
Research Article
J Plant Chem and Ecophysiol. 2024; 4(1): 1024.
Chemical Analysis of Solid Tree Resins of Protium Heptaphyllum and Protium Icicariba
Lumír Ondřej Hanuš¹*; Yoav Giladi²; Maria Luiza Ammirabile Martins (Tuca)²
¹Institute for Drug Research, School of Pharmacy, Faculty of Medicine, Hebrew University, Ein Kerem Campus, Jerusalem 91120, Israel
²Independent Researcher, Israel
*Corresponding author: Lumír Ondřej Hanuš, Institute for Drug Research, School of Pharmacy, Faculty of Medicine, Hebrew University, Ein Kerem Campus, Jerusalem 91120, Israel. Tel: +972-2-6439935 Email: lumirh@ekmd.huji.ac.il
Received: December 04, 2024; Accepted: December 19, 2024 Published: December 26, 2024
Abstract
Solid tree resins of Protium heptaphyllum and Protium icicariba were studied and analyzed volatile compounds compared. The absence of sabinene in the resin of the tree Protium icicariba is the main distinguishing feature.
Keywords: Protium heptaphyllum; Protium icicariba; Resin; Volatile compound; Terpenes; GC/MS
Introduction
Protium icicariba, commonly known as "Almescar" or "Icica," is a tropical tree native to South America, particularly found in the Amazon Rainforest. It is renowned for its resin, which has been traditionally used by indigenous communities for its medicinal properties and as an incense ingredient.
Protium heptaphyllum, often referred to as "Breu Branco”, “Mel Branco”, “Almesca” or "Almécega," is another tree species found in the Amazon Rainforest, and the Atlantic rainforest. It is well-known for producing a fragrant resin called "Breu" or "White Breu," which is used in traditional healing practices and as incense in spiritual rituals.
Both Protium icicariba and Protium heptaphyllum play significant roles in the cultural and traditional practices of indigenous peoples in their respective regions. Their resins have a long history of use for spiritual and medicinal purposes and continue to be valued for their aromatic and therapeutic qualities.
Almescar, also known as Breu Branco, is a fascinating resin (Figure 1) extracted from trees of the genus Protium, which are found in the Amazon rainforest, predominantly within Brazil. This resin has been an integral part of indigenous cultures for centuries, serving various traditional uses ranging from medicinal applications to spiritual practices. Almescar (Breu Branco) is particularly valued for its distinctive, pleasant aroma and its wide range of beneficial properties.

Figure 1: Resin secreted from the bark of Protium heptaphyllum.
Extraction and Uses
The resin is traditionally harvested by making incisions in the tree's bark, allowing the sap to exude and harden into a resinous mass. This method of extraction is sustainable, as it does not harm the tree when done correctly, and allows for continuous harvesting from the same individual over many years (Figure 1).
Almescar resin is known for its antiseptic, anti-inflammatory, analgesic, and gastroprotective properties [1-3], making it a valuable resource in traditional medicine for treating wounds, skin infections, and various other health issues. It is also used in aromatherapy due to its calming and grounding scent.
Environmental and Economic Impact
The sustainable harvesting of Almescar resin can have positive environmental and economic impacts. It provides a renewable resource that encourages the preservation of the Amazon rainforest and its biodiversity. Economically, it supports local communities by creating income opportunities that are aligned with the conservation of their natural environment. There's a growing interest in nontimber forest products like Almescar resin, as they offer a way to value the standing forest, providing an alternative to logging and other destructive practices.
Scientific Research
Protium heptaphyllum was for the first time published by Marchand [4] in 1873 and Protium icicariba by the same author [5] in 1867. Research into Almescar and other Amazonian resins is ongoing, with scientists exploring their potential applications in modern medicine and industry.
In this study we collected resin samples (Figure 2–5) of Protium heptaphyllum and Protium icicariba from different sources (Figure 6). We analyzed their chemical compositions and made a comparison of the results.

Figure 2: Almescar Sample 1 Botanical name: Protium heptaphyllum
Originated from: Caraíva - Bahia, Brazil
Sample type: fresh resin.

Figure 3: Almescar Sample 2 Botanical name: Protium heptaphyllum
Originated from: Jambreiro-Bahia Brazil.

