
Review Article
Austin J Lung Cancer Res. 2016; 1(1): 1001.
Anamorelin for the Treatment of Cancer Anorexia- Cachexia Syndrome of Lung Cancer
Hidefumi Sasaki*
Department of Oncology, Nagoya City University Graduate School of Medical Sciences, Japan
*Corresponding author: Hidefumi Sasaki, Global Medical Services, PAREXEL International, Tokyo, Japan
Received: November 17, 2015; Accepted: December 18, 2015; Published: January 04, 2016
Abstract
Anorexia and cachexia are among the most troubling and distressing symptoms of advanced cancer, for both patients and their families. Cancer anorexia-cachexia syndrome is a common debilitating condition, characterized by decreased body weight, mainly lean body mass and negatively impacts quality of life and prognosis. The condition is very common in patients with advanced lung cancer. Anamorelin HCl is a novel selective ghrelin receptor agonist with appetite-enhancing and anabolic activity. Anamorelin aims to address the symptoms by mimicking the effects of the so-called “hunger hormone” ghrelin, which is secreted by the stomach. The large, randomized controlled ROMANA 1 and 2 trials are the first phase III studies examining the impact of anamorelin on anorexia-cachexia in patients with advanced lung cancer.
Keywords: Cachexia; Anamorelin; Lung Cancer
Introduction
Alterations in body constructions that happen with chronic diseases are normally considered unwanted and are associated with loss of appetite, substantial weight loss, dramatic muscle loss and general weakness [1,2]. Thus the metabolic disorder, body overzealously breaks down skeletal muscle and adipose tissue may be associated with weight loss. Such unconscious weight loss has been termed cachexia. For example, cancer cells produce chemicals or other products that cause cachexia. Cachexia involves multiple pathways: procachectic and proinflammatory signals from cancer cells, systemic inflammation host and widespread changes. Cancer Anorexia-Cachexia Syndrome (CACS) was recently defined by an international expert consensus group as ‘‘amultifractorial syndrome characterized by an ongoing loss of skeletal mass (with or without loss of fat mass) that cannot be fully reversed by conventional support and leads to progressive functional impairment’’ [3]. CACS has severe consequences, such as reduction of treatment tolerance, reduction of response to therapy, and shortened survival, and can adversely affect a person’s quality of life [3-8]. Increased resting energy expenditure and alterations in metabolism of protein, fat, and carbohydrate are the reason for metabolic changes in cancer patients. Over expression of proinflammatory cytokines, such as Interleukin (IL)-1, IL-6, tumor necrosis factor, or interferon-γ, TNF-a, TGF-β, as well as macrophage inhibitory cytokine-1/growth differentiation factor 15 (MIC-1/GDF- 15) appear to be involved [9,10]. Inflammatory processes have been shown to maintain the wasting process in cachexia. Understanding the complex interplay of tumor and host factors will uncover new therapeutic targets. Activation of these factors has effects on peripheral (lipolysis, proteolysis, insulin resistance) as well as on central pathways (hypothalamic appetite regulation) [9,11] (Figure 1).
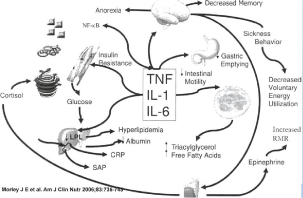
Figure 1: Mechanism of cachexia.
Anamorelin
Ghrelin is a 28-amino acid peptide hormone mostly produced in the stomach, but also in other gastrointestinal tissues [12,13] and is the endogenous ligand for the Ghrelin receptor (formally known as the Growth Hormone [GH] secretagogue type 1 a receptor) [14]. It induces the release of growth hormone from the pituitary gland, stimulates food intake and lowering energy expenditure [15,16]. Ghrelin also inhibits the production of the proinflammatory cytokines IL-1a, IL-6, and TNF, but induces the anti-inflammatory cytokine IL- 10 [17]. Administration of Ghrelin in animal studies has resulted in an improvement in food intake, body weight, and Learns Body Mass (LBM) retention [18,19]. Ghrelin administration has therapeutic appeal for its anabolic activities, and Ghrelin plasma levels have been assessed in several observational studies of cachexia in cancer patients [20]. However, due to its short half-life (approximately 30 min) and intravenous administration, the clinical effectiveness of Ghrelin remains limited.
