
Research Article
J Plant Chem and Ecophysiol . 2024; 4(1): 1023.
Exploring the Potent Triad: Antibacterial, Antifungal and Phytotoxic Activities of Ribes Himalense Decne from Thandiani, Abbottabad, Pakistan
Nimra Zeb¹; Asad Ullah¹; Safeer Ullah¹*; Ghulam Mujtaba Shah²; Zulfiqar Ahmad³; Maryam Shakir¹; Khaleel Ur Rehman¹; Nazia Zeb¹
¹Centre of Plant Biodiversity, University of Peshawar, Pakistan
²Department of Botany, Hazara University, Pakistan
³Department of Botany, GPGC No.1 Abbottabad, Pakistan
*Corresponding author: Safeer Ullah, Centre of Plant Biodiversity, University of Peshawar, Pakistan. Email: safeercpb1990@uop.edu.pk
Received: August 27, 2024 Accepted: September 17, 2024 Published: September 24, 2024
Abstract
The current studies were conducted to highlight biological activities of Ribes himalense Decne. including antibacterial, antifungal and phytotoxic potentials. Antibacterial studies revealed that the inhibitory effect for ethanolic crude extract of leaves of Ribes himalense was 16mm, 16mm, 14mm and 17mm against Escherichia coli, Proteus mirabilis, Streptococcus mutans and Bacillus subtilis respectively. The ethanoic extracts of stem showed 15mm, 18mm, 13mm and 16mm against, Escherichia coli, Proteus mirabilis, Streptococcus mutans and Bacillus subtilis respectively. It was noted that the ethanolic extract of leaves Ribes himalense showed significant inhibition of 15mm against Candida albicans while the inhibition zone of stem was 12mm. It was tested and revealed that the aqueous ethanolic extract of Ribes himalense showed inhibition against the growth of Lemna minor, which was used as test plant during the research. The phytotoxic activities of the plant showed that 10μI extract caused 0 inhibition of growth and 10% by the leaves and stem respectively. At 100μI extract the growth inhibition was showed by 30% and 10% by the leaves and stem extracts of Ribes himalense respectively. It was further studied that at 100μI extracts the inhibition of growth was 70% and 100% by the leaves and stem respectively. Cytotoxic studies of the research plant were conducted and revealed that the ethanlic extract of stem is more cytotoxic than the leaves. At 500μg/mL, 1000 μg/mL and 2000μg/mL ethanolic extrac of leaves showed about 90% cytotoxic effect while at 62. 5μg/mL, 125μg/mL and 250μg/mL cytotoxic effects were 30%, 50% and 70% respectively. In case of ethanolic extract of stem at the concentration of 2000μg/mL, 1000μg/mL, 500μg/mL, 250μg/mL and 125μg/mL cytotoxic effect was 90% for each, while 80% cytotoxicity was observed at the concentration of 62.5μg/mL.
Keywords: Antifungal; Antimicrobial; Biological activities; Cytotoxic; Phytotoxic; Ribes himalense
Introduction
Abbottabad is named after Major James Abbott, the first deputy commissioner of Hazara (1849-1853). The area mostly lies on Eurasian land plate and Iranian plateau but the eastern part is close to the Indian subcontinent [1]. The geographic coordinates of the area are 73°13′E longitude and 34°09′N latitude, at an elevation of 1,256 m (40121 ft). Summers are pleasant with heavy monsoon season followed by snowfall in winters [2]. Thandiani is located in the moist temperate forest in Pakistan with rich plant diversity [3]. Ribes himalense Decne. is a member of family Grossulariaceae, represented by one genus and eight species in Pakistan. Ribes himalense Decne. can be found in Kalam, Gilgit baltistan, Kaghan, Swat, Changli gali and Hazara. It is very commonly found in North Western Himalayas, between 2400-4000 m altitudes. Medicinal plant species have been widely used for many centuries across the globe [4]. Plant extracts are used in developing therapeutic medicines which work against wide range of microbes and infectious diseases [5]. Certain plant species contain polyphenols, alkaloids and volatile oils which are used as antibacterial agents. These plant extracts stop the growth of bacterial strains and kill them, leaving no harmful effects on human health [6]. Phytochemical extractions are very helpful against bacterial infections [7] and it is necessary to find new antibiotics because certain drug-resistant drugs are causing havoc and epidemics [8]. Pathogenic fungi is causing severe threats to animal’s and plant’s health [9]. Currently research interest has been on peak to isolate useful extracts from plant species to fight against pathogenic fungi which possess antifungal activities with least side effects on human and animals health [10]. Phytotoxic activity inhibits the growth, development/germination of plant or plant parts. Many chemicals, particularly phenolic and their products, possess high phytotoxic effects [11]. Phytotoxic chemicals released by plant species may be useful in weed management, either solitary or in combination [12]. Allelopathy is the study where plant toxic allelochemicals are studied which poses negative effects on neighboring plants [13]. Many plants are used as effective organic herbicides which have sufficient phytotoxic potency [14]. The focus of pharmacological research today is to use plant extracts to cure and prevent acute and chronic diseases [15,16]. Many in-vitro and in-vivo experiments revealed the anti-inflammatory, anti-cancer and antioxidant properties of bioactive compounds of plants origin [17,18]. Medicinal plants and drugs obtained from medicinal plants have been using by human since ancient times [19]. Cancer is generally known as multicellular disease, characterized by uncontrolled cell division [20]. To reduce the toxicity and side effects of chemotherapy, alternative medicines are used. A number of chemicals have been identified from different parts of the plants which possess anti-cancer potentials, helpful in controlling the cancer [21]. An estimation shows that about 70% of the anti-cancer drugs are of plants origin and more than 3000 plant species have been identified with anti-cancer qualities [22].