Figure 4: Almescar Sample 3 Botanical name: Protium icicariba Originated
from: caraíva Bahia Brazil Sample type: fresh resin.

Figure 5: Almescar Sample 4 Botanical name: Protium icicariba
Originated Belém- Pará Brazil Sample type: dry after curing.
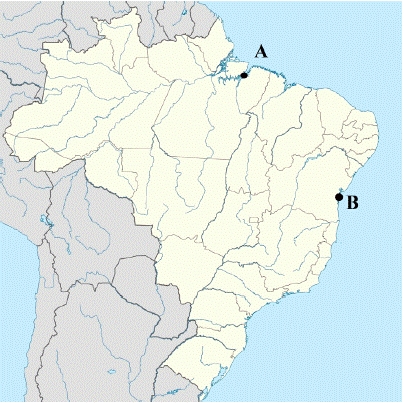
Figure 6: Illustration of the map of Brazil showing: location A where
samples 1-3 were collected and location B where sample 4 was collected.
Materials And Methods
Plant Materials
(Figure 2 to Figure 6)
Sample Preparation
120 mg of tree resin was used for analysis. Headspace gas chromatography/ mass spectroscopy analysis was used for the indirect determination of volatile constituents in solid samples by analyzing the associated vapor phase. Analytical method validation - selectivity, specificity, accuracy, precision, linearity, range, limit of detection, limit of quantification, ruggedness, and robustness are beyond the scope of this manuscript and will be published elsewhere.
General Experimental Procedures
Experimental conditions: For the GC/MS analysis, we employed an Agilent 7890B GC combined with an Agilent 5977B MSD and a PAL 3 (RSI 85) chromatograph. The column used was HP-5MS UI, 30 m × 0.25 mm with a film thickness of 0.25 μm, provided by Agilent Technologies, Inc. The analytical conditions were set with the column initially held at 35°C for 5 min. Subsequently, the temperature was programmed to rise from 35°C to 150°C at a rate of 5°C/min, then increasing by 15°C/min to 250°C, with a hold time of 90 min. The specific settings were as follows: Inlet temperature at 250°C, detector temperature at 280°C, split injection ratio of 1:5, initial temperature at 100°C, and initial time set to 4.0 min. Helium was used as the carrier gas with a flow rate of 1 mL/min. Incubation time: 6 min; Incubation temperature: 80ºC.
Identification
For compound detection and identification, we referenced various standards, retention times, and retention indices (Chromatogram 1,2, Table 1; Chromatogram 3,4, Table 2). Additionally, we consulted multiple libraries: NIST/EPA/NIH Mass Spectral Library 2017, Wiley Registry of Mass Spectral Data 11th Edition, FFNSC3, © 2015, and the Adams EO library, Mass Spectral Library, containing 2205 compounds. The identification of volatile compounds in the samples was based on a comparison of their mass spectra and retention times, along with their Kovats retention indices, either to those of injected standards or by referencing the National Institute of Standards and Technology’s Mass Spectral Library database.
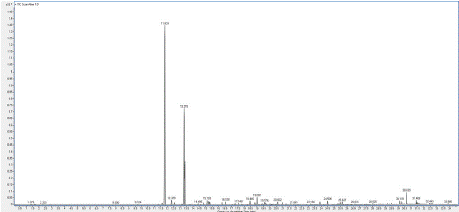
Chromatogram 1: GC/MS chromatogram of the sample 1 (Protium
heptaphyllum).
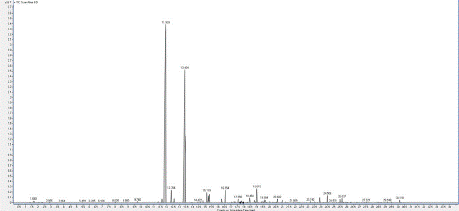
Chromatogram 2: GC/MS chromatogram of the sample 2 (Protium
heptaphyllum).