Ghrelin agonists, such as Anamorelin, carry potential in the treatment of CACS. Anamorelin HCL (ONO-7643: ANAM) is a potent and selective Ghrelin receptor agonist that mimics the N-terminal active core of Ghrelin [21]. ANAM has the advantage of being orally active and having a longer half-life (approximately 7hours) than Ghrelin [22,23].
Several clinical trials have found an increase growth hormone secretion following ANAM treatment in humans. Increasing growth hormone levels in the context of ANAM treatment in cancer patients raises concerns over potential for stimulating tumor growth. However, the significant increase in growth hormone produced in response to ANAM and even greater rise in growth hormone levels produced in response to Ghrelin following repeated daily dosing for 28 days were not associated with promotion of tumor growth in a murine model [24]. The A549 Non-Small Cell Lung Cancer (NSCLC) adenocarcinoma tumor cell line used in that study has been shown to possess a high degree of expression of the IGF-1 receptor [25]. Furthermore, in a Phase II clinical study in NSCLC patients, no statistically significant effect on long-term overall survival was noted after 12-weeks of treatment with 50 mg or 100mg ANAM compared with placebo [26].
NSCLC considerations
Lung cancer is the leading cause of cancer death worldwide with an estimated 1.37 million deaths attributed to lung cancer in 2008 [27]. Approximately 60 % of lung cancer patients show significant weight loss at the time of diagnosis, and more than 10 % of patients die with or from CACS itself. CACS and skeletal muscle wasting are commonly seen in NSCLC patients at baseline and are strongly associated with poor survival [28]. In addition, skeletal muscle wasting that develops during the course of chemotherapy (mostly caused by platinum therapy) in patients with advanced NSCLC had prognostic impact [29].
The large, randomized controlled ROMANA 1 and 2 trials are the first phase III international studies examining the impact of ANAM on CACS in patients with advanced NSCLC [30]. In the ROMANA studies, patients with unresectable stage III or IV NSCLC with cachexia were randomized (2:1) to receive either 100mg ANAM or placebo, given orally each day for 12 weeks. Among 484 participants in ROMANA 1, those taking ANAM experienced a median increase in LBM of 1.1kg in 12 weeks, compared to a loss of 0.44kg for those taking placebo. Body weight increased in the ANAM arm by an average of 2.2kg, compared to 0.14kg in the placebo arm of the study. Patient symptoms or concerns regarding anorexia-cachexia, including appetite, also significantly improved over 12 weeks in patients taking ANAM. The most drug-related adverse events included hyperglycemia (5.3%) and nausea (3.8%). In ROMANA 2, 495 participants with advanced NSCLC experienced similar benefits. Body weight increased by 0.95kg on average, compared to a loss of 0.57kg for those receiving placebo, and patient symptoms/concerns regarding anorexia-cachexia significantly improved over 12 weeks. Two Quality of Life (QoL) measures: the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) and the Functional Assessment of Anorexia/Cachexia Therapy (FAACT) were used. Two 4-item scales (fatigue/activity and appetite/eating) from the FACIT-F and FAACT questionares, respectively, demonstrated good internal consistency reliability, validity, and responsiveness [31].
Patients receiving ANAM did not experience improvements in their muscle strength, as measured by hand grip strength, but particular test can be difficult to administer in this patient population. The lack of effect of the drug on Hand-Grip Strength (HGS) as reported in the trial requires further explanation. HGS measures only upper but not lower extremity strength, and it does not inform enough about physical function and daily living. The populations studied are relatively young and in a good performance status, without information on multimodal management, namely reversible secondary nutrition impact symptoms. In addition, predominant male gender enrollment (ROMANA 1; 76.5%, ROMANA 2; 72.7%) might be affected the results. Further data need therefore to show whether the improved symptoms and concerns are related to the known mechanism of the oral Ghrelin agonist.
These studies had another limitation. The predominantly Caucasian (ROMANA 1; 98.8%, ROMANA 2; 96.8%) sample may limit the generalizability of the finding. Future Asian study will dissolve the problem. In addition, assessment for QoL methods has not been standardized yet. We have an interest in correlative biomarkers related to Ghrelin pathway and discovery of predictive biomarkers for ANAM.
