture at 25ºC for 5 minutes and read the absorbance at 560 nm against reagent blank. Quercetin was used as a positive control. The percentage inhibition was calculated by using the formula;
Inhibition (%) = (control - test) / control x 100
Nitric oxide radical scavenging assay
Nitric oxide radical scavenging was determined by the method of Garatt [19]. 2 mL of 10mM sodium nitroprusside was mixed with 0.5 mL Averrhoa bilimbi fruit extracts (lyophilized aqueous extract, petroleum ether, ethyl acetate, butanol and aqueous extracts) at various concentrations and incubated at 25ºC for 150 minutes. Then 0.5 mL of Griess reagent was added to 0.5 mL incubation mixture and absorbance was read at 540 nm after 30 minutes against reagent blank. Ascorbic acid, a potent free radical scavenger, was used as the reference standard. The percentage inhibition was calculated by using the formula;
Inhibition (%) = (control - test) / control x 100
Total reducing power
The reductive potential of the extract was determined by the method of Oyaizu [20]. The different concentrations of extracts and standard in 1ml of distilled water were mixed with phosphate buffer (0.2 M, pH 6.6) and potassium ferricyanide (1% w/v). The mixture was incubated at 50ºC for 20 minutes and then 10% of Trichloroacetic Acid (TCA) was added to the mixture, subjected to centrifugation for 10 minutes. The upper layer of solution was taken, mixed with distilled water and 0.1% FeCl3. Read the absorbance at 700 nm. Ascorbic acid was the reference standard.
Total antioxidant activity
The total antioxidant capacity of the extracts was evaluated by the phosphomolybdenum method according to the procedure described by Prieto et al. [21]. Briefly 0.3 mL of Averrhoa bilimbi fruit extracts (lyophilized aqueous extract, petroleum ether, ethyl acetate, butanol and aqueous extracts) was mixed with 3 mL of reagent solution (0.6 M sulphuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate). Reaction mixture was incubated at 95ºC for 90 minutes under water bath. Reading was taken at 695 nm after cooling to room temperature. Total antioxidant activity is expressed as the number of equivalents of ascorbic acid.
Statistical analysis
All experimental results were presented as mean ± SEM in triplicate. One way Analysis of Variance (ANOVA) was applied for comparison of the mean values. All statistical analyses were performed using SPSS software (SPSS 17 for windows; SPSS Inc., Chicago).
Qualitative analysis of Averrhoa bilimbi fruits
The phytochemical studies on Averrhoa bilimbi fruits revealed the presence of phenolics, alkaloids, flavonoids, steroids, terpenoids and tannins (Table 1).
Test
ABL
ABP
ABE
ABB
ABW
Alkaloids
++
+ +
+
+ +
+
Tannins
+
+
+
-
-
Saponins
+
+
+
-
+ +
Flavonoids
++
+
+ ++
+
+
Glycosides
++
+
+
+
+
Phytosterols
+
+
++
+++
-
Terpenoids
++
+
+
+
-
Phenols
+++
+
+ ++
+
+
Carbohydrate
+++
-
+ ++
+ +
+ ++
Table 1: Qualitative analysis of Averrhoa bilimbi fruits. The sign (+) indicates ‘trace’, (++) indicates ‘high’, (+++) indicates ‘abundant’ and (−) indicates ‘absence’. ABL, lyophilized aqueous extract; ABP, petroleum ether fraction of ABL; ABE, ethyl acetate fraction of ABL; ABB, butanol fraction of ABL; ABW, aqueous fraction of ABL.
Total phenolic and flavonoid content
From a linear calibration curve of gallic acid, in the range 20-100 μg/ml, total phenolic content was determined. Results showed that the phenolic content in lyophilized aqueous extract (ABL), petroleum ether (ABP), ethyl acetate (ABE), butanol (ABU) and aqueous (ABW) fractions were 20.50 ± 0.76 mg%, 4.92 ± 0.18 mg%, 31.26 ± 1.16 mg%, 12.81 ± 0.47 mg% and 9.22 ± 0.34 mg% respectively. The ethyl acetate fraction (ABE) exhibited the highest phenolic content as compared to ABL and other fractions (Figure 1).