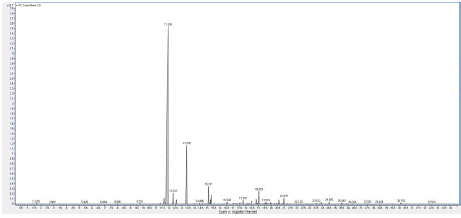
Chromatogram 3: GC/MS chromatogram of the sample 3 (Protium
icicariba).
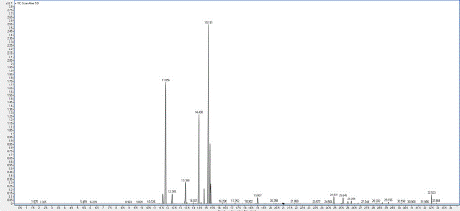
Chromatogram 4: GC/MS chromatogram of the sample 4 (Protium icicariba).
No
RT
Compound
1
2
%
%
1
2.301
2-methyl-3-butene-2-ol
0.06
2
3.864
3-ethyl-2,2-dimethyloxirane
0.03
3
6.205
3-methyl-2-butenal
0.02
4
6.758
hexenal
0.02
5
8.025
3,3,5,5-tetramethylcyclopentene
0.03
0.07
6
11.368
tricyclene
0.12
0.17
7
11.52
cumene
0.02
8
11.68
α-thujene
0.35
0.79
9
12.025
α-pinene
44.62
45.68
10
12.37
camphene
1.08
1.54
11
12.594
dehydrosabinene
0.4
0.47
12
13.428
sabinene
21.43
28.05
13
13.484
β-pinene
8.78
6.28
14
13.901
6-methyl-5-hepten-2-one
0.01
15
14.205
trans-p-menth-2-ene
0.02
16
14.422
α-phellandrene
0.12
17
14.614
o-methyl-anisole
0.04
0.09
18
14.847
α-terpinene
0.32
0.26
19
15.175
p-cymene
0.83
1.12
20
15.288
limonene
0.67
0.81
21
15.352
1,8-cineole
0.56
0.93
22
16.29
γ-terpinene
0.43
0.42
23
16.546
cis-sabinene hydrate
0.59
1.43
24
17.332
2-(2,2,4-trimethyl-1-cyclopent-3-enyl)acetaldehyde
0.17
0.13
25
17.644
linalool
0.01
0.02
26
17.813
3-thujen-2-ol
0.25
27
18.157
β-thujone
0.01
0.01
28
18.246
dehydrosabinaketone
0.04
29
18.454
α-campholenal
0.91
0.63
30
18.839
trans-pinocarveol
0.47
0.36
31
19.015
camphor
1.66
1.83
32
19.36
3-thujen-2-one
0.09
0.07
33
19.424
sabina ketone
0.2
0.16
34
19.512
pinocamphone
0.04
0.03
35
19.584
pinocarvone
0.35
36
19.673
endo-borneol
0.52
0.37
37
20.049
terpinen-4-ol
0.31
0.13
38
20.434
α-terpineol
0.05
0.03
39
20.61
myrtenal
0.62
0.43
40
20.987
verbenone
0.4
0.32
41
21.26
trans-carveol
0.13
0.08
42
21.861
cumin aldehyde
0.04
0.03
43
21.989
carvone
0.01
0.01
44
22.142
2,5-dimethoxytoluene
0.03
45
23.192
bornyl acetate
0.16
0.15
46
23.616
undecanal
0.03
47
24.603
δ-elemene
0.01
48
24.931
α-cubebene
0.03
49
25.3
butoxyethoxyethyl acetate
0.28
50
25.42
cyclosativene
0.03
51
25.637
copaene
0.51
0.49
52
25.741
daucene
0.06
53
25.885
α-bourbonene
0.06
0.07
54
26.005
β-cubebene
0.06
55
26.054
β-elemene
56
26.286
cyperene
0.02
57
26.126
tetradecane
0.04
58
26.374
dodecanal
0.1
59
26.631
β-ylangene
0.01
60
26.968
sibirene
0.01
61
27.368
guaia-6,9-diene
0.07
0.04
62
27.833
9-epi-(E)-caryophyllene
0.02
63
28.026
dodecanol
0.22
64
28.202
γ-muurolene
0.02
65
28.282
α-amorphene
0.02
66
28.443
β-selinene
0.05
0.06
67
28.531
δ-selinene
0.02
0.02
68
28.603
pentadecane
0.11
69
28.627
α-selinene
0.02
70
28.715
α-muurolene
0.01
0.03
71
28.996
γ-cadinene
0.01
0.03
72
29.148
δ-cadinene
0.11
0.08
73
29.268
dauca-4(11),8-diene
74
29.468
cis-dracunculifoliol
0.14
75
30.639
isopropyl laurate
1.6
76
31.408
lauryl acrylate
0.49
77
31.473
heptadecane
0.25
78
32.443
octadecane
0.13
79
33.493
14-methyl-pentadecanoic acid methyl ester
0.04
90.80%
94.90%
Table 1: Protium heptaphyllum (Aubl.) Marchand - concentrations of content compounds (% of total).