A comprehensive literature review was made and it was concluded that there is no research work available on the antimicrobial, phytotoxic and cytotoxic activities of Ribes himalense Decne. therefore, the present work was designed to identify the phytochemical potentials of the plant species.
Material And Methods
Plant Collection, Preservation and Identification
Ribes himalense Decne. is a medium shrub which belongs to family Grossulariaceae. Plant specimens and vigorous stem and roots were collected from the research area. The identification was carried out by reviewing the available literature [23-25]. Plant specimens were pasted on standard herbarium sheets (17 x 11 inches) and deposited at Centre of Plant Biodiversity Herbarium for ready reference.
Field surveys and Geo-referencing
Detailed field surveys were performed between the months of August and September in 2023. Ribes himalense was sampled across the research area and GPS (Garmin Vista ETrex) was used to measure the coordinates of plant species. The distribution map was generated by ArcGIS software.
Biological Assay
PCSIR Laboratory Complex Peshawar was used to perform Biological assay as follow.
Drying and grinding
The leaves and stem of the plant species were carefully collected. Leaves and stem were washed and dried in shad at room temperature. The dried leaves and stem were grinded with grinder separately and the powder were stored separately to avoid moisture and contamination.
Extraction
The standard procedure of Ahmad et al. [26] was adopted to prepare extracts. 255.8g of leaves and 363.2g of stem powder were taken in flasks and ethanol was added which was then incubated for 2 days with periodically shaking. The mixture was then filtered through a single layered filter paper. Equal amount of ethanol was added to the first extract, kept for a day and filtered again.
The extracts were combined and dried at 40°C in rotary evaporator to evaporate ethanol and obtain crude extract individually. The dried extracts were then stored in separate desiccators and kept at 2-8 °C for use. Finally, the crude extract was dissolved in 0.1% Dimethyl Sulfoxide (DMSO; 1 mg/μl 6), which is a non-toxic and universal solvent for many antimicrobial activities.
Antibacterial Assay
Material used during the procedure were, sterile borers, micropipette, sterile water, dimethyl sulphoxide, petri plates and bacterial strains. The antibiotic (Azithromycin & Ciprofloxacin) was used to cure bacterial infection. The bacterial strains used during studies are as follow. The microorganisms were put at 37°C in petri plates in nutritional medium and incubation was conducted for about 24 hours to increase the growth of microbes. Many microbial strains were grown in separate tubes.
Antibacterial screening
Antibacterial analysis was carried out using Agar well diffusion method. Plant extracts were dissolved in DMSO. For analysis Gram positive bacteria (Bacillus subtilis: clinical isolate & Streptococcus mutans: ATCC# 35668) and Gram negative bacteria (Proteus mirabilis: ATCC# 12553 & Escherichia coli: ATCC# 8738) were considered. The sub-cultured bacteria were placed in a saline medium for an incubation period of 30 minutes at 37 °C. Lawn was arranged through streaking on Mueller–Hinton Agar (MHA) plates by dipping sterile swabs of cotton in bacterial solution. Using sterile cork borer, wells of diameter 5 mm were formed and 100μL of sample were put in each. DMSO was used as solvent to prepare stock solution and Ciprofloxacin & Azithromycin were used as positive control. The plates were kept at 37°C for 24 hours after an incubation period of 2 hours in biosafety cabinet. The inhibition zone was calculated accordingly [27,28].