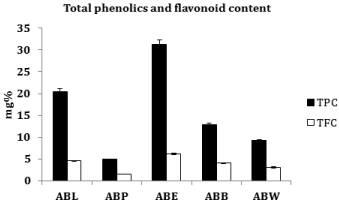
Figure 1: Total phenolics and flavonoid content.
Values are mean ± SEM (n = 3). ABL, lyophilized aqueous extract of A. bilimbi
fruits; ABP, petroleum ether fraction of ABL; ABE, ethyl acetate fraction of
ABL; ABB, butanol fraction of ABL; ABW, aqueous fraction of ABL.
Total Flavonoid Content (TFC) of A. bilimbi fruits was evaluated by aluminum trichloride-sodium nitrite colorimetric assay. TFC in ABL, ABP, ABE, ABB and ABW were 4.61 ± 0.17 mg%, 1.53 ± 0.05 mg%, 6.15 ± 0.23 mg%, 4.10 ± 0.15 mg% and 3.07 ± 0.11 mg% respectively. Among all the fractions ethyl acetate fraction (ABE) contained the highest amount of flavonoids (Figure 1).
DPPH radical scavenging activity
DPPH radical scavenging ability is broadly used as an index to assess the antioxidant potential of medicinal plants. Ethyl acetate fraction exhibited superior inhibition of DPPH radical followed by lyophilized aqueous extract (ABL), butanol (ABB), aqueous (ABW) and petroleum ether (ABP) fractions. The results were compared with reference standard Gallic Acid (GA) (Figure 2).
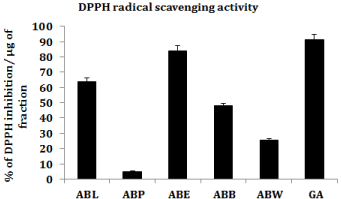
Figure 2: DPPH radical scavenging activity.
Values are mean ± SEM (n = 3). ABL, lyophilized aqueous extract of A. bilimbi
fruits; ABP, petroleum ether fraction of ABL; ABE, ethyl acetate fraction of
ABL; ABB, butanol fraction of ABL; ABW, aqueous fraction of ABL; GA, gallic
acid.
Superoxide radical scavenging activity
Superoxide radical scavenging activities of lyophilized aqueous extract of Averrhoa bilimbi fruits (ABL) and its different solvent fractions were assessed by the auto oxidation of hydroxylamine in the presence of NBT (nitroblue tetrazolium) and compared with reference compound quercetin (IC50 value 15.37 ± 0.57μg/ml). The decrease in absorbance at 560 nm with the plant extract and quercetin indicates the consumption of superoxide anion in the reaction mixture. Concentration of ABL, ABP, ABE, ABB and ABW required for 50% inhibition were found to be 82.00 ± 3.05, 153.75 ± 5.72, 71.75 ± 2.67, 92.25 ± 3.44 and 102.50 ± 3.82 μg/ml. The ethyl acetate fraction (ABE) was found to be an efficient scavenger of superoxide radicals than ABL and other fractions (Figure 3).
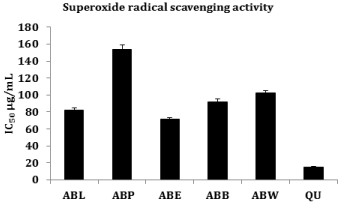
Figure 3: Superoxide radical scavenging activity.
Values are mean ± SEM (n = 3). ABL, lyophilized aqueous extract of A. bilimbi
fruits; ABP, petroleum ether fraction of ABL; ABE, ethyl acetate fraction of
ABL; ABB, butanol fraction of ABL; ABW, aqueous fraction of ABL; QU,
quercetin.
Nitric oxide radical scavenging activity
Lyophilized aqueous extract of Averrhoa bilimbi fruits (ABL) and its different solvent fractions exhibited a dose dependent inhibition of nitric oxide radicals. The IC50 values of ABL (71.75 ± 2.67 μg/ml), ABP (123.00 ± 4.58 μg/ml), ABE (61.50 ± 2.29 μg/ml), ABB (82.00 ± 3.05 μg/ml) and ABW (92.25 ± 3.43 μg/ml) were compared with the standard ascorbic acid (IC50 20.50 ± 0.76 μg/ml). Among all the fractions, ABE showed superior Nitric Oxide (NO) radical scavenging activity than other fractions (Figure 4).
