
Research Article
J Plant Chem and Ecophysiol. 2017; 2(2): 1017.
Inoculation with Pythium irregulare Increases the Water Use Efficiency of Wheat Exposed to Post-Anthesis Drought
Aldahadha AM*, Backhouse D and Warwick NWM
School of Environmental and Rural Science, University of New England, Australia
*Corresponding author: Aldahadha AM, School of Environmental and Rural Science, University of New England, Armidale, NSW 2351, Australia
Received: September 18, 2017; Accepted: October 16, 2017; Published: October 27, 2017
Abstract
The hypothesis that root rot caused by Pythium irregulare reduces the water use efficiency of wheat was tested in a system which simulated field conditions with late season water stress. Inoculation with Pythium significantly reduced transpiration during vegetative growth, so that plants entered post-anthesis drought with more available water. Although weekly transpiration rates were higher in inoculated plants than controls during the later stages of drought, infected plants were unable to make use of all of the extra water. There were no significant effects of inoculation on shoot biomass or grain yield, while total transpiration was reduced by 14%. Infected plants therefore had significantly higher integrated water use efficiency (grain yield relative to transpiration) than controls. Infected plants were significantly more stressed than controls during the drought, despite higher soil moisture, and showed reduced ability to use stomatal conductance to regulate leaf water potential. Pythium infection caused adverse changes to plant water use and water relations, but these did not translate into reductions in growth or yield. This, and the unexpected increase in water use efficiency, highlights the need to consider interactions with other environmental stresses when making assumptions about the effects of root diseases on crop productivity.
Keywords: Wheat; Pythium; Root rot; Water relations; Photosynthesis; Drought
Introduction
Wheat (Triticumaestivum) is one of the most important food crops in the world. In rainfed agriculture, the potential yield of wheat is limited by availability of water, with an attainable Water Use Efficiency (WUE) of 22 kg grainha-1mm-1 having been calculated for dry areas in Australia, North America, China and the Mediterranean basin (Sadras and Angus 2006). However, the average WUE in each of these areas is less than half of this (Sadras and Angus 2006). Among the constraints that potentially limit the ability of wheat to make effective use of available water is root disease. While the effect of various root diseases on the yield of wheat is well established, there has been very little examination of their effect on water relations and WUE.
[1] showed that cereal cyst nematode greatly reduced the transpiration of wheat plants, and that this effect could be mimicked by root pruning, suggesting that disease reduced the effective volume of the root system. On the other hand, [2] found a relatively small effect of inoculation with Gaeumannomyces graminis var. tritici (cause of take-all) on transpiration in wheat. However, there was a larger effect on carbon assimilation rates, leading to a reduction in instantaneous WUE (ratio of carbon assimilation to transpiration measured at the individual leaf level; [2]). In previous work [3] we have studied the effects of Pythium irregulare on water relations of wheat in a hydroponic system. Root infection with Pythium reduced rates of transpiration and carbon assimilation, and shoot biomass, but did not significantly affect WUE [3].
In the field, the effects of root damage caused by disease may be modified by variation in soil moisture. In seasons with higher soil moisture, water uptake by wheat affected by take-all may be higher than in drier seasons, even though the higher soil moisture increases disease severity [4]. Root pruning experiments on wheat [5,6] have shown that root damage prior to anthesis reduces transpiration during vegetative growth but under water-limited conditions allows increased transpiration during grain fill with consequent increases in grain yield and overall WUE. These observations suggest that the effect of root diseases on plant water relations could have complex interactions with the soil environment, especially the availability of water during key growth stages.
The objective of this study was to investigate the effect of infection with Pythium on WUE, water relations and other physiological parameters of wheat plants subjected to post-anthesis drought in pots which allowed rooting depths similar to those found in the field. The experiments tested the hypothesis that the infection with Pythium will reduce water uptake and water use efficiency. This study was divided into two experiments. It was unknown how much Pythium was needed for inoculating the plants. Therefore, different inoculum densities were used in the first experiment. In the second experiment, a few modifications were made due to high variability related to the effects of Pythium, therefore, more replicates with a single and higher inoculum dose were used. The watering regime was modified, based on experience with the first experiment, so that all pots received exactly the same amount of water during the course of the experiment, as would occur in the field.