Antifungal assay
The standard methods after Haq et al. [29] was followed. Candida albicans was collected from clinical isolates and PCSIR Laboratory Complex Peshawar, and was used to study the anti-fungal activity. The materials used during this activity includes, SDA (Sabouraud, Dextrose, Agar), antifungal drugs (Clotrimazole, Ciprofloxacin & Azithromycin), Autoclave, Incubator, test tubes and micropipettes.
S#
Strain which will be used
Type
ATCC#
1.
Escherichia coli
Gram negative
ATCC# 8738
2.
Proteus mirabilis
Gram negative
ATCC# 12553
3.
Streptococcus mutans
Gram positive
ATCC# 35668
4.
Bacillus subtilis
Gram positive
Clinical isolate
Table 1: Showing bacterial strains, type and ATCC#.
Phytotoxic Activity
Lemna minor L. was used to analyze the phytotoxic activities of Ribes himalense Decne. following protocols of Abbasi et al. [1]. Media composition was prepared (Table 2) and was used for the activity.
S.No
Salt
Grams
1.
Ferric chloride
0.056
2.
Boric acid
0.0028
3.
Potassium hydrogen phosphates
0.68
4.
Potassium nitrates
1.6
5.
Copper sulphates
0.023
6.
Manganese chlorides
0.0036
7.
Calcium nitrates
1.17
8.
Ethylene diamine tetra acetic acids
0.0113
9.
Manganese sulphates
0.496
10.
Zinc sulphates
0.0023
11.
Sodium molybdates
0.0012
Table 2: Composition of E-media.
Plant species
Locality
Coordinates
Altitude
Distribution in Pakistan
Ribes himalense Decne.
Thandiani-1
34. 231129o
73. 352325o2703m
Kaghan, Gilgit Agency, Swat, Kalam, Hazara, Changla Gali and Thandiani
Thandiani-2
34. 23109o
73. 352420o2704m
Table 3: Showing Plant Localities, Coordinates, Altitude and Distribution in Pakistan.
30 mg plant extract were mixed into 1.5 ml of ethanol to make stock solution. 10 plants of Lemna minor L. with three fronds were placed in 30 ml of medium. Concentrations (10μl, 100μl, 1000μl) were used for positive control medium, and extracts were put into the medium containing plants in petri dishes and were exposed to sunlight for seven days. Fronds number were examined after 7 days and contrast to the positive control. The procedure was repeated 3 times and percentage of inhibition seen was calculated by using following formula.
Cytotoxic Activity
The cytotoxic activities of Ribes himalense Decne. was performed against brine shrimp nauplii, provided by PCSIR laboratories Complex Peshawar. 20 mg of plant extract was dissolved in 2 ml ethanol to prepare stock solution and procedures were adopted after Abbasi et al. [1] & Haq et al. [30]. 38 g of sea salts, dissolved in 1000 ml of distilled water and brine shrimp eggs were placed in container with perforated divider. The eggs were placed on the dark side of the container and the hatching nauplii migrated to the light side, later on. An estimated time of 2 days were given for the hatching eggs. Test tubes were filled with stock solution in different concentrations (62.5, 125, 250, 500, 1000 and 2000 μg/mL) after 2 days and 10 freshly hatched shrimps were added to each test tube containing different concentrations of stock solution. Magnifying glass was used to see live and dead brine shrimps after 24 hours [31] [32].
Results
Field data were collected from the available sites of Ribes himalense Decne. during field visit.
The plant was found at 2 localities Thandiani-1 at 34o424/ N latitude, 73o247/ E longitude and Thandiani-2 at 34o424/ N latitude and 73o47/ E longitude at an altitude 1282 m and 1285 m respectively. The data was geo referenced with the help of GPS (Garmin Vista ETrex). Distribution, locality and sub locality maps were developed with the help of ArcGIS software (Table 1 & Figure 1).
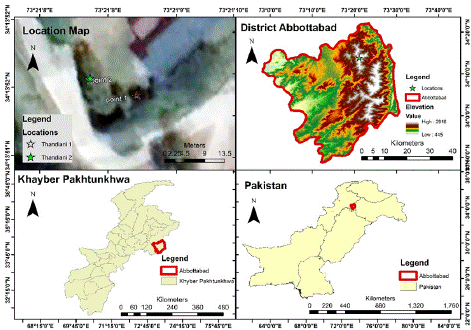
Figure 1: Location Map and geo-referencing of Ribes himalense in the study area showing 2 localities i.e., (Thandiani-1 and Thandiani-2).
Biological Assay
PCSIR Peshawar was used to carry out biological studies of Ribes himalense Decne.