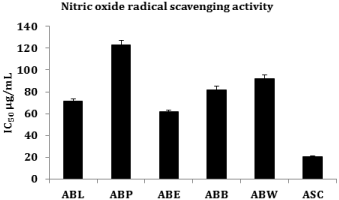
Figure 4: Nitric oxide radical scavenging activity.
Values are mean ± SEM (n = 3). ABL, lyophilized aqueous extract of A. bilimbi
fruits; ABP, petroleum ether fraction of ABL; ABE, ethyl acetate fraction of
ABL; ABB, butanol fraction of ABL; ABW, aqueous fraction of ABL; ASC,
ascorbic acid.
Total reducing power
The reducing power of the lyophilized aqueous extract of Averrhoa bilimbi fruits (ABL), ABL fractions and the reference compound, ascorbic acid increased linearly with increasing concentrations. ABE exhibited superior reducing power than other fractions and this may be due to the presence of phenolics and flavonoids which possess potent hydrogen donating abilities (Figure 5).
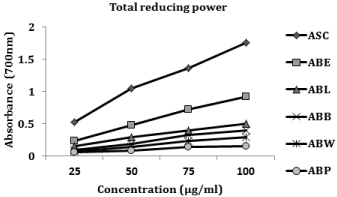
Figure 5: Total reducing power.
Values are mean ± SEM (n = 3). ABL, lyophilized aqueous extract of A. bilimbi
fruits; ABP, petroleum ether fraction of ABL; ABE, ethyl acetate fraction of
ABL; ABB, butanol fraction of ABL; ABW, aqueous fraction of ABL; ASC,
ascorbic acid.
Total antioxidant activity
The phosphomolybdenum method has been routinely used to assess the antioxidant capacity of plant extracts and was expressed as the number of equivalents of ascorbic acid. Ethyl acetate fraction (ABE) exhibited higher antioxidant activity with the value 82.00 ± 3.05 μg of ascorbic acid/mg of extract (Figure 6).
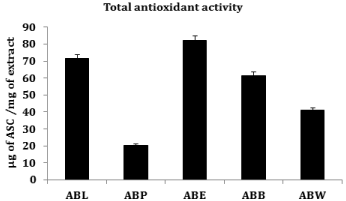
Figure 6: Total antioxidant activity.
Values are mean ± SEM (n = 3). ABL, lyophilized aqueous extract of A. bilimbi
fruits; ABP, petroleum ether fraction of ABL; ABE, ethyl acetate fraction of
ABL; ABB, butanol fraction of ABL; ABW, aqueous fraction of ABL.
Discussion
Screening of traditionally used plants and discovery of their active components with antioxidant properties would be useful in the treatment of various disorders [22]. Phenolics are composed of several classes of compounds including flavonoids (flavones, isoflavones and flavonones), anthocyanins and catechins. According to Afolayan et al [23], high phenolic content of plant extracts could be responsible for their free radical scavenging activity. Previous studies on fruits and vegetables also show a relationship between phenolic content and antioxidant activities [24]. The antioxidant potential of polyphenols arises from their high reactivity as hydrogen or electron donors, which can stabilize and delocalize the unpaired electron and chelate metal ions [25]. In agreement with the above report the ethyl acetate fraction (ABE) exhibited the highest phenolic content when compared with lyophilized aqueous extract, petroleum ether, butanol and aqueous fractions. These results suggested that the phenolic compounds significantly contributed to the antioxidant capacity of the Averrhoa bilimbi fruits.
Flavonoids play a vital role in antioxidant system in plants. The antioxidant potential of flavonoids is due to several mechanisms, such as scavenging of free radicals, chelation of metal ions and inhibition of enzymes responsible for free radical generation [26]. The ethyl acetate fraction of A. bilimbi has been shown to possess the highest flavonoid content than lyophilized aqueous extract (ABL) and other fractions. The high content of these phytochemicals in A. bilimbi may be the contributing factor for its high free radical scavenging activity.