Materials and Methods
Preparation of inoculum
Pythium irregulare was isolated from a seedling of triticale (xTriticosecale) at the Laureldale Research Farm, Armidale NSW, Australia. Millet seeds were soaked in distilled water for 12 h, drained and then placed in sterilized glass Petri dishes. These were wrapped with aluminum foil and autoclaved on two consecutive days. Each plate was inoculated with three plugs of Pythium irregulare grown previously on Potato Dextrose Agar (PDA) media. Penicillin G (1 ml at 0.005 g ml-1) was added to each plate after filtering with a MILLEX®GS (0.22 μm pore size) filter, to inhibit bacterial growth. All the plates were kept in an incubator for 7-10 d at 25ºC in the dark and then dried for 30 min under a filtered air flow.
Soil preparation and soil inoculation with Pythium
Pots were made from lengths of PVC pipe, 15 cm in diameter x 100 cm in height, with a cap with drainage holes at the bottom. The depth of these pots is within the range of maximum rooting depths reported for wheat in field experiments [7]. Pots were placed in a glasshouse bay which was set with an average maximum temperature of 25ºC and an average minimum of 18ºC. The average relative humidity was approximately 60%. In the first experiment, five levels of Pythium inoculum were used (0, 0.1, 0.5, 2 and 5 gpot-1) and mixed well into the top 20 cm of soil (sandy loam: peat (3:1 V/V)). Soil pH was adjusted to 6.4 with agricultural lime and Granular N:P:S (14.3:12:10.5) Starter 15 fertiliser was applied to the soil mixture at a rate of 13 g m-2. Six replicates were used for each inoculum density treatment. The original weight of the soil was about 16 kg for each pot before watering. Pots were watered to field capacity with 6 l of water before sowing. Three surface sterilized spring wheat seeds cv. Janz were sown in each pot on 18 December 2009, and later thinned to two plants at the two-leaf stage, GS 12 on the Zadoks scale [8]. This gave a plant density equivalent to 125 plants m-2. Plastic beads were placed on the soil surface to a depth of 2 cm to reduce soil evaporation.
In the second experiment, 12 pots were prepared with Pythium inoculum and 12 pots for controls. Each pot was filled with 16 kg of soil. The same type of soil and pots, and growing conditions were used as in the first experiment. Ten grams of Pythium inoculum was mixed into the soil to a depth of 30 cm and another 10 g of autoclaved millet seeds were added to uninfected (control) pots. Water was added to each pot to bring them to field capacity just before sowing. Three sterilized wheat seeds (cv. Janz) were sown in each pot on 1 May 2010, and later thinned to two plants at the two-leaf stage, GS 12 on the Zadoks scale. Plastic beads were placed on the soil surface to a depth of 2 cm to reduce soil evaporation.
Water regime
In both experiments, the Evapotranspiration (ET) of all pots was measured regularly during the experiment every 2-3 d. In the first experiment, the pots were re-watered to field capacity. Each pot was weighed prior to re-watering to determine the amount of water lost since last watering. The pots were then watered to excess and allowed to drain overnight. The following morning they were re-weighed. The amount of water lost through soil evaporation was monitored by weighing six unplanted pots, with polystyrene bead coverings. Transpiration was determined by subtracting water loss of unplanted pots from that of planted pots. Drought was imposed by completely withholding watering from full anthesis stage, GS 69 on the Zadoks scale (8 February 2010) until harvest on 15 March 2010 (approximately six weeks).
In the second experiment, pots were watered according to the amount of water lost by the treatment with lowest mean ET, which was the inoculated treatment. This ensured that both treatments were supplied with the same volume of available water during the experiment, as would occur in the field. Drought was imposed by completely withholding watering from the full anthesis stage, GS 69 on the Zadoks scale (7 July 2010) until maturity or harvest on 18 August 2010 (approximately six weeks).
Measurements
In both experiments, counts were made of heads and grain and shoot mass was also determined by drying for 2 days at 80ºC. Harvest index was calculated as grain weight divided by the total of grain weight plus shoot dry weight. Integrated water use efficiency was calculated based on both a grain weight and total shoot (grain plus vegetative) dry weight divided by total transpiration.