No
RT
Compound
3
4
RI
%
%
1
5.459
toluene
0.04
0.07
763
2
6.229
3-methyl-2-butenal
0.01
782
3
8
3,3,5,5-tetramethylcyclopentene
0.08
857
4
10.726
bornylene
0.02
906
5
11.247
5,5-dimethyl-1-vinylbicyclo[2.1.1]hexane
0.02
921
6
11.351
tricyclene
0.19
0.07
925
7
11.512
cumene
0.02
927
8
11.616
α-thujene
1.79
1.56
929
9
11.856
α-pinene
66.61
20.43
937
10
12.345
camphene
1.89
1.74
952
11
12.586
dehydrosabinene
0.64
0.05
956
12
13.388
β-pinene
12.25
3.22
979
13
13.7
trans-p-menth-2-ene
0.04
0.04
981
14
13.901
6-methyl-5-hepten-2-one
0.04
986
15
14.277
Δ2-carene
0
1001
16
14.43
α-phellandrene
13.05
1005
17
14.606
Δ3-carene
0.13
0.24
1011
18
14.847
α-terpinene
2.06
1017
19
15.007
p-ment-1-ene
0.05
1025
20
15.191
p-cymene
2.67
39.5
1025
21
15.239
limonene
0.68
1030
22
15.296
β-phellandrene
7.73
1031
23
15.352
1,8-cineole
1.35
1.99
1032
24
15.64
cis-β-ocimene
0
1038
25
16.29
γ-terpinene
0.06
0.09
1060
26
16.554
cis-sabinene hydrate
0.32
0.06
1070
27
17.252
terpinolene
0.17
1088
28
17.324
2-(2,2,4-trimethyl-1-cyclopent-3-enyl)acetaldehyde
0.08
29
17.821
3-thujen-2-ol
0.61
0.03
1107
30
18.149
β-thujone
0.11
1114
31
18.462
α-campholenal
0.6
0.03
1125
32
18.823
trans-pinocarveol
0.75
1139
33
18.911
verbenol
0.16
1140
34
19.007
camphor
0.89
1142
35
19.32
menthone
0
1154
36
19.52
pinocamphone
0.08
0.02
1160
37
19.592
pinocarvone
0.29
0.02
1164
38
19.68
borneol
0.19
0.01
1166
39
19.937
cis-pinocamphone
0.22
0.01
1173
40
20.033
terpinen-4-ol
0.07
0.03
1177
41
20.258
p-cymen-8-ol
0.07
0.14
1183
42
20.426
α-terpineol
0.12
0.09
1189
43
20.61
myrtenal
0.56
0.05
1193
44
20.795
homomyrtenol
0.06
1194
45
20.995
verbenone
0.88
0.02
1205
46
21.252
trans-carveol
0.07
1217
47
21.725
thymol methyl ether
0.01
1235
48
21.869
cumin aldehyde
0.03
0.07
1239
49
21.981
carvone
0.03
1242
50
21.989
carvacrol methyl ether
0.03
1244
51
22.101
carvotanacetone
0.03
1246
52
22.158
thymoquinone
0.02
1250
53
22.133
2,5-dimethoxytoluene
0.05
1251
54
22.286
piperitone
0.07
1253
55
22.615
3,5-dimethoxytoluene
0.01
1274
56
23.184
bornyl acetate
0.08
1285
57
23.288
p-cymen-7-ol
0.03
1289
58
23.577
carvacrol
0.03
1299
59
24.603
δ-elemene
0.01
1338
60
24.931
α-cubebene
0.87
1351
61
25.428
cyclosativene
0.01
1368
62
25.525
ylangene
0.15
1372
63
25.645
copaene
0.03
0.79
1376
64
25.741
daucene
0
1381
65
25.893
α-bourbonene
0.09
0.03
1384
66
26.014
β-cubebene
0.38
1389
67
26.286
cyperene
0.39
1399
68
26.535
α-gurjunene
0.01
0.05
1409
69
26.879
γ-maaliene
0.02
1435
70
27.16
α-bergamotene
0.03
1435
71
27.248
α-guaiene
0.01
1439
72
27.368
guaia-6,9-diene
0.04
1450
73
27.649
α-humulene
0.03
1454
74
28.202
γ-muurolene
0.13
1477
75
28.314
α-curcumene
0.02
1483
76
28.442
β-selinene
0.04
0.03
1486
77
28.523
δ-selinene
0.01
1490
78
28.627
α-selinene
0.02
1494
79
28.715
α-muurolene
0.02
0.16