DPPH (1, 1-diphenyl-2-picrylhydrazyl) is a stable free radical usually used to test preliminary radical scavenging activity of a compound or a plant extract [27]. DPPH has characteristic absorbance maxima at 517 nm, which decreases with the scavenging of the proton radical. This property has been extensively used to evaluate the free radical scavenging effect of natural antioxidants [28]. In the present study lyophilized aqueous extract (ABL) and different solvent fractions exhibited considerable DPPH radical scavenging activity as indicated by their IC50 values. This suggests that the ABL and its fractions have compounds that are capable of donating hydrogen to a free radical in order to eliminate odd electron which is responsible for radical’s reactivity [29]. Among different solvent fractions of ABL, ethyl acetate fraction (ABE) showed maximum scavenging activity followed by lyophilized aqueous extract, butanol, aqueous and petroleum ether fractions.
Superoxide anions are harmful reactive oxygen species generated either by auto-oxidation processes or by enzymes [30]. The concentration of superoxide increases under conditions of oxidative stress [31]. In the PMS/NADH-NBT system, superoxide anion liberated from dissolved oxygen by PMS/NADH coupling reaction reduces NBT to a blue colored product called formazon. Antioxidants are able to inhibit the formazon formation. The decrease in absorbance at 560 nm with antioxidants indicates the scavenging of superoxide anion in the reaction mixture [32]. Consistent with the above reports lyophilized aqueous extract (ABL) and ABL fractions caused a dose dependent inhibition of superoxide radicals, of which ethyl acetate fraction (ABE) showed superior superoxide radical scavenging activity in comparison with other fractions and ABL. The possible mechanism of scavenging the superoxide anions may be owed to the inhibitory effect of the A. bilimbi fruits towards generation of super oxides in the in vitro system.
Nitric Oxide (NO) has shown to play a vital role in various physiological processes such as smooth muscle relaxation, neuronal signaling, inhibition of platelet aggregation and regulation of cell mediated toxicity. It is a diffusible free radical, which act as an effector molecule in diverse biological processes, with vasodilatation, antimicrobial and antitumor activities. The toxicity of NO increases significantly when it reacts with superoxide radical, forming the highly reactive peroxynitrite anion (ONOO-) [33]. The nitric oxide liberated from sodium nitroprusside reacts with oxygen to form nitrite. The phytochemicals have the ability to counteract nitric oxide formation and thereby inhibit the bad effects of excessive NO generation in the human body [34]. In agreement with the above reports lyophilized aqueous extract (ABL) and ABL fractions caused a dose dependent inhibition of nitric oxide radicals. Among different solvent fractions of ABL, ethyl acetate fraction (ABE) has potent nitric oxide scavenging activity (IC50 value 59.96 ± 2.23 μg/ml) and petroleum ether fraction (ABP) showed the least nitric oxide scavenging activity (IC50 value 119 ± 4.46 μg/ml).
Reducing power is frequently used to assess the ability of natural antioxidant to donate electron [35,36]. In the reducing power assay, the presence of reluctant (antioxidants) in the fractions facilitates the reduction of ferric (Fe3+) to ferrous (Fe2+) form. Several reports revealed that there is a direct correlation between antioxidant activities and reducing power of certain plant extracts [37,38]. In agreement with the above reports, the reducing power of the lyophilized aqueous extract (ABL), ABL fractions and the reference standard, ascorbic acid increased linearly with increasing concentrations. The superior reducing power was shown by ethyl acetate fraction (ABE) and this may be due to the presence of phenolics and flavonoids which possess potent hydrogen donating abilities.
The phosphomolybdenum method has been routinely used to evaluate the antioxidant activity of plant extracts [39]. The total antioxidant activity of different solvent fractions of A. bilimbi was estimated from their ability to reduce Phosphate/Mo (VI) complex to Phosphate/Mo (V). Previous reports indicate a positive relationship exists between total phenols and antioxidant activity in many plant species [40]. The statement has been justified in the current study were the ethyl acetate fraction of the ABL (ABE) showed the superior antioxidant capacity (in term of ascorbic acid equivalent) with maximum phenol content.
The present study revealed that Averrhoa bilimbi fruits exhibit good antioxidant and free radical scavenging activity. Significant antioxidant activities showed by Averrhoa bilimbi fruits provide a scientific validation for the traditional use of this plant. Hence, there is a need for further studies concerning the active compounds present in Averrhoa bilimbi fruits. It is therefore concluded that Averrhoa bilimbi fruits can be used as a viable source of natural antioxidants with health benefits and might be utilized as a functional food/ nutraceutical.
