
Review Article
Austin J Lung Cancer Res. 2016; 1(1): 1002.
Cigarette Smoking and Nicotine Addiction
Valayil JM*
Department of Biochemistry, Indian Institute of Science, India
*Corresponding author: Valayil JM, Department of Biochemistry, Indian Institute of Science, Bangalore, India
Received: November 29, 2015; Accepted: December 22, 2015; Published: January 04, 2016
Abstract
Tobacco smoking is apparently the major reason for the increasing incidence of lung cancers. Nicotine, an alkaloid in present in tobacco accounts for the addictive nature of tobacco. Nicotine is an exogenous agonist to the nicotinic Acetylcholine Receptors (nAChRs) distributed throughout the central and peripheral nervous system. Upon administration, nicotine binds to the nAChRs and modulates the neurotransmitter release. Studies relate the pleasurable effects of cigarette smoking to the nicotine induced increase in dopamine levels in the mesocorticolimbic system in mammals. Understanding the molecular mechanisms underlying nicotine addiction enables the development of effective strategies for smoking cessation.
Keywords: Lung cancer; Tobacco; Nicotine; Dopamine; Nicotinic acetyl choline receptors; Synaptic plasticity
Introduction
Lung cancer was a rare disease at the start of the 20th century before the massive introduction of cigarettes [1]. Currently, lung cancer accounts for more than one quarter of cancer deaths worldwide [2]. Epidemiological studies unassailably demonstrate a consistent correlation between tobacco smoking and the incidence of lung cancer [3]. Tobacco smoke is a cocktail of several toxic chemicals of which more than 60 are known to bind and mutate DNA [4,5]. While mainstream smoking is undoubtedly the leading risk factor, several studies associate the incidence of lung cancer to Environmental Tobacco Smoke (ETS) exposure in non-smokers [6,7].
Tobacco smoke is a complex and dynamic chemical mixture whose composition varies with tobacco type and preparation, characteristics of cigarette and several other factors [8,9]. Among the different carcinogens in cigarette smoke, polycyclic aromatic hydrocarbons and tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3- pyridyl)-1-butanone are known to play the major roles [10]. In addition to the chemicals that directly contribute to its mutagenic and carcinogenic effects, tobacco smoke also contains chemicals like nicotine, responsible for its addiction and rein forcibility [11]. Studies report that nearly 80% of the smokers attempting to quit smoking fail within a year, and those who succeed usually have tried to self restraint recurrently [12]. It is this seductive addiction to tobacco that makes the disease, a chronic health issue. A detailed understanding of the pharmacological effects of nicotine that causes tobacco addiction is a necessary basis for optimal smoking cessation intervention. This article reviews the pharmacological effects of nicotine and the underlying cellular events that account for tobacco addiction.
History of tobacco smoking
The cultivation and usage of the tobacco plant, Nicotiana, ages back as early as 5000 BC in the Americas. However, the populations used the plant largely for its medicinal properties and in ceremonies rather than its pleasurable effects. Later, it was introduced in Europe in the 16th century by the early explorers from where it spread around the globe with the progress of European civilization. Tobacco was an inevitable part of medical practice and was celebrated as a ‘holy herb’ curing various diseases [13]. Prior to the 1820s, tobacco was chewn, drunk or sniffed. Tobacco smoking became popular in the 19th century with the advent of handmade and machine manufactured cigarettes.
Even in the first flush of enthusiasm for the medicinal properties of tobacco, people criticized its efficacy. With the isolation of nicotine, a dangerous alkaloid from tobacco leaves in 1828, the medical world began to doubt the usage of tobacco as a general treatment. Although many reported the adverse effects of tobacco smoking, the criticisms were largely ignored and out shadowed by the massive profit from tobacco trade. The rates of cigarette smoking increased dramatically in the 20th century and the medical practitioners observed an increasing incidence of lung cancer in 1920s and 1930s [14]. The first conclusive evidence on the adverse health effects of cigarette smoking came up in the 1950s with the publication of retrospective studies on the prevalence of lung cancer in tobacco smokers [15-18]. In 1957, the US public health service issued its first statement on tobacco smoking as a cause of lung cancer [19] and in 1962 the Royal College of Physicians of London reviewed the scientific evidence on smoking and health and concluded that tobacco smoking was undoubtedly a cause of lung cancer [20]. The US Surgeon General commissioned further investigations which also came to the same conclusions [21,22]. Ever since, there have been accumulating evidence on the toxicology of tobacco smoke and its health impacts on smokers as wells as non smokers.
Chemistry and toxicology of tobacco smoke
Cigarette smoke is a complex mixture of more than 4000 different chemical compounds [23]. The composition of cigarette smoke varies with cigarette design as well as the chemical nature of the product. Upon pyrolysis, the chemicals in tobacco are distilled into smoke, or react to form constituents that are distilled to smoke. Cigarette smoking imparts serious threats of air pollution, thereby extending its health hazards to nonsmokers as well. The air in the immediate vicinity of an active smoker contains a mixture of exhaled mainstream smoke released from the butt end of the burning cigarette and side stream smoke that results from the burning cigarette coal when it smolders [24]. Gases, mainly nitrogen, oxygen and carbon dioxide constitute 95% of the smoke. The particulate phase contains thousands of compounds, among which the carcinogenicity of nearly 60 are well documented [25].
Poly Amino Hydrocarbons (PAHs) and Tobacco Specific Nitrosamines (TNSAs) are among the well studied tobacco carcinogens. These compounds undergo metabolic activation and form DNA adducts which, unless repaired result in miscoding and mutations. Although there exist specific competing detoxification mechanisms, the delicate balance between metabolic activation and detoxification determines tumor induction. Mutations in critical growth control genes such as epidermal growth factor gene, RAS and the tumor suppressor gene p53 are known to alter the normal cellular growth control mechanisms, promoting the development of cancer [26-28].
Benzo[a] pyrene, a PAH is the most extensively studied carcinogen whose potency to induce lung tumors is well studied [29-31]. Two aza-arenes, bibenz[a,h] acridine and 7H-dibenzo[c,g] -carbazole, although present in minor concentrations in cigarette smoke, are known to induce lung tumors in rats and hamsters [32,33]. Among the TNSAs, 4-(methylnitrosamino) -1-(3-pyridyl) -1-butanone (NNK), is a potent lung carcinogen shown to induce pulmonary tumor formation in rats, mice and hamsters [34]. Investigations have demonstrated that 1,3 - butadiene, a chemical in tobacco smoke imparts high cancer risks [35]. In addition, tobacco smoke also contains metals like nickel, chromium, cadmium and arsenic and the radioactive compound polonium (210Po) which are potent lung carcinogens. Substantial concentrations of co-carcinogens such as catechols, methylcatechols, pyrogallol, decane, undecane, pyrene, benzo [e] pyrene and fluoranthene are also detected in tobacco smoke [36]. It has been reported that cyanide, arsenic and cresols present in tobacco smoke increase the incidence of cardiovascular diseases. Acrolein and acetaldehyde are known to cause respiratory irritations. Apart from the toxic carcinogenic and mutagenic chemicals, tobacco smoke contains stable and unstable free radicals and Reactive Oxygen Species (ROS) in the particulate and gas phase which can cause extensive oxidative damage in cells [37]. Taken together, tobacco smoke contains plethora of toxic chemicals, the chronic exposure to which can cause cancers and other serious health issues.