Antibacterial Assay
Four strains of bacteria i.e. Escherichia coli, Proteus mirabilis, Streptococcus mutans and Bacillus subtilis were used to study the antibacterial activities. To study the zone of inhibition two replicates were used each for leaves and stem with 100mg/ml concentration. The inhibitory zone for ethanolic crude extract of leaves of Ribes himalense was scored as 16, 16, 14 and 17mm against Escherichia coli, Proteus mirabilis, Streptococcus mutans and Bacillus subtilis respectively. Maximum inhibition zone of 17mm of leaves extract was noted against Bacillus subtilis. For the ethanolic extract of stem of the Ribes himalense Decne. also showed the antibacterial activity against Escherichia coli, Proteus mirabilis, Streptococcus mutans and Bacillus subtilis which is 15, 18, 13 and 16mm respectively. Maximum inhibition zone of 18mm of stem extract was noted against Proteus mirabilis.
Antifungal Assay
Candida albicans was used as a test organism for the antifungal activity of the crude extract of stem and leaves of Ribes himalense Decne. and it showed effective results. The ethanolic extract of plant leaves significantly inhibit C. albicans fungus with a zone of inhibition of 15mm.The inhibition zone for the stem extract was 12mm. Azithromycin, Ciprofloxacin and Clotrimazole were used as a standard for antifungal activity.
The comparative antimicrobial activity of ethanolic extracts of Ribes himalense Decne. and standard antibiotics Azithromycin, ciprofloxacin and Clotrimazole against test microorganisms is shown in the (Figure 2,3 & Table 4).
S.No.
Strains used
Zone of inhibition(mm) at concentration (100mg/ml)
Standards
Leaves
Stem
Azith.
Cipro.
Clot.
(25µg)
(25µg)
(30µg)
1.
Escherichia coli,
16
15
30
43
NA
2.
Proteus mirabilis
16
18
41
43
NA
3.
Streptococcus mutans
14
13
31
43
NA
4.
Bacillus subtilis
17
16
22
40
NA
5.
Candida albicans
15
12
30
50
32.5
Table 4: Showing strains used, zone of inhibition, concentration and standards.
SOV
d.f.
S.S.
M.S.
F-Value
P-Value
Microbes
4
96.889
24.222
1.3490
0.2750
Extracts
2
4874.178
2437.089
135.7290
0.0000
Interaction
8
197.378
24.672
1.3741
0.2476
Error
30
538.667
17.956
Total
44
5707.111
Coefficient of variation (CV) = 18.79%, α value @ 0.05
Table 5: ANOVA for antimicrobial activities of leaves and stem extracts.
Figure 2: RPlot showing correlation among different standards.
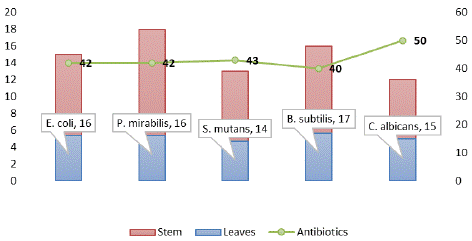
Figure 3: Antimicrobial activity of Ribes himalense Decne.
The ANOVA reveals that significant difference exists among the plant extracts and antibiotics. The leaves extract is more effective than stem extract while all the microbes are equally affected with the application of plant extract. Whereas, the interaction of both factor is non-significant.
Phytotoxic Activity
To evaluate the phytotoxic potential of the leaf extracts of R. himalense Decne. a growth bioassay was conducted against Lemna minor L. The aqueous ethanolic extracts of R. himalense Decne. markedly inhibited the growth of the test plant. The strength of the phytotoxicity of the plant extracts greatly varied by concentration. The growth of Lemna minor L. declined with increasing extract concentration (Figure 4,5). At 10 μl extract the growth of inhibition shown 0 and 10% by leaves and stem extract respectively. At 100 μl extract the growth of inhibition shown 30% and 10% by leaves and stem extract respectively. At 1000μl extract the growth of inhibition shown 70 and 100% by leaves and stem extract respectively. Paraquat solution was used as a positive control and growth inhibition of leaves and stem was 100% each. The negative control DMSO showed no phytotoxic activity as shown in the Table 6 (Figure 4 & 5).
S. No.
Extract Concentrations (µl)
Growth Inhibition by
leaves (%)Growth Inhibition by stem (%)
1.
10
0
10
2.
100
30
10
3.
1000
70
100
4.
Neg. Control Methanol
-
-
5.
Paraquat solution
100
100
Table 6: Showing extract concentration for phytotoxic activities, inhibition by leaves and stem of Ribes himalense Decne.