Acknowledgement
This work was financially supported by Promotion of University Research and Scientific Excellence (PURSE) programme, Department of Science and Technology (DST), Government of India.
References
- Rosy BA, Joseph H. Phytochemical, pharmacognostical, antimicrobial activity of Indigofera spalathoids Vahl., (Fabaceae) Int J Biol Technol. 2010; 1: 12-15.
- Brown JE, Rice-Evans CA. Luteolin-Rich artichoke extract protects low density lipoprotein from oxidation in vitro. Free Radic Res. 1998; 29: 247-255.
- Cerutti PA. Oxidant stress and carcinogenesis. Eur J Clin Invest. 1991; 21: 1-5.
- Halliwell B. Oxidative stress, nutrition and health. Experimental strategies for optimization of nutritional antioxidant intake in humans. Free Radical Res. 1996; 25: 57-74.
- Maritim AC, Sanders RA, Watkins III JB. Diabetes, Oxidative Stress, and Antioxidants: A Review. J Biochem Mol Toxicol. 2003; 17: 24-38.
- Shekhar HU, Goto M, Watanabe J, Konishide-Mikami I, Bari ML, Takano- Ishikawa Y. Multi food functionalities of Kalmi Shak (Ipomoea aquatica) grown in Bangladesh. Agric Food Anal Bacteriol. 2011; 1: 24-32.
- Samatha T, Acharya RS, Srinivas P, Ramaswamy N. Quantification of total phenolic and total flavonoid contents in extracts of Oroxylum indicum L. Kurz. Asian J Pharm Clin Res. 2012; 5: 177-179.
- Patay EB, Sali N, Koszegi T, Csepregi R, Bal´azs VL, Nemeth TS, et al. Antioxidant potential, tannin and polyphenol contents of seed and pericarp of three Coffea species. Asian Pac J Trop Med. 2016; 9: 366-371.
- Shirwaikar A, Kuppusamy R, Punitha IS, Rajendran K. In-vitro Antioxidant Studies on Tetra Isoquinoline Alkaloid Berberine. Biol Pharm Bull. 2006; 29: 1906-1910.
- Goh SH, Chuah CH, Mok JSL, Soepadmo E. Malaysian medicinal plants for the treatment of cardiovascular diseases, Pelanduk, Malaysia. 1995; 63.
- Mackeen MM, Ali AM, El Sharkawy SH, Manap MY, Salleh KM, Lajis NH, et al. Antimicrobial and cytotoxic properties of some Malaysian traditional vegetables (ulam), International Journal of Pharmacognosy. 1997; 35: 174- 178.
- Ong HCU, Nordiana M. Malay ethno-medico botany in Machang, Kelantan, Malaysia. Fitoterapia. 1999; 70: 502-513.
- Alsarhan AN, Sultana N, Kadir MRA, Aburjai T. Ethno pharmacological Survey of Medicinal Plants in Malaysia, the KangkarPulai Region. Int J Pharmacol. 2012; 8: 679-686.
- Sofowara A. Medicinal plants and Traditional medicine in Africa, Spectrum Books Ltd. Ibadan, Nigeria. 1993.
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic acid-phosphotungstic acid reagents. Am J Enol Viticult. 1965; 16: 44-158.
- Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in Mulberry and their scavenging effect on superoxide radicals. Food chem. 1999; 64: 555-559.
- Hollman Peter CH. Evidence for health benefits of plant phenols: local or systemic effects. J Sci Food Agric. 2001; 81: 842-852.
- Robak J, Gryglewski RJ. Flavonoids are scavengers superoxide anions. Biochem Pharmacol. 1998; 37: 837-841.
- Garatt DC. The quantitative analysis of drugs. 3rd Edn. Japan: Chapman and Hall: 1964.
- Oyaizu M. Studies on products of browning reaction: antioxidant activities of products of browning reaction prepared from glucoseamine. Jpn J Nutr. 1986; 44: 307-315.
- Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem. 1999; 269: 337- 341.
- Jia W, Gao W, Tang L. Antidiabetic herbal drugs officially approved in China. Phytother Res. 2003; 17: 1127-1134.