Tobacco addictives
Nicotine, an alkaloid present in tobacco leaves account for much of its addictive nature [38-40]. It is a tertiary amine consisting of a pyridine and a pyrrolidine ring. Nicotine molecule partly resembles the neurotransmitter acetylcholine and hence serves as an agonist to the nicotinic Acetylcholine Receptors (nAChRs) found throughout the central and peripheral nervous system [41]. The psycoactive effects of nicotine results from its action on these brain nicotinic cholinergic receptors facilitating the release of neurotransmitters (dopamine and others), producing pleasure, stimulation and mood modulation. Tobacco also contains several other pharmacologically active minor alkaloids such as anatabine, anabasine, N-methylanabasine, anabaseine and nornicotine. Dwoskin and colleagues reported that these alkaloids induce dopamine release from striatal brain tissue in rats [42]. Although these chemicals can be addictive when administered alone at sufficient concentrations, their minor concentrations in tobacco leave suggest their contributions towards the addictive nature of tobacco to be meagrer. These alkaloids probably act in synergy with nicotine to generate the addictive effects.
Nicotine is a volatile alkaloid, the absorption and renal excretion of which largely depends on pH. At alkaline pH, nicotine exists in the nonionized state and hence can readily cross the lipoprotein membranes, whereas at acidic pH, nicotine remains ionized and hence is poorly absorbed across the membranes. When a person inhales cigarette smoke, nicotine is distilled from tobacco and is carried to the lungs. The physiological pH of pulmonary fluid and the large surface area of alveoli favors the rapid absorption of nicotine into the pulmonary venous circulation. It then enters the arterial circulation and moves to the brain where it binds to the nicotinic cholinergic receptors opening the channels, facilitating the entry of cations and subsequent release of neurotransmitters. Nicotine in oral products having alkaline pH are readily, but gradually absorbed across the oral mucosa compared to the absorption across the alveolar surface. When nicotine is swallowed, the acidic pH in the stomach does not favor its absorption, whereas the alkaline pH in small intestine supports its absorption. However, unlike when swallowed, the bioavailability of nicotine is higher when absorbed through lungs and oral mucosa since it reaches the systemic circulation before passing through the liver.
The neurobiology of nicotine addiction
The mechanisms mediating nicotine dependence are an active area of research since nicotine is the major addictive agent in tobacco. Nicotine dependence has two aspects – 1) the persistence of smoking behavior and 2) the emergence of withdrawal symptoms upon cessation [43,44]. A better understanding of the neuromodulatory effects of nicotine forms the basis of an effective smoking cessation intervention.
Neuronal nicotinic acetyl choline receptors (nAChRs)
\nAChRs play a key role in the neuroadaptations mediating tobacco dependence. nAChRs are ligand-gated ion channels composed of five membrane spanning subunits arranged like a rosette around a water filled pore [45,46]. Different combinations of nAChR subunits generate receptors that differ in their pharmacokinetics and functional properties. The neuronal α subunit exists in nine isoforms (α2 to α10) and the neuronal β subunit exists in three isoforms (β2, β3, and β4). α2 to α6 nAChR subunits combine with β2 to β6 subunits to form hetero oligomeric receptors, some of which share similar pharmacokinetic properties [47]. Subunits α7 to α9 form only homo oligomeric receptors and α7 is dominantly expressed in the mammalian central nervous system [48].
The subunit composition of nAChRs is a crucial factor determining its specialized functions and properties such as ligand affinity, ion permeability and desensitization. Studies have illustrated that although nicotine serves as an exogenous agonist to various nAChR subtypes, the one containing α4 and β2 subunits bind nicotine with highest affinity [49,50]. Ligand affinity also varies with different stochiometric combinations of subunits. Among the different stochiometric combinations of α4β2 receptor subtype, such as (α4)2(β2)3, (α4)3(β2)2 and (α4)2(β2)2(α5), (α4)2(β2)3 nAChR is most sensitive to upregulation by nicotine [51]. Studies reveal that the inclusion of α5 subunit in α4β2 receptor subtype enhances receptor assembly and expression, reduce the relative magnitude of ligandmediated upregulation and facilitate receptor channel closure [52,53]. In the Ventral Tegmental Area (VTA) and substantia nigra, α6 and possibly β3 subunits are included in α4β2 nAChR complexes resulting in high affinity receptors [54]. α7 homomeric nAChRs desensitize rapidly and facilitate comparatively high Ca2+:Na+ permeability ratio [55]. As a result, the opening of α7 nAChR channels can mediate the increased release of neurotransmitters and impact on several Ca2+ dependent mechanisms, including activation of second messenger pathways quite distinct from other nAChRs [56].
Neuronal nAChRs are predominantly located in presynaptic terminals and hence are believed to modulate the release of various neurotransmitters including acetylcholine, glutamate, GABA and dopamine [57]. Nevertheless nAChRs situated on post synaptic sites contribute a small minority of fast excitatory transmission [58]. Several studies have revealed that nAChRs are involved in maintaining the resting membrane potential, modulation of synaptic transmission and mediation of fast excitatory transmission. The role of nAChRs in the development of synaptic plasticity, learning, memory and attention are well documented [59-61].
Nicotinic modulation of neurotransmitter release
The pharmacological effects of nicotine in mammals result from its binding at the nAChRs and subsequent modulation of neurotransmitter release. Nicotine from tobacco bathes all of the brain and hence reaches nAChRs at synaptic as well as nonsynaptic locations. Nicotine influences many neuronal circuits and functions due to the diverse distribution and the roles of functionally distinct nAChRs. It has been shown that nicotine enhances the levels of dopamine in the mesocorticolimbic system by increasing the firing rate of dopaminergic neurons [62]. Blocking dopamine release in the nucleus accumbens with antagonists abrogates the rewarding effects of nicotine, as indicated by reduced self-administration in rats [59]. Rat brain slices, when administered with nicotine at concentrations as obtained from tobacco smoke showed activation of nAChRs on mesolimbic dopaminergic neurons and subsequent modulation of neurotransmitter release [63,64].