Plant water relations and photosynthetic measurements were made only in the second experiment. Pre-dawn and mid-day water potential (Ψ) were measured once a week for the four week drought period. Pre-dawn Ψ was measured at 6.30 am and midday Ψ between 11 am and 12 pm, using a Soil Moisture Equipment Corporation Scholander type pressure chamber. Two flag leaves were sampled (one flag leaf for predawn and the other for midday Ψ) from each of three replicates of either treatment (controls and Pythium). The leaves were wrapped with aluminum foil in the glasshouse and returned immediately to the laboratory for measurement of water potential.
Photosynthesis (carbon assimilation), stomatal conductance, internal carbon dioxide concentration and transpiration rate were measured using a portable photosynthesis system (LICOR-6400XT). The measurements were taken between 10:30 am and 11:30 am on days with full sun, except during the last week of drought when there was full cloud cover, from 6 replicates of each treatment.
The experimental design was a completely randomised design. Treatment effects were analysed by ANOVA in the first experiment. Treatments were compared by t-tests in the second experiment.
Results
Experiment 1: water use and yield
There was no overall significant effect of inoculum density on Transpiration Per Week (TPW), except in the weeks ending 40 and 66 Days After Sowing (DAS) (Figure 1). Controls and 0.1 g treatments had significantly higher transpiration (P < 0.05) than higher inoculum densities at 40 DAS (during tillering). However, controls and 0.1 g treatments had significantly lower transpiration (P < 0.05) than other inoculum densities in the week ending 66 DAS (during grain fill). There was no significant effect of inoculum density of Pythium on Cumulative Transpiration (CT) when compared with controls (data not shown) at any time from tillering until harvest.
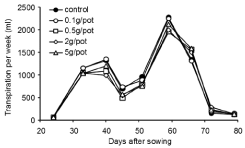
Figure 1: Experiment 1: Effect of inoculum density of Pythium on weekly
transpiration rate of wheat cv. Janz from tillering until harvest.
There was no significant effect of inoculum density on yield components or on integrated WUE calculated on either a grain weight or total shoot dry weight basis (data not shown).
Experiment 2: water use and yield
There was no significant difference between treatments in the Transpiration Per Week (TPW) during vegetative growth from the weeks ending 17 DAS until 47 DAS (Figure 2). However, TPW was significantly higher (P < 0.01) for controls than Pythium during reproductive growth from the weeks ending 55 DAS until 74 DAS, except in the week ending 67 DAS where P = 0.056. There were no significant differences in TPW between controls and Pythium in the weeks ending 82 to 87 DAS, which were 7-14 days after drought was imposed. Transpiration was very low in the week ending 87 DAS because of heavily overcast conditions. At the later stages of grain fill, plants inoculated with Pythium had significantly higher TPW than controls in the weeks ending 95 DAS (P < 0.01) and 101DAS (P < 0.05).
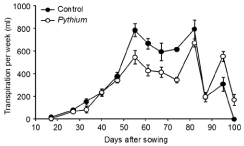
Figure 2: Experiment 2: Effect of inoculation with Pythium at 10 g/pot on
transpiration per week of wheat cv. Janz from the three-leaf stage (17 DAS)
until harvest (101 DAS). Error bars show standard errors (n = 12).
Cumulative transpiration of the control treatment from the threeleaf stage to late anthesis, when drought was imposed, was 2907 ml. This was significantly (P < 0.01) reduced to 2128 ml by inoculation with Pythium, so that the Pythium treatment entered the drought with almost 780 ml more water per pot than the controls. Cumulative transpiration at harvest was significantly (P < 0.001) higher in the controls (4809 ml) than in the Pythium treatment (4117 ml). During the period of water stress from anthesis until harvest, there was no significant difference between treatments in the total amount of water transpired. Inoculated pots contained approximately 600 ml more water than control pots at harvest maturity.
There were no significant differences between controls and Pythium for yield components (Table 1). However, plants inoculated with Pythium had significantly higher integrated WUE (P <0.01) than controls, when based on grain yield, and significantly higher WUE (P <0.05), when based on dry matter (shoot plus grain).
Treatments
HN/pot
GW (g)
TDW (g)
WUEgrain (g/l)
WUEDM (g/l)
HI
Control
12.7
10.3
20.6
2.15
4.3
0.50
Pythium
12.5
11.2
22.4
2.7
5.2
0.52
Level of significance
ns
ns
ns
<0.01
<0.05
ns
Table 1: The number of heads per pot (HN/pot), Grain Weight (GW), Total Dry Weight (TDW), Water Use Efficiency based on grain (WUE grain), Water Use Efficiency based on total dry weight (WUE DM), and Harvest Index (HI) for wheat cv. Janz after inoculation with 10 g of Pythium per pot.