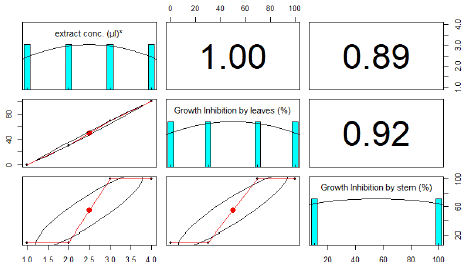
Figure 4: RPlot between growth inhibition of leaves and stem.
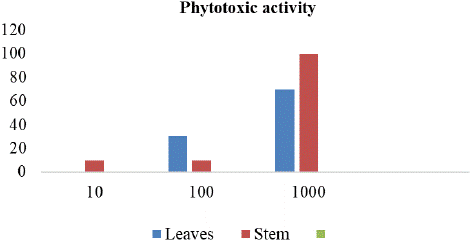
Figure 5: Phytotoxic effect of Ribes himalense Decne.
Cytotoxic Activity
Brine shrimp larvae were the test organisms for the current cytotoxicity investigation. Cytotoxic studies of the Ribes himalense Decne. was carried out and the result showed that the ethanolic extract of the stem is more cytotoxic than the leaves. At 2000, 1000 and 500 μg/mL ethanolic extract of leaves showed 90% cytotoxic effect of each, at 250, 125 and 62. 5 μg/mL cytotoxic effect was 70, 50 and 30% respectively. In case of ethanolic extract of stem at the concentration of 2000, 1000, 500, 250 and 125 μg/mL cytotoxic effect was 90% for each, while 80% cytotoxicity was observed at the concentration of 62.5 μg/mL (Table 7 & Figure 6).
Sample
% mortality after 24h (µg/mL)
2000
1000
500
250
125
62.5
Leaves
90%
90%
90%
70%
50%
30%
Stem
90%
90%
90%
90%
90%
80%
Table 7: Percent mortality of brine shrimps nauplii after 24h (μg/mL).
SOV
d.f.
S.S.
M.S.
F-Value
P-Value
Concentration
5
6125.000
1225.000
639.1304
0.0000
Extracts
1
3025.000
3025.000
1578.2609
0.0000
Interaction
5
3725.000
745.000
388.6957
0.0000
Error
24
46.000
1.917
Total
35
12921.000
Coefficient of variation (CV) = 1.75%, α value@ 0.05
Table 8: ANOVA for different concentrations of leaves and stem extracts for cytotoxic activity.
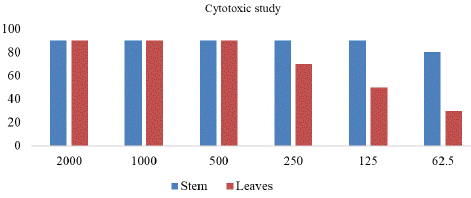
Figure 6: Cytotoxic effect of Ribes himalense Decne.
The ANOVA shows that there is significant difference among the different treatment means of concentrations and extracts from different portions (leaves and stem). Also, the interaction effect is significant. It reflects that increasing the concentration of plant extract up to certain level (500 μg/mL) will increase the cytotoxicity. The stem extract is more cytotoxic than leaves extract at low concentration (250 μg/mL and below).
Discussion
Ribes himalense Decne. belongs to family Grossulariaceae which is represented in Pakistan by 1 genus and 8 species. It is found in Kaghan, Gilgit Agency, Swat, Kalam, Hazara, Changla Gali and Thandiani. Moreover, it is very common in NW Himalayas from 2400-4000 m [33]. During the present study the Ribes himalense was found at the two localities Thandiani-1 (2703m) and Thandiani-2 (2704m), showing considerable decrease in its population. During the field studies Ribes himalense was found at Thandiani-1 and Thandiani-2 during the geo-referencing at 2703m and 2704m altitude respectively. Geo-referencing of the Punica granatum in Dir district, Khyber Pakhtunkhwa, Pakistan has been carried out by Ali et al. [34]. Similarly, scientist i.e., Ahmad et al. [35,36] carried out geo-referencing studies of some other important plant species from the other similar ecological zones.
Many researchers carried out work on the antimicrobial activities of various species. They reported that various parts of the plant are showing antimicrobial potential [37,38]. The antimicrobial activities of the Aloe vera were studied by Danish et al. [39] and Haq et al. [40], it was noted that the plant extract showed considerable results. It is noted during this study that the ethanolic extracts of leaves and stem of Ribes himalense Decne. showed more inhibitory impact on the test organisms. When both extracts were evaluated for antimicrobial potential, both shown different degrees of antimicrobial activity against the tested bacterial and fungal species. The extracts were compared with three standards antibiotics including azithromycin, ciprofloxacin and clotrimazole. The findings presented in this study are supporting the studies carried out by Muktar et al. [41] and Bereksi et al. [42]. The ethanolic extracts of plant leaves and stem significantly inhibit C. albicans which coincide with the findings of Rabie et al. [37] and Rong et al. [43].