Extensive studies have demonstrated the involvement of the mesolimbic system in the mammalian midbrain in the reinforcing effects of drug abuse [65]. The region contains dopaminergic neurons originating from the VTA and projecting into the nucleus accumbens and the frontal cortex. A common feature of addictive drugs is that they increase dopamine levels in the nucleus accumbens by regulating the activity of these dopaminergic projections [66, 67]. The activity of VTA dopaminergic neurons is primarily regulated by the release of the excitatory neurotransmitter glutamate from neuronal projections originating from the nucleus accumbens and the frontal cortex. Other inputs that regulate the activity of the mesolimbic system are γ-Amino Butyric Acid (GABA) inhibitory interneurons located within the VTA and the nucleus accumbens and cholinergic projections from brainstem nuclei to the VTA. To date, three cell types in the VTA have been shown to express nAChRs: Dopamine neurons, GABA neurons, and glutamatergic presynaptic terminals that synapse onto dopamine neurons.
The neurons within the VTA have a wide variety of nAChRs, the differential distribution of which has important functional consequences on the nicotine signalling in the mesolimbic system [68]. The concentration of agonist required activating and desensitizing the receptor, the speed of activation, the ionic permeability, the rates of desensitization and recovery from desensitization, all depends on the subunit composition of the mature receptor. Dopaminergic neurons of the VTA express α2–α7 and β2–β4 subunits, which can give rise to at least three pharmacologically distinct nAChR subtypes, of which one is a homomeric α7 receptor [69,70]. Nearly 25% of the GABA neurons express the α3, α5, α6 and β4 subunits, indicating that most nAChRs of these VTA neurons contain α4β2 receptors [71]. Less than half of the VTA neurons express homomeric α7 nAChRs [72].
The nicotine concentration in a smoker’s blood reaches 300–500 nM, a few minutes after the initiation of smoking and eventually concentrations close to 250 nM are sustained [73]. This low nicotine concentration is sufficient enough to activate the high-affinity α4β2 and α3β2 nAChR subtypes on VTA dopaminergic neurons leading to their increased firing and dopamine release. However, the sustained nicotine concentrations cause these receptors to desensitize in few minutes [74]. Recent studies show that the α6β2 nAChRs expressed in the VTA are necessary for the effects of nicotine on dopaminergic neuron activity and dopamine dependent behaviors such as locomotion and reinforcement [75]. The early, acute effects of nicotine in the VTA activates the GABA neurons as well, but similar to the nAChRs on VTA dopaminergic neurons, the ones that are associated with these cells also desensitize rapidly relieving the inhibitory control [76,77]. Desensitization not only prevents further activation of nAChRs by nicotine, but also precludes their responsiveness to endogenous cholinergic signaling. As a result, the dopaminergic neurons in the VTA receive less inhibitory GABAergic input than prior to nicotine’s arrival in the VTA, and this decrease of inhibitory tone results in increases in action potential firing. The rapid desensitization of VTA dopaminergic neuron receptors suggests the existence of additional mechanisms that induces the long-term enhancement of dopamine release. The glutamergic terminal in the VTA expresses α7 nAChRs and the low nicotine concentrations associated with tobacco use induce much less desensitization of these receptors. This results in the enhanced glutamergic signaling and the subsequent excitation of VTA dopaminergic neurons [78]. This nicotine-induced glutamate release when paired with a postsynaptic depolarization of the dopaminergic neurons, relieves the Mg2+ block of the NMDA receptors, resulting in long-term synaptic potentiation. Once the afferent glutamergic transmission is potentiated, it continues to excite the dopaminergic neurons, resulting in high dopamine levels in the mesolimbic system. The nicotinic activity resulting in the long term potentiation is capable of influencing synaptic plasticity in the dopaminergic system [79]. The long-term nicotine exposure causes an increase in the number of nAChRs in the brains of humans, rats and mice, especially those subtypes with a high nicotine binding affinity [80,81].
The molecular mechanisms underlying nicotinic signaling can well explain the common smoking behavior and patterns (Figure 1). Most smokers report that the first cigarette of the day is the most pleasurable [82]. This is because, following overnight abstinence, nicotine concentrations in the brain is at their lowest level and the nAChRs have mostly recovered from desensitization. Thus, smoking the first cigarette strongly activates nAChRs, likely inducing the greatest dopamine release and contributing to the most pleasurable impact. After a few cigarettes, there is much receptor desensitization, causing less impact from additional cigarettes. Most smokers maintain 2-5 hours of interval between the episodes of cigarette smoking. During these intervals, the plasma nicotine levels drop and some nAChRs recover from desensitization [83,84]. As already discussed, long term exposures to nicotine results in the increased expression of nAChRs as a homeostatic response to the increased nAChR desensitization [85]. It has been reported that in chronic smokers, avoidance of nicotine for few weeks lowers the number of nAChRs towards the lower value as seen in nonsmokers. However, the complete desensitization of nAChRs after a night of abstinence induces carving due to which most smokers fail to quit. After years of smoking, the neuroadaptations and long-term synaptic changes results in the learned behaviors, some of which are associated with the environmental cues associated with smoking. Studies suggest that nicotinic mechanisms influencing synaptic plasticity underlie learning and memory, providing a link between nicotine addiction and learned associates within the context of tobacco usage [86]. Even after years of abstinence, this remodeling of brain circuits induces the rein forcibility of smoking and the extraneous factors associated with smoking can become independent motivators of rewarding behavior [87,88].
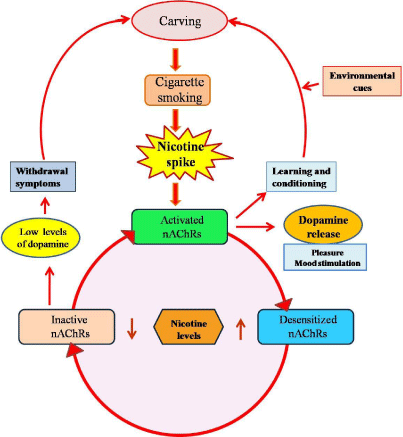
Figure 1: Nicotine depletion repletion cycle.
Nicotine acts on nicotinic acetyl choline receptors (nAChRs), triggering the
release of dopamine and other neurotransmitters that produce rewarding
psychoactive effects. Desensitization of the receptors following the nicotine
spike results in short-term tolerance of nicotine and reduced satisfaction
from smoking. Nicotinic activation of nAChRs also results in neural plasticity
leading to behavioral conditioning. Due to this neuronal remodulation, the
smoking associated environmental cues can serve as stimuli inducing
carving. In the absence of nicotine, nAChRs regain their sensitivity to nicotine
and become reactivated in response to a new dose. In the intervals between
smoking episodes or after abstinence, brain nicotine levels decline, this leads
to reduced levels of dopamine resulting in depression, tension and other
withdrawal symptoms, including craving.