Plant water relations
The results of predawn water potential (Ψ) (Figure 3a) showed no significant differences between controls and Pythium during 4 weeks of water stress. There was also no significant difference in midday water potential (Ψ) between both treatments at 0 and 7 days after drought but the difference was significant at 14 and 21 days after withholding water (Figure 3b). After 14 and 21 days of drought, controls had significantly higher Ψ (P <0.05) than Pythium inoculated plants. Water potential of plants at the end of drought was lower than at the beginning of stress. In general, the average of predawn Ψ was higher than midday Ψ for both treatments and during all the days of measurement.
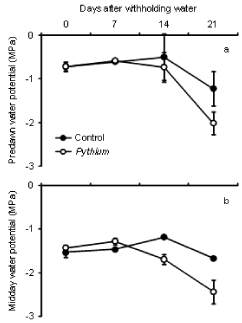
Figure 3: Effect of inoculation with Pythium on predawn (a) and midday (b)
water potential of wheat cv. Janz after withholding water. Error bars show
standard errors (n = 3).
Physiological measurements
Pythium inoculation only had a significant effect on photosynthetic rate (A) (P < 0.01) at the beginning of the drought period (day 0 after withholding water). The photosynthetic rate of controls (Figure 4a) was significantly higher (21.65 μmol CO2 m-2s-1) than Pythium (13.62 μmol CO2m-2s-1). However, there was no significant (P > 0.05) effect of disease on photosynthetic rate at 7, 14 and 21 days of drought. The photosynthetic rate decreased as the drought period lengthened and reached a minimum (<0 μmol CO2m-2s-1) after three weeks of stress as the measurement was taken under full cloud cover.
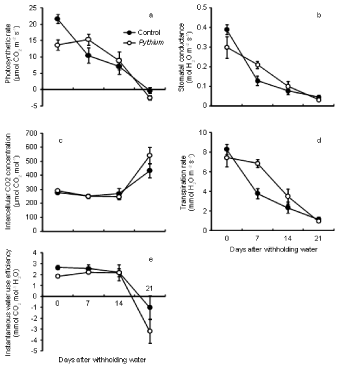
Figure 4: Effect of inoculation with Pythium on photosynthetic rate (a), stomatal conductance (b), intercellular CO2 concentration (c), transpiration rate (d) and
instantaneous water use efficiency (e) of wheat cv. Janz after withholding water. Error bars show standard errors (n = 6).
There was no significant effect of disease on stomatal conductance (gs) at all days of water stress period except 7 days after the drought began, where plants inoculated with Pythium had significantly higher gs (P < 0.05) than controls (Figure 4b). Stomatal conductance was decreased for both treatments after 7 days of drought, as compared with the beginning of stress. The lowest stomatal conductance was 0.03 mol H2O m-2 s-1 for Pythium inoculated plants and 0.05 mol H2O m-2 s-1 for controls after 3 weeks of drought.
Pythium treatment had no significant effect on intercellular CO2 concentration [CO2]i during the drought period (Figure 4c). [CO2] i ranged from 252 to 279 μmol CO2mol-1 in controls and from 247 to 306 μmol CO2mol-1 in Pythium-inoculated plants after two weeks of water stress. However, [CO2]i increased above ambient levels at 21 days, presumably due to respiration.
Instantaneous transpiration rate (E) did not differ significantly between both treatments during water stress except after 7 days of drought, when the transpiration rate of Pythium (5.9 mmol H2O m-2s- 1) was significantly higher (P < 0.01) than controls (3.8 mmol H2O m-2s-1) (Figure 4d).
Instantaneous water use efficiency (A/E) did not differ significantly between Pythium inoculated plants and controls during stress, except at day 0 of the drought, when control plants had significantly higher values (P < 0.01) than Pythium (Figure 4e). Instantaneous WUE varied from 2.2 to 2.6 mmol CO2/mol H2O for controls, and from 1.3 to 2.2 mmol CO2/mol H2O for Pythium inoculated plants. However, A/E was reduced at 21 days to less than 0 mmol CO2/mol H2O for both treatments because of negative carbon assimilation values during cloudy weather.