In ROMANA studies, pooled overall survival was not significantly different between ANAM and placebo group (Hazard ratio=1.06 (95% CI 0.89-1.26), p=0.47). There is a hypothesis that eliminating the CACS might improve the prognosis of NSCLC. Thus the survival result was another discussion point. ANAM treatment for procahexiamight is more beneficial.
Other considerations in the study include permission of patients treated with concomitant chemotherapy or targeted therapy. In ROMANA studies, more than 70% subjects received concomitant chemotherapy (platinum based doublet or single agent) while only 3% received oral tyrosine kinase therapy. However, in Asian cohort, more subjects will be received molecular targeted therapies. Additionally, the thinking regarding the recent progress of immunotherapy for NSCLC has changed somewhat: proinflammatory cytokines and anti-inflammatory cytokines circumstances. These points have to be considered in future study.
Conclusion
Anorexia and cachexia are among the most troubling and distressing symptoms of advanced cancer, for both patients and their families. Symptoms of the wasting syndrome can include a loss of weight and muscles, together with fatigue, weakness, and loss of appetite. The condition is very common in patients with advanced lung cancer. Anamorelin aims to address the symptoms by mimicking the effects of the so-called “hunger hormone” ghrelin, which is secreted by the stomach. A new drug, anamorelin, improves appetite and body mass in patients with advanced lung cancer who are suffering cancer anorexia and cachexia, according to phase III data.
References
- Von Haehling S, Anker SD. Cachexia vs obesity: where is the real unmet clinical need? J Cachexia Sarcopenia Muscle. 2013; 4: 245-246.
- Vaughan VC, Martin P, Lewandowski PA. Cancer cachexia: impact, mechanisms and emerging treatments. J Cachexia Sarcopenia Muscle. 2013; 4: 95-109.
- Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011; 12: 489-495.
- Donohoe CL, Ryan AM, Reynolds JV. Cancer cachexia: mechanisms and clinical implications. Gastroenterol Res Pract. 2011; 601434.
- Dewys WD, Begg C, Lavin PT, Band PR, Bennett JM, Bertino JR, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med. 1980; 69: 491-497.
- Kumar NB, Kazi A, Smith T, Crocker T, Yu D, Reich RR, et al. Cancer cachexia: traditional therapies and novel molecular mechanism-based approaches to treatment. Curr Treat Options Oncol. 2010; 11: 107-117.
- Andreyev HJ, Norman AR, Oates J, Cunningham D. Why do patients with weight loss have a worse outcome when undergoing chemotherapy for gastrointestinal malignancies? Eur J Cancer. 1998; 34: 503-509.
- Ross PJ, Ashley S, Norton A, Priest K, Waters JS, Eisen T, et al. Do patients with weight loss have a worse outcome when undergoing chemotherapy for lung cancers? Br J Cancer. 2004; 90: 1905-1911.
- Tan CR, Yaffee PM, Jamil LH, Lo SK, Nissen N, Pandol SJ, et al. Pancreatic cancer cachexia: a review of mechanisms and therapeutics. Front Physiol. 2014; 5: 88.
- Tsai VW, Husaini Y, Manandhar R, Lee-Ng KK, Zhang HP, Harriott K, et al. Anorexia/cachexia of chronic diseases: a role for the TGF-β family cytokine MIC-1/GDF15. J Cachexia Sarcopenia Muscle. 2012; 3: 239-243.
- Soenen S, Chapman IM. Body weight, anorexia, and undernutrition in older people. J Am Med Dir Assoc. 2013; 14: 642-648.
- Sakuma K, Yamaguchi A. Sarcopenia and cachexia: the adaptations of negative regulators of skeletal muscle mass. J Cachexia Sarcopenia Muscle. 2012; 3: 77-94.
- von Haehling S, Steinbeck L, Doehner W, Springer J, Anker SD. Muscle wasting in heart failure: An overview. Int J Biochem Cell Biol. 2013; 45: 2257-2265.
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999; 402: 656-660.