Pharmacotherapy for smoking cessation
As already discussed, effective smoking cessation requires the treatment of positive reinforcement associated with nicotine dependence as well as the negative reinforcement associated with its withdrawal. Smoking cessation strategies often aim to lower the urge to smoke, at each stage than to remain abstinent. Nicotine Replacement Therapies (NRTs), were the first pharmacological approaches approved by the Food and Drug Administration (FDA) for use in smoking cessation therapy [89]. NRT comes in various formats such as nicotine patches, chewing gums, nasal sprays, inhalers and tablets. The goal of NRT is to replace nicotine obtained from cigarette smoking and thereby reduce the motivation to smoke and ease the transition from cigarette smoking to abstinence [90]. Transdermal nicotine patches, maintain a steady state infusion of nicotine and the peak plasma nicotine concentrations are reached over 4-6 hours. In contrast, the other forms are faster acting and require repeated administration, but are found to be effective in relieving cue provoked acute carvings. Hence combination NRTs with nicotine patch combined with short-acting forms prove more effective than single form NRTs in smoking cessation [91].
Although NRT remains the first line drug treatment for smoking cessation, few other non-nicotine drugs such as bupropion and varenicline have also proved to be potent (Table 1). Bupropion, initially introduced as an antidepressant was subsequently noticed to attenuate the urge to smoke cigarettes in clinical trials. Although the precise mechanism of action of bupropion as a smoking cessation aid remains unclear, extensive investigations suggest that the compound exerts its effect primarily through the inhibition of dopamine reuptake into neuronal synaptic vesicles in nucleus accumbens [92,93]. This dopamine uptake inhibition reduces the severity of dopamine deficiency and withdrawal symptoms upon abstinence. Moreover, studies report the ability of bupropion to act as a noncompetitive antagonist at the postsynaptic nAChRs and thereby block the pharmacological effects of nicotine [94]. Bupropion is also found to inhibit nicotine induced vesicular release of dopamine. All these contribute to the efficacy of bupropion as an anti smoking agent. A systematic review of randomized trials shows that varenicline, a partial nicotinic agonist supports smoking cessation more effectively compared to single form NRT and bupropion [95]. Varenicline stimulates and blocks nicotinic receptors in the brain. Hence, while smoking it blocks nicotine from binding to the VTA nAChRs and by that attenuates the reward from cigarettes and undermines the learnt drive to smoke [96].
Pharmacotherapy
Common side-effects
Dosage
Duration
Availability
Nicotine patches
Local skin reaction,
insomnia, vivid dreams
7 , 14 and 21 mg patches /24h,
15 mg patch/16h
8 - 10 weeks
Nicoderm CQ,
Nicotrol
Nicotine inhaler
Headache, dyspepsia,
mouth and throat irritation,
cough, rhinitis
6-16 4 mg cartridges/day.
Puff each cartridge for up to 20 min
Up to 12 weeks
Nicotrol inhaler
Nicotine gum
Headache, dyspepsia,
nervousness
2 or 4 mg /piece
Week 1-6: 1 piece every 1-2 h
Week 7-9: 1 piece every 2-4 h
Week 10-12: 1 piece every 4-8 h
12 weeks
Nicotex,
Nicogum
Nicotine lozenge
Dyspepsia, xerostomia
2 or 4 mg /lozenge
Week 1-6: 1 lozenge every 1-2 h
Week 7-9: 1 lozenge every 2-4 h
Week 10-12: 1 lozenge every 4-8 h
12 weeks
Commit lozenge
Nicotine nasal spray
Sneezing, coughing,
watery eyes, throat irritation
1 dose = 1 spray/nostril
8-40 doses/day
1-2 doses/h, not to exceed 5 doses/h
or 40 doses/day
3 - 6 months
Nicotrol NS
Bupropion
Tachycardia, headache,
insomnia, xerostomia,
weight loss, dizziness
150 mg/day for 3 days
followed by 150 mg twice daily
7 - 12 weeks,
maintainance up to
6 months
Zyban
Varenicline
Nausea, insomnia, headache
0.5 mg/day for 3 days
followed by 0.5 mg twice daily
for 4 days and then 1 mg twice daily
3 months,
maintainance up to
6 months
Chantix
Table 1: Smoking cessation first-line pharmacotherapy.
Second line medications for smoking cessation include nonnicotine drugs such as nortiptyline and clonidine. Both the agents help to ameliorate the symptoms of tobacco withdrawal. While nortiptyline brings about its effects by inhibiting noradrenaline and serotonin reuptake, clodinine acts by down turning noradrenaline release [97,98]. The administration of nicotinic receptor antagonists should provide a means of extirpating both primary and secondary rein forcers associated with smoking. Thus, nicotine blockade therapy using nicotinic antagonists such as mecamylamine presents a promising new approach to smoking cessation [99]. NicVax, an experimental conjugate vaccine has been shown effective in treating nicotine addiction in clinical trials [100,101]. NicVax consists of the hapten 3’-aminomethylnicotine conjugated Pseudomonas aeruginosa exoprotein A. NicVAX stimulates the immune system to make antibodies that bind to nicotine molecules, forming complexes that are too big to cross the blood-brain barrier and preventing them from reaching brain nicotine receptors. Effective smoking cessation interventions require a combination of behavioral support with pharmacotherapies.
Genetics of nicotine dependence
In the recent years, several studies have brought to light the involvement of genes in the development of nicotine dependence. Studies conducted in twins, adoption and separated twins, have consistently suggested a strong genetic influence on smoking behavior [102,103]. Attempts to identify the genes underlying nicotine addiction are rather complicated due to the involvement of multiple genes and environmental factors. So far, genes coding for nicotine receptor subtypes, dopamine receptors and dopamine transporters, GABA receptors, opiate and cannabinoid receptors and other types of receptors have been associated with different aspects of smoking behavior [104]. Genome wide association studies reveal the genes within the α5/α3/β4 nicotinic cholinergic receptor gene complex on chromosome 15 to be the most prominent genetic determinant of nicotine dependence [105,106]. The variants in the α5/α3/β4 gene region are found to have a profound influence on the number of cigarettes smoked per day, plasma levels of cotinine (a biomarker of nicotine intake), urine levels of tobacco-smoke carcinogens and the risks of smoking-related diseases [107]. Nicotine metabolism is profoundly influenced by the genes coding for the hepatic enzymes cytochrome P450 2A6 (CYP2A6) and cytochrome P450 2D6 (CYP2D6) of which CYP2A6 is responsible for about 90% of the metabolic inactivation of nicotine to cotinine [108]. It has been demonstrated that CYP2D6 polymorphisms enhance the metabolic detoxification of nicotine and hence the probability of being addicted to smoking [109]. Several genes affecting cell adhesion and extracellular matrix molecules have also been identified as having a strong genetic association to smoking behaviour [110]. These findings support the idea that neural plasticity and learning are also crucial determinants of individual differences in vulnerability to nicotine dependence [111]. Overall, variants in two broad classes of genes influence smoking behavior – 1) genes that influence nicotine response (nicotine metabolism, nicotinic receptors) and 2) genes that predispose to addictive behaviour due to their effects on key neurotransmitter pathways (dopamine, serotonin, opioid). Individuals with reduced nicotine metabolism and high dopamine levels are likely to be less addicted to smoking, whereas those with increased nicotine metabolism and lower dopamine levels have more chances of developing addiction [112,113].