1499
80
28.996
γ-cadinene
0.02
0.05
1513
81
29.156
δ-cadinene
0.03
0.18
1524
82
29.316
cadina-1,4-diene
0
1533
83
29.509
α-calacorene
0.01
1542
84
29.837
β-calacorene
0
1563
85
30.158
caryophyllene oxide
0.06
1581
86
30.527
humulene epoxide I
0.01
1604
87
30.968
δ-cadinol
0.02
1645
94.15%
97.50%
Table 2: Protium icicariba (DC.) Marchand - concentrations of content compounds (% of total) in samples 3 and 4.
Commercially available standards for α-pinene, camphene, β-pinene, myrcene, Δ3-carene, α-terpinene, p-cymene, limonene, 1,8-cineole, α-ocimene, trans-β-ocimene, γ-terpinene, terpinolene, linalool, isopulegol, geraniol, β-caryophyllene, α-humulene, cis-nerolidol, trans-nerolidol, caryophyllene oxide, guaiol, and α-bisabolol were obtained from Restek (Bellefonte, PA, USA).
Results
All samples contained high amounts of pinene (α and β). Both samples of P. heptaphyllum contained high amounts of sabinene while the two samples of P. icicariba did not revealed any sabinene. Sample 4 of P. icicariba contained a unique chemical composition with high amounts of p-cymene and α- and β-phellandrene.
As we did not have all the main compounds as standards, it was impossible to quantify all these volatiles exactly. Percentage compositions (in Tables as % were obtained from electronic integration measurements without considering relative response factors).
(Chromatogram 1 to 4 & Table 1 & 2)
Discussion
Previous studies suggest that the outcomes of resin analysis are influenced by multiple factors, including the freshness of the sample, preservation methods, processing techniques (e.g., steam distillation, hydrodistillation, organic solvent extraction), seasonal variation, and the geographic origin of the collected samples. These variables significantly impact the chemical composition of the resin..In resin produced by Protium heptaphyllum, the predominant terpenes and terpenoids identified in previous studies include limonene [6-8], terpinolene [9-14], p-cymene [13,15-17], and p-cymen-8-ol [7,9,11- 13], with α-pinene [10,13-15], 1,8-cineole [3,7,8,18], α-phellandrene [8,10,13,18], and sabinene [3,7-10,15,18] observed in smaller concentrations.Protium icicariba has only been analyzed in three scientific papers. Siani et al. [19] analyzed the essential oils from the oleoresin in three different years.
The main compounds were p-cymene (20-40%), p-cymen-8-ol (10-26%), terpinolene (5.8-31.0%), limonene (5.8-8.0%), α-pinene (5.6-7.7%), and sabinene (1.6-2.3%). Pereira et al. [20] studied dehiscent/indehiscent ripe fruits and found as the main compounds E-β-ocimene (31.07/28.14%), α-fenchene (27.67/12.83%), limonene (16.10/21.29%), and α-phellandrene (8.86/12.50%). da Paz Lima et al. [21] found in Protium icicariba lupeol, and α- and β-amyrin.