- Afolayan AJ, Jimoh FO, Sofidiya MO, Koduru S, Lewu FB. Medicinal potential of the root of Arctotis arctotoides. Pharm Biol. 2007; 45: 486-493.
- Omken B, Sigva HO, Mutlu S, Doganlar S, Yemenicioglu A, Frary A. Total antioxidant activity and total phenolic contents in different Turkish eggplant (Solanum melongena L.) cultivars. Int J Food Prop. 2009; 12: 616-624.
- Rice-Evans C, Miller N, Paganga G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997; 2: 152-159.
- Benavente-Garcia O, Castillo J, Marin FR, Ortuno A, Del-Rio JA. Uses and properties of Citrus flavonoids. J Agric Food Chem. 1997; 45: 4505-4515.
- Nanjo F, Goto K, Seto R, Suzuki M, Sakai M, Hara Y. Scavenging effects of tea catechins and their derivatives on 1,1- diphenyl-2-picrylhydrazyl radical. Free Radic Biol. 1996; 21: 895-902.
- Jao CH, Ko WC. 1, 1-Diphenyl-2-Picrylhydrazyl (DPPH) radical scavenging by protein hydrolyzates from tuna cooking juice. Fish Sci. 2002; 68: 430-435.
- Tung YT, Wub HJ, Hsieh C, Ping-Sheng CPS, Chang ST. Free radicalscavenging phytochemicals of hot water extracts of Acaciaconfusa leaves detected by an on-line screening method. Food Chem. 2009; 115: 1019-1024.
- Lekshmi RK, Mini S. Evaluation of antioxidant and antiglycation activities of various solvent fractions of Cissus quadrangularis stems. Int J Pharm Bio Sci. 2013; 4: 1259-1268.
- Lee JC, Kim HR, Kim J, Jang YS. Antioxidant property of an ethanol extract of the stem of Opuntia ficus-indica var. saboten. J Agric Food Chem. 2002; 50: 6490-5496.
- Perez Gutierrez RM, Flores Cotera LB, Neira Gonzalez AM. Evaluation of the Antioxidant and Anti-glication Effects of the Hexane Extract from Piper auritum Leaves in vitro and Beneficial Activity on Oxidative Stress and Advanced Glycation End-Product-Mediated Renal Injury in Streptozotocin- Treated Diabetic Rats. Molecules. 2012; 17: 11897-11919.
- Huie R, Padmaja S. The reaction NO with superoxide. Free Rad Res Comms. 1993; 18: 195-199.
- Shahriar M, Akhter S, Hossain I, Haque A, Bhuiyan MA. Evaluation of in vitro antioxidant activity of bark extracts of Terminalia arjuna. J Med Plants Res. 2012; 6: 5286-5298.
- Yildirim A, Mavi A, Oktay M, Kara AA, Algur OF, Bilaloglu V. Comparison of antioxidant and antimicrobial activities of Tilia (Tilia argentea Desf Ex DC), Sage (Salvia triloba L.), and bark Tea (Camellia sinensis) extracts. J Agirc Food Chem. 2000; 48: 5030-5034.
- Dorman HJD, Peltoketo A, Hiltunen R, Tikkanen MJ. Characterization of the antioxidant properties of deodorization aqueous extracts from selected Lamiaceae herbs. Food Chem. 2003; 83: 255-256.
- Duh PD, Tu YY, Yen. Antioxidant activity of the aqueous extract of harng Jyur (Chrysanthemum morifolium Ramat.). Lebensmittel-Wissenschaft and Techno. 1999; 32: 269-277.
- Nisha P, Mini S. In Vitro Antioxidant and Antiglycation Properties of Methanol Extract and Its Different Solvent Fractions of Musa paradisiaca L. (Cv. Nendran) Inflorescence. Int J Food Prop. 2014; 17: 399-409.
- Jayaprakasha GK, Singh RP, Sakariah KK. Antioxidant activity of grape seed (Vitis vinifera) extracts on peroxidation models in vitro. Food Chem. 2001; 73: 285-290.
- Oktay M, Gulcin I, Kufrevioglu OI. Determination of in vitro antioxidant activity of fennel (Foeniculum vulgare) seed extracts. LWT - Food Sci. Technol. 2003; 36: 263-271.