Genetic variation strongly affects the efficacy of pharmacotherapies employed for smoking cessation. Individuals with decreased dopamine or serotonin levels due to decreased synthesis, increased re-uptake or increased metabolism are likely to exhibit severe withdrawal symptoms upon abstinence and hence seem to achieve better cessation rates with antidepressants such as bupropion and nortriptyline [114-116]. Smokers with increased or normal number and activity of nAChRs or decreased nicotine detoxification exhibit better responses with NRT as well as varenicline [117-120]. Varenicline also proves effective for individuals with high nicotine detoxification for whom NRTs are inadequate [121]. In addition, the differences in the metabolism or elimination of drugs can also influence their potency. For instance, smokers with increased bupropion metabolism and high expression of nicotinic receptors exhibit better quit rates with bupropion [122]. Variants of Organic Cation Transporter 2 (OCT2), resulting in a high varenicline transporter activity and elimination, benefit more from NRT [123]. All these point to the fact that genotyping smokers can provide better directions in determining the most effective treatment strategy.
Conclusion
The pharmacopsycological effects of nicotine underlie the sustained tobacco smoking behavior. A great deal of progress has been made in the understanding of molecular and cellular basis of nicotine addiction. Accumulating evidences point to the nAChRs and the levels of dopamine in the mesolimbic system as the major factors influencing nicotine dependence. These advances have paved way to the successful development of novel and effective therapeutics for smoking cessation. For instance, varenicline is a partial agonist at the high affinity α4β2 nAChRs, the administration of which maintains a modest level of dopamine in the brain and hence reduces the withdrawal symptoms and blocks the reinforcing effects of smoking during abstinence [124]. Further detailed understanding of nicotine addiction with special focus on neuroadaptations and neuronal plasticity can enable the development of better medications and intervention strategies to help people quit and stay abstinent. Unraveling the genetic basis of smoking behavior would enable genetically tailored, personalized smoking cessation therapies in the coming decades.
References
- Stewart BW, Kleihues P. World cancer report. Lyon: IARC press. 2003.
- Siegel RL, Miller KD, Jemal A. Cancer statistics. CA: a cancer journal for clinicians. 2015; 65: 5-29.
- Cornfield J, Haenszel W, Hammond EC, Lilienfeld AM, Shimkin MB, Wynder EL. Smoking and lung cancer: recent evidence and a discussion of some questions. 1959. Int J Epidemiol. 2009; 38: 1175-1191.
- Pfeifer GP, Denissenko MF, Olivier M, Tretyakova N, Hecht SS, Hainaut P. Tobacco smoke carcinogens, DNA damage and p53 mutations in smoking-associated cancers. Oncogene. 2002; 21: 7435-7451.
- DeMarini DM. Genotoxicity of tobacco smoke and tobacco smoke condensate: a review. Mutat Res. 2004; 567: 447-474.
- Dockery DW, Trichopoulos D. Risk of lung cancer from environmental exposures to tobacco smoke. Cancer Causes Control. 1997; 8: 333-345.
- Hackshaw AK, Law MR, Wald NJ. The accumulated evidence on lung cancer and environmental tobacco smoke. BMJ. 1997; 315: 980-988.
- Fowles J, Dybing E. Application of toxicological risk assessment principles to the chemical constituents of cigarette smoke. Tob Control. 2003; 12: 424-430.
- Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat Rev Cancer. 2003; 3: 733-744.
- Hecht SS. Tobacco smoke carcinogens and lung cancer. J Natl Cancer Inst. 1999; 91: 1194-1210.
- Benowitz NL. Nicotine addiction. N Engl J Med. 2010; 362: 2295-2303.
- Balfour DJ, Fagerström KO. Pharmacology of nicotine and its therapeutic use in smoking cessation and neurodegenerative disorders. Pharmacol Ther. 1996; 72: 51-81.
- Dickson SA. Panacea or Precious Bane. Tobacco in 16th Century Literature. New York: New York Public Library. 1954.
- White C. Research on smoking and lung cancer: a landmark in the history of chronic disease epidemiology. Yale J Biol Med. 1990; 63: 29-46.
- Doll R, Hill AB. Smoking and carcinoma of the lung; preliminary report. Br Med J. 1950; 2: 739-748.
- Levin ML, Goldstein H, Gerhardt PR. Cancer and tobacco smoking; a preliminary report. J Am Med Assoc. 1950; 143: 336-338.
- Schrek R, Baker LA, Ballard GP, Dolgoff S. Tobacco smoking as an etiological factor in disease. I. Cancer. Cancer Res. 1950; 10: 49–58.
- Wynder EL, Graham EA. Tobacco smoking as a possible etiological factor in bronchogenic carcinoma. A study in six hundred and eighty four proved cases. J Am Med Assoc. 1950; 143: 329–336.
- US Public Health Service. Smoking and Health. Report of the Advisory Committee to the Surgeon General of the Public Health Service (Washington). US Department of Health Education and Welfare (PHS). 1964.
- Evans PA. Smoking and health. Summary of a report of the Royal College of Physicians of London on smoking in relation to cancer of the lung and other diseases. Cent Afr J Med. 1962; 8: 234-236.
- USDHEW. The Health Consequences of Smoking. A Public Health Service Review. 1967: PHS 1696.
- USDHEW. The Health Consequences of Smoking. A Public Health Service Review. 1969.
- Rodgman A, Perfetti TA. The Chemical Components of Tobacco and Tobacco Smoke. Boca Raton (FL): CRC Press. Taylor & Francis Group. 2009.
- Guerin MR, Higgins CE, Jenkins RA. Measuring environmental emissions from tobacco combustion: side stream cigarette smoke literature review. Atmospheric Environment. 1987; 21: 291–297.
- Hoffmann D, Djordjevic MV, Hoffmann I. The changing cigarette. Prev Med. 1997; 26: 427-434.