(Table 3)
P. heptaphyllum sample 1 (%)
P. heptaphyllum
P. icicariba
P. icicariba
RI
sample 2 (%)
sample 3 (%)
sample 4 (%)
α-thujene
0.35
0.79
1.79
1.56
929
α-pinene
44.62
45.68
66.61
20.43
937
camphene
1.08
1.54
1.89
1.74
952
sabinene
21.43
28.05
0
0
974
β-pinene
8.78
6.28
12.25
3.22
979
α-phellandrene
0
0.12
0
13.05
1005
p-cymene
0.83
1.12
2.67
39.5
1025
limonene
0.67
0.81
0.68
0
1030
β-phellandrene
0
0
0
7.73
1031
1,8-cineole
0.56
0.93
1.35
1.99
1032
cis-sabinene hydrate
0.59
1.43
0.32
0.06
1070
terpinolene
0
0
0
0.17
1088
camphor
1.66
1.83
0
0.89
1142
isopropyl laurate
1.6
0
0
0
1618
Table 3: The main compounds and the main differences in samples of P. heptaphyllum and P. icicariba.
The evaluation and the ranking of the most prominent substances in all four samples is compared in Table 3. All four samples contained very high amounts of α-pinene and high amounts of β-pinene. Both samples of P. heptaphyllum contained high amounts of sabinene while the two P. icicariba samples contained no sabinene at all. P. icicariba sample 4 contained a unique chemical composition with high amounts of p-cymene and α- and β-phellandrene. Some compounds reported in previous studies, such as limonene [6-8] terpinolene [9-14], and p-cymen-8-ol [7,9,11-13], were found in negligible concentrations or were not detected in our samples. This discrepancy may stem from factors beyond sample processing, such as genetic variability, environmental conditions, or differences in collection and preservation methods.
The research of these substances in P. heptaphyllum and P. icicariba deserves greater attention due to the frequent use of their resins in indigenous cultures. Further research is essential to fully explore their chemical composition and potential applications.
Author Statements
Conflict of Interest Statement
No potential conflict of interest was reported by the author.
References
- Aragao GF, Pinheiro MCC, Bandeira PN, Lemos TLG, de Barros Viana GS. Analgesic and Anti-Inflammatory Activities of the Isomeric Mixture of Alphaand Beta-Amyrin from Protium heptaphyllum(Aubl.) March. Journal of Herbal Pharmacotherapy. 2007; 7: 31-47.
- Oliveira FA, Vieira-Júnior GM, Chaves MH, Almeida FRC, Florêncio MG, Lima Jr RCP, et al. Gastroprotective and anti-inflammatory effects of resin from Protium heptaphyllum in mice and rats. Pharmacological Research. 2004; 49: 105-111.
- Amaral MPM, Braga FAV, Passos FB, Almeida FRC, Oliveira RCM, Carvalho AA, et al. Additional Evidence for the Anti-inflammatory Properties of the Essential Oil of Protium heptaphyllum Resin in Mice and Rats. Latin American Journal of Pharmacy. 2009; 28: 775-782.
- Marchand L. Fam. Burseraceae. Videnskabelige meddelelser fra den Naturhistoriske forening i Kjöbenhavn for Aaret. Udgivne af Selskabets Bestyrelse, Kjöbenhavn, Bianco Lunos Bogtrykkeri. 1873; 54-57: 1873-74.
- Marchand L. Recherches sur l’organisation des Burséracées. Adansonia; recueil d’observations botaniques. Rédigé par le Dr H. Baillon, Tome huitième, Paris, France, Imprimerie de E. Martinet, rue Mignon, 2. 5, rue de l’Ancienne-Comédie et chez F. Savy, 24, rue Hautefeuille, Septembre – Aout. 1867: 17-60.
- Amaral MPM, Braga FAV, Passos FFB, Almeida FRC, Oliveira RCM, Carvalho AA, et al. Additional Evidence for the Anti-inflammatory Properties of the Essential Oil of Protium heptaphyllum Resin in Mice and Rats. Latin American Journal of Pharmacy. 2009; 28: 775-782.