The present study showed that the extract of Ribes himalense Decne. has phytotoxic potential. Similar findings are noted during screening of 81 species for their phytotoxic effects [44]. Results of this study showed similarity with the study of [45]. Cytotoxic activities are recorded both in case of leaves and stem extracts of Ribes himalense Decne. Like other plant species investigated by Khor et al. [46] & Nejad et al. [47] also showed cytotoxic activities including leaf extract of Moringa oleifera.
Conclusion
The ecological studies showed that the distribution range of the RH has reduced and facing severe ecological issues. The population has been drastically decreased and no regeneration of Ribes himalense at any stage has been noticed in the research area and the plant has facing regeneration issues. The ethanolic extract of leaves and stem of the plant has shown considerable antimicrobial activities. The plant has shown phytotoxic and cytotoxic activity. The various parts of plant including leaves and stem have shown considerable phytotoxic and cytotoxic activities. The research area has rich phytodiversity and it is a tourist, picnic spot and due to anthropogenic activities, the flora of the area is facing severe problems. During the studies it was observed that habitat loss is occurring due to mass scale construction, widening and melting of excess roads. Which has resulted in floral and faunal habitat loss in the research area.
Author Statements
Author Contributions
Conceptualization, N.Z., A.U., and S.U; software, S.U; validation, N. Z., and S.U.; resources, N.Z., G. M. S., Z. A., M. S. and writing—original draft preparation, N.Z., S.U. writing—review and editing, A.U., S.U., K. R. and N. Z. visualization S.U., and N. Z; supervision S.U., A. U., and N. Z.
All authors have read and agreed to the published version of the manuscript.
References
- Abbasi AM, Khan MA, Ahmad M, Qureshi R, Arshad M, Jahan S, et al. Ethnobotanical Study of Wound Healing Herbs among the Tribal Communities in Northern Himalaya Ranges District Abbottabad, Pakistan. Pak J Bot. 2010; 42: 3747-3753.
- Ullah S, Ullah A. Vascular Plant Diversity and Biological Spectrum of Galiyat Valley, District Abbottabad, Khyber Pakhtunkhwa, Pakistan. J Xi’an Shiyou Univ Nat Sci. 2023; 19: 775-788.
- Khan W, Khan SM, Ahmad H, Alqarawi AA, Shah GM, Hassain M, et al. Life Forms, Leaf Size Spectra and Diversity Indices of Plant Species Grown in the Thandiani Forests, District Abbottabad, Khyber Pakhtunkhwa, Pakistan. Saudi J Biol Sci. 2018; 25: 94-100.
- Chaachouay N, Dourira A, Zidane L. Herbal Medicine Used in the Treatment of Human Diseases in the Rif, Northern Morocco. Arab J Sci Eng. 2022; 47: 131-153.
- Shah Z, Ilyas M, Khan M, Ahmad A, Khan M, Khan N. Antimicrobial activities of selected medicinal plants collected from Northern districts of Khyber Pakhtunkhwa, Pakistan. J Pharm Res. 2012; 5: 1729-1733.
- Lee CK, Kim H, Moon KH, Shin KH. Screening and isolation of antibiotic resistance inhibitors from herb materials-resistance inhibition of volatile components of Korean aromatic herbs. Arch Pharm Res. 1998; 21: 62-66.
- Kumar A, Singh D, Rehman H, Sharma NR, Mohan A. Antibacterial, antioxidant, cytotoxicity and qualitative phyto-chemical evaluation of seed extracts of nigella sativa and its silver nanoparticles. IJPSR. 2019; 10: 4922-4931.
- Sharma A, Flores-Vallejo RC, Cardoso-Taketa A, Villarreal ML. Antibacterial activities of medicinal plants used in Mexican traditional medicine. J Ethnopharmacol. 2017; 208: 264-329.
- Fisher MC, Henk DA, Briggs CJ, Brownstein JS, Madoff LC, McCraw SL, et al. Emerging Fungal Threats to Animal, Plant and Ecosystem health. Nature. 2012; 484: 186-194.
- Tabassum N, Vidyasagar G. Antifungal Investigations on Plant Essential Oils. A Review. Int J Pharm Sci. 2013; 5: 19-28.