- Riely GJ, Marks J, Pao W. KRAS mutations in non-small cell lung cancer. Proc Am Thorac Soc. 2009; 6: 201-205.
- Petitjean A, Mathe E, Kato S, Ishioka C, Tavtigian SV, Hainaut P, et al. Impact of mutant p53 functional properties on TP53 mutation patterns and tumor phenotype: lessons from recent developments in the IARC TP53 database. Human mutation. 2007; 28: 622-629.
- Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007; 7: 169-181.
- Thyssen J, Althoff J, Kimmerle G, Mohr U. Inhalation studies with benzo[a]pyrene in Syrian golden hamsters. J Natl Cancer Inst. 1981; 66: 575-577.
- Wolterbeek AP, Schoevers EJ, Rutten AA, Feron VJ. A critical appraisal of intratracheal instillation of benzo[a]pyrene to Syrian golden hamsters as a model in respiratory tract carcinogenesis. Cancer Lett. 1995; 89: 107–116.
- Deutsch-Wenzel RP, Brune H, Grimmer G, Dettbarn G, Misfeld J. Experimental studies in rat lungs on the carcinogenicity and dose-response relationships of eight frequently occurring environmental polycyclic aromatic hydrocarbons. J Natl Cancer Inst. 1983; 71: 539-544.
- Deutsch-Wenzel RP, Brune H, Grimmer G. Experimental studies on the carcinogenicity of five nitrogen containing polycyclic aromatic compounds directly injected into rat lungs. Cancer Lett. 1983; 20: 97-101.
- Sellakumar A, Stenbäck F, Rowland J, Shubik P. Tumur induction by 7H-dibenzol[c,g] carbazole in the respiratory tract of Syrian hamsters. J Toxicol Environ Health. 1977; 3: 935-939.
- Hecht SS. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem Res Toxicol. 1998; 11: 559-603.
- Fowles J, Dybing E. Application of toxicological risk assessment principles to the chemical constituents of cigarette smoke. Tob Control. 2003; 12: 424-430.
- Hecht SS. Carcinogenic effects of cigarette smoke on the respiratory tract. Roth RA, editor. In: Comprehensive toxicology: toxicology of the respiratory system. Oxford (U.K.): Elsevier Science. 1997; 437–451.
- Valavanidis A, Vlachogianni T, Fiotakis K. Tobacco smoke: involvement of reactive oxygen species and stable free radicals in mechanisms of oxidative damage, carcinogenesis and synergistic effects with other respirable particles. International Journal of Environmental Research and Public Health. 2009; 6: 445-462.
- Benowitz NL. Drug therapy. Pharmacologic aspects of cigarette smoking and nicotine addition. N Engl J Med. 1988; 319: 1318-1330.
- Fagerstrom KO, Heatherton TF, Kozlowski LT. Nicotine addiction and its assessment. Ear Nose Throat J. 1990; 69: 763-765.
- Fagerstrom KO, Heatherton TF, Kozlowski LT. Nicotine addiction and its assessment. Ear Nose Throat J. 1990; 69: 763-765.
- Laviolette SR, van der Kooy D. The neurobiology of nicotine addiction: bridging the gap from molecules to behaviour. Nat Rev Neurosci. 2004; 5: 55-65.
- Dwoskin LP, Teng L, Buxton ST, Ravard A, Deo N, Crooks PA. Minor alkaloids of tobacco release [3H]dopamine from superfused rat striatal slices. Eur J Pharmacol. 1995; 276: 195-199.
- Levine DG. "Needle freaks": compulsive self-injection by drug users. Am J Psychiatry. 1974; 131: 297-300.
- Koob GF, Markou A, Weiss F, Schultheis G. Opponent process and drug dependence: neurobiological mechanisms. Seminars in Neuroscience. 1993; 5: 351–358.
- Jackson KJ, Martin BR, Changeux JP, Damaj MI. Differential role of nicotinic acetylcholine receptor subunits in physical and affective nicotine withdrawal signs. Journal of Pharmacology and Experimental Therapeutics. 2008; 325: 302–312.
- Paterson D, Nordberg A. Neuronal nicotinic receptors in the human brain. Prog Neurobiol. 2000; 61: 75-111.
- Gotti C, Zoli M, Clementi F. Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol Sci. 2006; 27: 482-491.
- Lindstrom J, Anand R, Gerzanich V, Peng X, Wang F, Wells G. Structure and function of neuronal nicotinic acetylcholine receptors. Prog Brain Res. 1996; 109: 125-137.
- Flores CM, DeCamp RM, Kilo S, Rogers SW, Hargreaves KM. Neuronal nicotinic receptor expression in sensory neurons of the rat trigeminal ganglion: demonstration of a3ß4, a novel subtype in the mammalian nervous system. J Neurosci 1996; 16: 7892–7901.
- McCallum SE, Collins AC, Paylor R, Marks MJ. Deletion of the ß2 nicotinic acetylcholine receptor subunit alters development of tolerance to nicotine and eliminates receptor upregulation. Psychopharmacology. 2006; 184: 314–327.
- Nelson ME, Kuryatov A, Choi CH, Zhou Y, Lindstrom J. Alternate stoichiometries of alpha4beta2 nicotinic acetylcholine receptors. Mol Pharmacol. 2003; 63: 332-341.
- Mao D, Perry DC, Yasuda RP, Wolfe BB, Kellar KJ. The alpha4beta2alpha5 nicotinic cholinergic receptor in rat brain is resistant to up-regulation by nicotine in vivo. J Neurochem. 2008; 104: 446-456.
- Ramirez-Latorre J, Yu CR, Qu X, Perin F, Karlin A, Role L. Functional contributions of alpha5 subunit to neuronal acetylcholine receptor channels. Nature. 1996; 380: 347-351.
- Quik M, Bordia T, O'Leary K. Nicotinic receptors as CNS targets for Parkinson's disease. Biochem Pharmacol. 2007; 74: 1224-1234.
- Castro NG, Albuquerque EX. alpha-Bungarotoxin-sensitive hippocampal nicotinic receptor channel has a high calcium permeability. Biophys J. 1995; 68:516–524.
- Quik M, Philie J, Choremis J. Modulation of alpha7 nicotinic receptor-mediated calcium influx by nicotinic agonists. Mol Pharmacol. 1997; 51: 499-506.
- Marchi M, Grilli M. Presynaptic nicotinic receptors modulating neurotransmitter release in the central nervous system: functional interactions with other coexisting receptors. Progress in neurobiology. 2010; 92: 105-111.
- Sher E, Chen Y, Sharples TJ, Broad LM, Benedetti G, Zwart R, et al. Physiological roles of neuronal nicotinic receptor subtypes: new insights on the nicotinic modulation of neurotransmitter release, synaptic transmission and plasticity. Curr Top Med Chem. 2004; 4: 283–297.