- Jarkovska Z, Krsek M, Rosieka M, Marek J. Endocrine and metabolic activities of a recently isolated peptide hormone ghrelin, an endogenous ligand of the growth hormone secretagogue receptor. EndocrRegul. 2004; 38: 80-86.
- Fanzani A, Conraads VM, Penna F, Martinet W. Molecular and cellular mechanisms of skeletal muscle atrophy: an update. J Cachexia Sarcopenia Muscle. 2012; 3: 163-179.
- Akamizu T, Kangawa K. Ghrelin for cachexia. J Cachexia Sarcopenia Muscle. 2010; 1: 169-176.
- Wang W, Andersson M, Iresjö BM, Lönnroth C, Lundholm K. Effects of ghrelin on anorexia in tumor-bearing mice with eicosanoid-related cachexia. Int J Oncol. 2006; 28: 1393-1400.
- DeBoer MD, Zhu XX, Levasseur P, Meguid MM, Suzuki S, Inui A, et al. Ghrelin treatment causes increased food intake and retention of lean body mass in a rat model of cancer cachexia. Endocrinology. 2007; 148: 3004-3012.
- Legakis I, Stathopoulos J, Matzouridis T, Stathopoulos GP. Decreased plasma ghrelin levels in patients with advanced cancer and weight loss in comparison to healthy individuals. Anticancer Res. 2009; 29: 3949-3952.
- Morozumi N, Hanada T, Habara H, Yamaki A, Furuya M, Nakatsuka T, et al. The role of C-terminal part of ghrelin in pharmacokinetic profile and biological activity in rats. Peptides. 2011; 32: 1001-1007.
- Kumor K, Polvino W. Biologic activity of RC-1291, a novel oral ghrelin mimetic for cancer anorexia/cachexia: results from a Phase I randomized, double-blind, placebo-controlled trial in healthy volunteers. Support Care Cancer. 2006; 14: 593. Abstract 03-009.
- Blum R, Polvino W. Pharmacokinetic (PK) profile of RC-1291, a novel oral ghrelin mimetic for the treatment of cancer anorexia/cachexia. Support Care Cancer. 2006; 14: 593. Abstract 03-010.
- Northrup R, Kuroda K, Duus EM, Barnes SR, Cheatham L, Wiley T, et al. Effect of ghrelin and anamorelin (ONO-7643), a selective ghrelin receptor agonist, on tumor growth in a lung cancer mouse xenograft model. Support Care Cancer. 2013; 21: 2409-2415.
- Gong Y, Yao E, Shen R, Goel A, Arcila M, Teruya-Feldstein J, et al. High expression levels of total IGF-1R and sensitivity of NSCLC cells in vitro to an anti-IGF-1R antibody (R1507). PLoS One. 2009; 4: e7273.
- Temel J, Bondarde S, Jain M, Allen S, Mann W. Efficacy and safety of anamorelin HCL in NSCLC patients: results from a randomized, double-blind, placebo-controlled, multicenter phase II study. Eur J Cancer. 2013; 49 (Suppl 2): Abstract 1308.
- World Health Organization. Cancer-Fact sheet No. 297. In : Editor (ed) (eds) Book Cancer-Fact sheet No. 297, City. 2013.
- Ross PJ, Ashley S, Norton A, Priest K, Waters JS, Eisen T, et al. Do patients with weight loss have a worse outcome when undergoing chemotherapy for lung cancers? Br J Cancer. 2004; 90: 1905-1911.
- Kimura M, Naito T, Kenmotsu H, Taira T, Wakuda K, Oyakawa T, et al. Prognostic impact of cancer cachexia in patients with advanced non-small cell lung cancer. Support Care Cancer. 2015; 23: 1699-1708.
- Temel JS, Currow DC, Fearon KF, Yan Y, Friend J, Abernethy AP. Phase III trials of anamorelin in patients with advanced non-small cell lung cancer (NSCLC) and cachexia (ROMANA 1 and 2). J ClinOncol 2015; 33: Suppl Abstract 9500.
- Salsman JM, Beaumont JL, Wortman K, Yan Y, Friend J, Cella D. Brief versions of the FACIT-fatigue and FAACT subscales for patients with non-small cell lung cancer cachexia. Support Care Cancer. 2015; 23: 1355-1364.