There was a significant relationship between midday leaf water potential and stomatal conductance in the Pythium treatment but not in controls (Figure 5a,b). Stomatal conductance varied over a wide range of values in controls, while midday water potential remained in a narrow range. In inoculated plants, stomatal conductance decreased as midday water potential decreased.
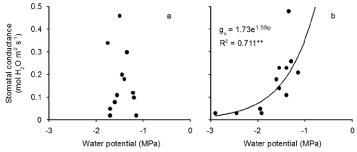
Figure 5: Relationship between stomatal conductance and midday leaf water potential during the 21 day water with holding period after anthesis of wheat cv. Janz
either uninoculated (a) or inoculated (b) with Pythium. ** R2 significant at P< 0.01.There was no significant relationship between gs and water potential in Figure 5a.
The relationships between photosynthesis or carbon assimilation (A) and transpiration rate (E) (Figure 6a,b) and between photosynthesis and stomatal conductance (gs) (Figure 7a,b) are presented for both inoculated and uninoculated plants during 4 weeks of water stress. In both treatments, as E increased, A also increased. Similarly, A increased as gs increased. The effect of inoculation on the relationships between photosynthesis and transpiration rate was tested using multiple regression. Photosynthetic rate was regressed against ln(E) and ln(E) x inoculation. A similar analysis was done for stomatal conductance. A significant interaction term indicates that the relationship differs between the two treatments. Inoculation had a significant (P < 0.01) effect on the relationship between photosynthesis and transpiration rate, but not on the relationship between photosynthesis and stomatal conductance. Photosynthetic rate was higher for a given E in controls than it was in the Pythium treatment.
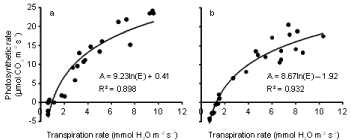
Figure 6: Relationship between photosynthetic rate and transpiration rate during the 21 day water withholding period after anthesis of wheat cv. Janz either
uninoculated (a) or inoculated (b) with Pythium. Both R2 are significant at P< 0.01.
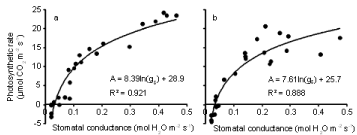
Figure 7: Relationship between photosynthetic rate and stomatal conductance during the 21 day water withholding period after anthesis of wheat cv. Janz either
uninoculated (a) or inoculated (b) with Pythium. Both R2 are significant at P< 0.01.
Discussion
The first experiment had been set up to test for interactions between root damage caused by Pythium and drought stress after anthesis on water relations of wheat. While the inoculation treatments generally reduced transpiration prior to anthesis, the effect was small and was only significant during tillering. However, there was an unexpected result in that the higher levels of inoculum increased transpiration during the second week of the drought treatment. This led to a modified hypothesis that reduction of transpiration by root disease during early growth could allow more water to be available during post-anthesis drought. This was tested in the second experiment, which was modified by using a higher rate of inoculum, and by controlling the total amount of water available to the plants rather than regularly rewatering to field capacity prior to imposing drought.
In the second experiment, there was a clear reduction in transpiration rate from tillering until early post-anthesis drought due to inoculation with Pythium. Transpiration was higher in the inoculated plants at the end of the drought period, but overall there was a reduction of 14% of total water use in the Pythium treatment when compared with controls. The infected plants entered the drought with significantly more water available. As a consequence, yield was not significantly affected and calculated water use efficiency increased. However, the infected plants were not able to make use of all of the extra water available after anthesis.
The effects of Pythium on water use of wheat in these experiments were similar to the effects of root pruning reported by [5,6]. However, in the root pruning experiments there was an increase in yield, presumably because continued growth of roots after anthesis allowed access to more of the extra water stored in the soil. It is likely that infection with Pythium prevented root systems from continuing to explore all of the soil in the pots after anthesis, although it was not possible to extract root systems in a way that could have confirmed this. Pythium species are known to decay root tips and reduce rates of root extension in wheat (Charmswarng and Cook 1985). In a hydroponic system, P. irregulare also reduced the ability of a given mass of root to uptake water [3].