- Abd-ElGawad MA, Elshamy AI, Al-Rowaily SL, El-Amier YA. Habitat affects the chemical profile, allelopathy, and antioxidant properties of essential oils and phenolic enriched extracts of the invasive plant Heliotropium curassavicum. Plants. 2019; 8: 482.
- Puig CG, Reigosa MJ, Valentão P, Andrade PB, Pedrol N. Unravelling the bioherbicide potential of Eucalyptus globulus Labill: Biochemistry and effects of its aqueous extract. PLoS ONE. 2018; 13: 0192872.
- Pan L, Li XZ, Yan ZQ, Guo HR, Qin B. Phytotoxicity of umbelliferone and its analogs: Structure–activity relationships and action mechanisms. Plant Physiol. Biochem. 2015; 97: 272–277.
- Dastan D, Salehi P, Ghanati F, Gohari AR, Maroofi H, Alnajar N. Phytotoxicity and cytotoxicity of disesquiterpene and sesquiterpene coumarins from Ferula pseudalliacea. Ind Crop Prod. 2014; 55: 43–48.
- Aye MM, Aung HT, Sein MM, Armijos C. A Review on the Phytochemistry Medicinal Properties and Pharmacological Activities of 15 Selected Myanmar Medicinal Plants. Mol. 2019; 24: 293.
- Oppedisano F, Macrì R, Gliozzi M, Musolino V, Carresi C, Maiuolo J, et al. The Anti-Inflammatory and Antioxidant Properties of n-3 PUFAs: Their Role in Cardiovascular Protection. Biomed. Res. J. 2020; 8: 306.
- Tabassum N, Ahmad F. Role of natural herbs in the treatment of hypertension. Pharmacogn. 2011; 5: 30–40.
- Khan K, Rahman IU, Calixto ES, Ali N, Ijaz F. Ethnoveterinary Therapeutic Practices and Conservation Status of the Medicinal Flora of Chamla Valley, Khyber Pakhtunkhwa, Pakistan. Front Vet Sci. 2019; 6: 1-122.
- Zaman W, Ye J, Ahmad M, Saqib S, Shinwari ZK, Chen Z. Phylogenetic exploration of traditional Chinese medicinal plants: A case study on Lamiaceae (angiosperms). Pak J Bot. 2022; 54: 1033–1040.
- Dubey A, Yadav P, Peeyush P, Verma P, Kumar R. Investigation of Proapoptotic Potential of Ipomoea carnea Leaf Extract on Breast Cancer Cell Line. JDDT. 2022; 12: 51-55.
- Radha AHM, Kadhim JN. Diversity of Medicinal Plants Used as Male Contraceptives: An Initiative Towards Herbal Contraceptives. Indian J Tradit Knowl Arch Razi Inst. 2021; 76: 659–666.
- Alonso-Castro AJ, Villarreal ML, Salazar-Olivo LA, Gomez-Sanchez M, Dominguez F, Garcia-Carranca A. Mexican Medicinal Plants Used for Cancer Treatment: Pharmacological, Phytochemical and Ethnobotanical Studies. J. Ethnopharmacol. 2011; 133: 945–972.
- Ali SI, Nasir YJ. (Eds.). 1989-1991. Flora of Pakistan. Nos. 191-193. Department of Botany, University of Karachi, Karachi Pakistan.
- Ali SI, Qaiser M. (Eds.). 1993-2022. Flora of Pakistan. Nos. 194-223. Department of Botany, University of Karachi, Karachi Pakistan.
- Nasir E, Ali SI. (Eds.). 1970-1989. Flora of Pakistan. Nos. 1-190. Pakistan Agriculture Research Council, Islamabad.
- Ahmad N, Muhammad J, Khan K, Ali W, Fazal H, Ali M, et al. Silver and gold nanoparticles induced diferential antimicrobial potential in calli cultures of Prunella vulgaris. BMC Chemistry. 2022; 16: 20.
- Ali AM, Saeed AAM, Fdhel TA. Phytochemical analysis and antimicrobial screening of selected Yemeni folk medicinal plants. J Med Plants Stud. 2019; 7: 108-114.
- Saeed F, Younas M, Fazal H, Mushtaq S, Rahman FU, Shah M, et al. Green and chemically synthesized zinc oxide nanoparticles: effects onin-vitroseedlings and callus cultures of Silybum marianumand evaluation of their antimicrobial and anticancer potential. Artif Cells Nanomed Biotechnol. 2021; 49: 450-460.
- Haq MNU, Wazir SM, Ullah F, Khan RA, Shah MS, Khatak A. Phytochemical and Biological Evaluation of Defatted Seeds of Jatropha Curcas. Sains Malays. 2016; 45: 1435-1442.