- Rózsa B, Katona G, Kaszás A, Szipöcs R, Vizi ES. Dendritic nicotinic receptors modulate backpropagating action potentials and long-term plasticity of interneurons. Eur J Neurosci. 2008; 27: 364-377.
- McKay BE, Placzek AN, Dani JA. Regulation of synaptic transmission and plasticity by neuronal nicotinic acetylcholine receptors. Biochem Pharmacol. 2007; 74: 1120-1133.
- Kenney JW, Gould TJ. Modulation of hippocampus-dependent learning and synaptic plasticity by nicotine. Mol Neurobiol. 2008; 38: 101-121.
- Grenhoff J, Aston-Jones G, Svensson TH. Nicotinic effects on the firing pattern of midbrain dopamine neurons. Acta Physiol Scand. 1986; 128: 351-358.
- Corrigall WA, Franklin KB, Coen KM, Clarke PB. The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology (Berl). 1992; 107: 285-289.
- Calabresi P, Lacey MG, North RA. Nicotinic excitation of rat ventral tegmental neurones in vitro studied by intracellular recording. Br J Pharmacol. 1989; 98: 135-140.
- Pidoplichko VI, DeBiasi M, Williams JT, Dani JA. Nicotine activates and desensitizes midbrain dopamine neurons. Nature. 1997; 390: 401-404.
- Wise RA. Neurobiology of addiction. Curr Opin Neurobiol. 1996; 6: 243-251.
- Markou A. Pathways and systems involved in nicotine dependence. Bock G, Goode J, editors. In: Understanding Nicotine and Tobacco Addiction. Novartis Foundation Symposium 275. John Wiley & Sons. 2006; 1: 132–152.
- Pontieri FE, Tanda G, Orzi F, Di Chiara G. Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature. 1996; 382: 255-257.
- Wooltorton JR, Pidoplichko VI, Broide RS, Dani JA. Differential desensitization and distribution of nicotinic acetylcholine receptor subtypes in midbrain dopamine areas. J Neurosci. 2003; 23: 3176-3185.
- Charpantier E, Barnéoud P, Moser P, Besnard F, Sgard F. Nicotinic acetylcholine subunit mRNA expression in dopaminergic neurons of the rat substantia nigra and ventral tegmental area. Neuroreport. 1998; 9: 3097-3101.
- Klink R, de Kerchove d'Exaerde A, Zoli M, Changeux JP. Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci. 2001; 21: 1452-1463.
- Henningfield JE, Stapleton JM, Benowitz NL, Grayson RF, London ED. Higher levels of nicotine in arterial than in venous blood after cigarette smoking. Drug Alcohol Depend. 1993; 33: 23-29.
- Pidoplichko VI, DeBiasi M, Williams JT, Dani JA. Nicotine activates and desensitizes midbrain dopamine neurons. Nature. 1997; 390: 401-404.
- Laviolette SR, van der Kooy D. GABAA receptors in the ventral tegmental area control bidirectional reward signaling through dopaminergic and non-dopaminergic neural motivational systems. Eur J Neurosci. 2001; 13: 1009–1015.
- Gotti C, Guiducci S, Tedesco V, Corbioli S, Zanetti L, Moretti M, et al. Nicotinic acetylcholine receptors in the mesolimbic pathway: primary role of ventral tegmental area a6ß2* receptors in mediating systemic nicotine effects on dopamine release, locomotion, and reinforcement. The Journal of Neuroscience. 2010; 30: 5311-5325.
- Steffensen SC, Lee RS, Stobbs SH, Henriksen SJ. Responses of ventral tegmental area GABA neurons to brain stimulation reward. Brain Res. 2001; 906: 190-197.
- Radcliffe KA, Fisher JL, Gray R, Dani JA. Nicotinic modulation of glutamate and GABA synaptic transmission of hippocampal neurons. Ann N Y Acad Sci. 1999; 868: 591-610.
- Mansvelder HD, McGehee DS. Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron. 2000; 27: 349-357.
- Staley JK, Krishnan-Sarin S, Cosgrove KP, Krantzler E, Frohlich E, Perry E, et al. Human tobacco smokers in early abstinence have higher levels of beta2* nicotinic acetylcholine receptors than nonsmokers. J Neurosci. 2006; 26: 8707-8714.
- Marks MJ, McClure-Begley TD, Whiteaker P, Salminen O, Brown RW, Cooper J, et al. Increased nicotinic acetylcholine receptor protein underlies chronic nicotine-induced upregulation of nicotinic agonist binding sites in mouse brain. J Pharmacol Exp Ther. 2011; 337: 187-200.
- Fenster CP, Whitworth TL, Sheffield EB, Quick MW, Lester RA. Upregulation of surface a4ß2 nicotinic receptors is initiated by receptor desensitization after chronic exposure to nicotine. The journal of neuroscience. 1999; 19: 4804-4814.
- DiFranza JR, Wellman RJ. A sensitization-homeostasis model of nicotine craving, withdrawal, and tolerance: integrating the clinical and basic science literature. Nicotine Tob Res. 2005; 7: 9-26.
- Dani JA, Ji D, Zhou FM. Synaptic plasticity and nicotine addiction. Neuron. 2001; 31: 349-352.
- Balfour DJ, Wright AE, Benwell ME, Birrell CE. The putative role of extra-synaptic mesolimbic dopamine in the neurobiology of nicotine dependence. Behavioural brain research. 2000; 113: 73-83.
- Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000; 25: 515-532.
- Gilbert DG, Gilbert BO. Personality, psychopathology, and nicotine response as mediators of the genetics of smoking. Behav Genet. 1995; 25: 133-147.
- Stitzel JA, Lu Y, Jimenez M, Tritto T, Collins AC. Genetic and pharmacological strategies identify a behavioral function of neuronal nicotinic receptors. Behavioural Brain Research. 2000; 113: 57–64.
- Berrettini W, Yuan X, Tozzi F, Song K, Francks C, Chilcoat H, et al. Alpha-5/alpha-3 nicotinic receptor subunit alleles increase risk for heavy smoking. Mol Psychiatry. 2008; 13: 368-373.
- Raupach T, van Schayck CP. Pharmacotherapy for smoking cessation: current advances and research topics. CNS Drugs. 2011; 25: 371-382.
- Silagy C, Mant D, Fowler G, Lodge M. Meta-analysis on efficacy of nicotine replacement therapies in smoking cessation. Lancet. 1994; 343: 139-142.
- Bohadana A, Nilsson F, Rasmussen T, Martinet Y. Nicotine inhaler and nicotine patch as a combination therapy for smoking cessation: a randomized, double-blind, placebo-controlled trial. Archives of Internal Medicine. 2000; 160: 3128-3134.