Grain yield depends on water availability at critical times. Passioura [9] indicated that the grain yield of wheat depends more on water use after anthesis than on the total amount of water used by the plant. This was clearly seen in the second experiment. There was no difference between infected and uninfected plants in the total amount of water transpired between anthesis and harvest, which was reflected in the similar yields in the two treatments.
The effect of Pythium on water use efficiency differed between integrated WUE and instantaneous WUE. When total biomass or grain production was compared with total transpiration, WUE was increased. This measure is of most relevance to overall crop management. However, direct measurements of instantaneous WUE at the start of and during the drought period did not show significant differences between controls and inoculated plants, consistent with previous studies [3]. When the relationship between photosynthesis and transpiration rate was plotted, there was evidence of higher WUE in control plants. Measurements of WUE at single points in time can therefore give a misleading idea of the effect that disease is having on the overall ability of the plant to make best use of the available water. This is consistent with the argument of [10] that effective use of water may be a more important goal in dealing with drought stress than increased water use efficiency.
Pythium significantly reduced midday water potential 14 and 21 days after watering was stopped. During this period, the difference in pot weight (due to increased water in the Pythium treatment) between the treatments was at its greatest. This confirms that Pythium was increasing water stress due to drought even though more water was present in the pots than in controls. In this study, there was a significant relationship between midday leaf water potential (Ψ) and stomatal conductance (gs) in wheat infected with Pythium but not in controls because uninfected plants varied gs to maintain constant leaf Ψ while inoculated plants appeared unable to do this [11].
The results indicated that the inoculation of plants with Pythium caused a significant reduction in photosynthetic rate per unit leaf area at the time when watering stopped but not during the drought. Balota et al, (2005) found higher reduction in carbon assimilation under high soil moisture than low moisture in wheat diseased by the soil-borne fungus Gaeumannomyces graminis. This suggests that diseased plants under high moisture conditions experienced more severe stress from the disease than plants in drier soil. This could be due to higher levels of disease severity in wetter soils, but this effect would not apply in the experiment described here. The most likely explanation for a lack of difference in photosynthetic rate between inoculated plants and controls during the drought was that inoculated plants had higher stomatal conductance and transpiration.
References
- Amir J, Sinclair TR, Thomas R. Cereal cyst nematode effects on wheat water use, and on root and shoot growth. Field Crops Research. 1996; 47: 13-20.
- Balota M, Rush CM, Payne WA, Lazar MD. The effect of take-all disease on gas-exchange rates and biomass in two winter wheat lines with different drought response. Plant and Soil. 2005; 275: 337-348.
- Aldahadha AM, Warwick NWM, Backhouse D. Effects of Pythium irregulare and root pruning on water-use efficiency of hydroponically grown wheat under PEG-induced drought. Journal of Phytopathology. 2012; 60: 397-403.
- Pillinger C, Paveley N, Foulkes MJ, Spink J. Explaining variation in the effects of take-all (Gaeumannomyces graminis var. tritici) on nitrogen and water uptake by winter wheat. Plant Pathology. 2005; 54: 491-501.
- Fang Y, Xu B, Turner NC, Li F. Grain yield, dry matter accumulation and remobilization, and root respiration in winter wheat as affected by seeding rate and root pruning. European Journal of Agronomy. 2010; 33: 257-266.
- Ma SC, Li FM, Xu BC, Huang ZB. Effect of lowering the root/shoot ratio by pruning roots on water use efficiency and grain yield of winter wheat. Field Crops Research. 2010; 115: 158-164.
- Kirkegaard JA, Lilley JM. Root penetration rate - a benchmark to identify soil and plant limitations to rooting depth in wheat. Australian Journal of Experimental Agriculture. 2007; 47: 590-602.
- Zadoks JC, Chang TT, Konzak CF. A decimal code for the growth stages of cereals. Weeds Research. 1974; 14: 415-421.
- Passioura JB. Grain yield, harvest index, and water use of wheat. Journal of the Australian Institute of Agricultural Science. 1977; 43: 117-120.
- Blum A. Effective Use of Water (EUW) and not Water-Use Efficiency (WUE) is the target of crop yield improvement under drought stress. Field Crops Research. 2009; 112: 119-123.
- Jones HG. Stomatal control of photosynthesis and transpiration. Journal of Experimental Botany. 1998; 49: 387-398.