- Haq A, Mushtaq S, Khan A, Islam A, Khan H, Malik ZA, et al. Evaluation of Phytochemical, Bioactive, and Antifungal Potential of Jatropha Curcas Seed Oil and De Oiled Seed Cake Extracts Against Phytopathogenic Fungi. Plant Pathol J. 2021; 103: 863-873.
- Mbata T, Debiao L, Saikia A. Antibacterial activity of the crude extract of Chinese green tea (Camellia sinensis) on Listeria monocytogenes. Afr J Biotech. 2008; 7: 1-4.
- Meyer B, Ferrigni N, Putnam J, Jacobsen L, Nichols D, McLaughlin JL. Brine Shrimp: A Convenient General Bioassay for Active Plant Constituents. Planta med. 1982; 45: 31-34.
- Siddiqi MA. Grossulariaceae in Nasir and Ali’s Flora of West Pakistan. Botany Department Gordon College, Rawalpindi. 1972; 27: 1-10.
- Ali K, Khan S, Khan N, Khan W, Rahman IU, Ullah F, et al. Ethnobotanical and Ecological Study of Punica granatum in Dir District, Khyber Pakhtunkhwa, Pakistan. Regul Mech Biosyst. 2017; 8: 656–661.
- Ahmad A, Saeed A, Gulshan AB, Yousaf W, Zafar I. Envisaging Natural Vegetation in Contrasting Environments (Piedmont and Alluvial) of Dera Ghazi Khan, Pakistan. Pak J Bot. 2023; 55: 2231-2241.
- Iqbal IM, Balzter H, Bareen FE, Shabbir A. Mapping Lantana Camara and Leucaena Leucocephala in Protected Areas of Pakistan: A Geo-Spatial Approach. Remote Sens. 2023; 15: 1-20.
- Rabie G, Taha M, Youssef K. Antifungal Activity of Petroleum Ether and Ethanol Extracts of Moringa Oleifera Seeds. Asian J Appl Sci. 2019; 7: 56-62.
- Nyarko RO, Kumar R, Sharma S, Chourasia A, Roy A, Saha P. Antibacterial Activity of Herbal Plant- Tinospora Cordifolia and Catharnthus Roseus. World J pharm. pharm sci. 2022; 11: 1973-1977.
- Danish P, Ali Q, Hafeez MM, Malik A. Antifungal and Antibacterial Activity of Aloe Vera Plant Extract. Biol Clin Sci Res J. 2020; 4: 1-8.
- Haq A, Ali Q, Rashid MS, Waheed F, Hayat S, Malik A. Antibacterial and Antifungal activity of Aloe vera plant. Life Sci J. 2020; 17: 76-82.
- Muktar Y, Mohammadnur M, Wondimu A, Hiko A. In vitro antibacterial activity of crude methanol ex¬tracts of various parts of Parthenium hysterophorus against pathogenic bacterial strains. Ethiop. Vet. J. 2017; 21: 89-101.
- Bereksi MS, Hassaine H, Bekhechi C, Abdelouahid DE. Evaluation of antibacterial activity of some medicinal plants extracts commonly used in algerian traditional medicine against some pathogenic bacteria. Pharmacogn J. 2018; 10: 507-512.
- Rong S, Xu H, Li L, Chen R, Gao X, Xu Z. Antifungal Activity of endophytic Bacillus safensis B21 and its potential application as a biopesticide to control rice blast. Pestic Biochem Physiol. 2018; 162: 69-77.
- Gilani SA, Fujii Y, Shinwari ZK, Adnan M, Kikuchi A, Watanabe KN. Phytotoxic Studies of Medicinal Plant Species of Pakistan. Pak J Bot. 2010; 42: 987-996.
- Maqbool R, Anwar I, Nadeem MA, Inqalabi TEI, Raza A, Khan BA, et al. Exploring the Phytotoxic Effects of Glycyrrhiza glabra L. on Emergence and Seedling Growth of Pisum sativum L. Pak J Weed Sci Res. 2022; 28: 213-220.
- Khor KZ, Joseph J, Shamsuddin F, Lim V, Moses EJ, Samad NA. The Cytotoxic Effects of Moringa oleifera Leaf Extract and Silver Nanoparticles on Human Kasumi-1 Cells. Int J Nanomed. 2020; 15: 5661- 5670.
- Nejad AE, Fazilati M, Daneshmand D, Habibollahi S. Cytotoxic Effects of Moringa oleifera Leaf Extract on Human Hepatoma Cell Line HepG-2. Jentashapir J Cell Mol Biol. 2020; 11: e108527.