- Mobin M, de Lima SG, Almeida LTG, Filho JCS, Rocha MS, Oliveira AP, et al. Gas Chromatography-Triple Quadrupole Mass Spectrometry Analysis and Vasorelaxant Effect of Essential Oil from Protium heptaphyllum (Aubl.) March. BioMed Research International. 2017: 1928171.
- Mobin M, De Lima SG, Almeida LTG, Takahashi JP, Teles JB, Szeszs MW, Martins MA, Carvalho AA, Melhem MSC. 2016. MDGC-MS analysis of essential oils from Protium heptaphyllum (Aubl.) and their antifungal activity against Candida specie. Rev. Bras. Pl. Med., Campinas. 2016; 18: 531-538.
- de Lima EM, Cazelli DSP, Pinto FE, Mazuco RA, Kalil IC, Lenz D, et al. Essential Oil from the Resin of Protium heptaphyllum: Chemical Composition, Cytotoxicity, Antimicrobial Activity, and Antimutagenicity. Pharmacognosy Magazine. 2016; 12: S42-S46.
- Bandeira PN, Fonseca AM, Costa SMO, Lins MUDS, Pessoa ODL, Monte FJQ, et al. Antimicrobial and Antioxidant Activities of the Essential Oil of Resin of Protium heptaphyllum. Natural Product Communications. 2006; 1: 117-120.
- Marques DD, Sartori RA, Lemos TLG, Machado LL, de Souza JSN, Monte FJQ. Chemical composition of the essential oils from two subspecies of Protium heptaphyllum. Acta Amazonica. 2010; 40: 227 – 230.
- Siani AC, Ramos MFS, Menezes-de-Lima Jr. O, Ribeiro-dos-Santos R, Fernadez-Ferreira E, Soares ROA, et al. Evaluation of anti-inflammatoryrelated activity of essential oils from the leaves and resin of species of Protium. Journal of Ethnopharmacology. 1999; 66: 57–69.
- Albino RC, Oliveira PC, Prosdocimi F, da Silva OF, Bizzo HR, Gama PE, et al. Oxidation of monoterpenes in Protium heptaphyllum oleoresins. Phytochemistry. 2017; 136: 141-146.
- Bandeira PN, Machado MIL, Cavalcanti FS, Lemos TLG. Essential Oil Composition of Leaves, Fruits and Resin of Protium heptaphyllum (Aubl.) March. Journal of Essential Oil Research. 2001; 13: 33-34.
- Tafurt-García G, Muñoz-Acevedo A. Metabolitos volátiles presentes en Protium heptaphyllum (Aubl.) March. colectado en Tame (Arauca - Colombia). Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas. 2012; 11: 223–232.
- Siani AC, Ramos MFS, Guimarães AC, Susunaga GS, Zoghbi M das GB. Volatile Constituents from Oleoresin of Protium heptaphyllum (Aubl.) March. Journal of Essential Oil Research. 1999; 11: 72-74.
- Silva JR de A, Zoghbi M das GB, da C Pinto A, Godoy RLO, Amaral ACF. Analysis of the Hexane Extracts from Seven Oleoresins of Protium Species. Journal of Essential Oil Research. 2009; 21: 305-308.
- Rao VS, Maia JL, Oliveira FA, Lemos TLG, Chaves MH, Santos FA. Composition and Antinociceptive Activity of the Essential Oil from Protium heptaphyllum Resin. Natural Product Communications. 2007; 2: 1199-1202.
- Siani AC, Garrido IS, Monteiro SS, Carvalho ES, Ramos MFS. Protium icicariba as a source of volatile essences. Biochemical Systematics and Ecology. 2004; 32: 477–489.
- Pereira IF, da Costa APF, Srbek-Araujo AC, Guimarães LJ, Merencio AF, da Silva AG. The Dispersion of Diaspores of Protium icicariba (Burseraceae) - a Networked or Multifactorial System? Journal of Chemical Ecology. 2020; 46: 163–175.
- da Paz LM, de Castro FBG, da Silva MF, das GF, Ferreira AG, Fo ER, et al. Phytochemistry of Crepidospermum rhoifolium, Tetragastris altissima, Protium icicariba and P. apiculatum: Chemosystematic implications. Revista Latinoamericana de Quimica. 2001; 29: 135-144.