- Balfour DJ. The pharmacology underlying pharmacotherapy for tobacco dependence: a focus on bupropion. Int J Clin Pract. 2001; 55: 53-57.
- Richmond R, Zwar N. Review of bupropion for smoking cessation. Drug Alcohol Rev. 2003; 22: 203-220.
- Paterson NE. Behavioural and pharmacological mechanisms of bupropion's anti-smoking effects: recent preclinical and clinical insights. Eur J Pharmacol. 2009; 603: 1-11.
- Garrison GD, Dugan SE. Varenicline: a first-line treatment option for smoking cessation. Clin Ther. 2009; 31: 463-491.
- Wardhan N, Chopra D, Rehan HS. Varenicline: a novel drug for smoking cessation. International Journal of Mental Health and Addiction. 2007; 5: 244-247.
- Hughes JR, Stead LF, Lancaster T. Nortriptyline for smoking cessation: a review. Nicotine Tob Res. 2005; 7: 491-499.
- Glassman AH, Covey LS, Dalack GW, Stetner F, Rivelli SK, Fleiss J, et al. Smoking cessation, clonidine, and vulnerability to nicotine among dependent smokers. Clinical Pharmacology & Therapeutics. 1993; 54: 670-679.
- Clarke PB. Nicotinic receptor blockade therapy and smoking cessation. Br J Addict. 1991; 86: 501-505.
- Cerny EH, Cerny T. Vaccines against nicotine. Hum Vaccin. 2009; 5: 200-205.
- Raupach T, Hoogsteder PH, Onno van Schayck CP. Nicotine vaccines to assist with smoking cessation: current status of research. Drugs. 2012; 72: e1-16.
- Saccone SF, Hinrichs AL, Saccone NL, Chase GA, Konvicka K, Madden PA, et al. Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Hum Mol Genet. 2007; 16: 36–49.
- Bierut LJ, Madden PA, Breslau N, Johnson EO, Hatsukami D, Pomerleau OF, et al. Novel genes identified in a high-density genome wide association study for nicotine dependence. Hum Mol Genet. 2007; 16: 24-35.
- Ho MK, Tyndale RF. Overview of the pharmacogenomics of cigarette smoking. Pharmacogenomics J. 2007; 7: 81-98.
- Uhl GR, Liu QR, Drgon T, Johnson C, Walther D, Rose JE. Molecular genetics of nicotine dependence and abstinence: whole genome association using 520,000 SNPs. BMC Genet. 2007; 8: 10.
- Amos CI, Wu X, Broderick P, Gorlov IP, Gu J, Eisen T, et al. Genome-wide association scan of tag SNPs identifies a susceptibility locus for lung cancer at 15q25.1. Nat Genet. 2008; 40: 616-622.
- Keskitalo K, Broms U, Heliövaara M, Ripatti S, Surakka I, Perola M, et al. Association of serum cotinine level with a cluster of three nicotinic acetylcholine receptor genes (CHRNA3/CHRNA5/CHRNB4) on chromosome 15. Hum Mol Genet. 2009; 18: 4007-4012.
- Messina ES, Tyndale RF, Sellers EM. A major role for CYP2A6 in nicotine C-oxidation by human liver microsomes. J Pharmacol Exp Ther. 1997; 282: 1608-1614.
- Saarikoski ST, Sata F, Husgafvel-Pursiainen K, Rautalahti M, Haukka J, Impivaara O, et al. CYP2D6 ultrarapid metabolizer genotype as a potential modifier of smoking behaviour. Pharmacogenetics and Genomics. 2000; 10: 5-10.
- Hung RJ, McKay JD, Gaborieau V, Boffetta P, Hashibe M, Zaridze D, et al. A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nature. 2008; 452: 633-637.
- Le Marchand L, Derby KS, Murphy SE, Hecht SS, Hatsukami D, Carmella SG, et al. Smokers with the CHRNA lung cancer-associated variants are exposed to higher levels of nicotine equivalents and a carcinogenic tobacco-specific nitrosamine. Cancer Res. 2008; 68: 9137–9140.
- Quaak M, van Schayck CP, Knaapen AM, van Schooten FJ. Genetic variation as a predictor of smoking cessation success. A promising preventive and intervention tool for chronic respiratory diseases? Eur Respir J. 2009; 33: 468-480.
- Quaak M, van Schayck CP, Knaapen AM, van Schooten FJ. Implications of gene-drug interactions in smoking cessation for improving the prevention of chronic degenerative diseases. Mutat Res. 2009; 667: 44-57.
- Swan GE, Valdes AM, Ring HZ, Khroyan TV, Jack LM, Ton CC, et al. Dopamine receptor DRD2 genotype and smoking cessation outcome following treatment with bupropion SR. Pharmacogenomics J. 2005; 5: 21-29.
- Lerman C, Jepson C, Wileyto EP, Ep¬stein LH, Rukstalis M, Patterson F, et al. Role of functional genetic variation in the dopamine D2 receptor (DRD2) in response to bupropion and nicotine replacement therapy for tobacco depend¬ence: results of two randomized clinical trials. Neuropsychopharmacology. 2006; 31: 231–242.
- Quaak M, van Schayck CP, Postma DS, Wagena EJ, van Schooten FJ. Genetic variants in the serotonin transporter influence the efficacy of bupropion and nortriptyline in smoking cessation. Addiction. 2012; 107: 178-187.
- Lerman C, Tyndale R, Patterson F, Wileyto EP, Shields PG, Pinto A, et al. Nicotine metabolite ratio predicts efficacy of transdermal nicotine for smoking cessation. Clin Pharmacol Ther. 2006; 79: 600-608.
- Hutchison KE, Allen DL, Filbey FM, Jepson C, Lerman C, Benowitz NL, et al. CHRNA4 and tobacco dependence: from gene regulation to treatment outcome. Arch Gen Psychiatry. 2007; 64: 1078-1086.
- Ozaki S, Oyama T, Isse T, Kagawa N, Uramoto H, Sugio K, et al. Smoking cessation program and CYP2A6 polymorphism. Front Biosci. 2006; 11: 2590-2597.
- Malaiyandi V, Lerman C, Benowitz NL, Jepson C, Patterson F, Tyndale RF. Impact of CYP2A6 genotype on pretreatment smoking behaviour and nicotine levels from and usage of nicotine replacement therapy. Mol Psychiatry. 2006; 11: 400-409.
- Hays JT, Ebbert JO. Varenicline for tobacco dependence. N Engl J Med. 2008; 359: 2018-2024.
- Lee AM, Jepson C, Hoffmann E, Epstein L, Hawk LW, Lerman C, et al. CYP2B6 genotype alters abstinence rates in a bupropion smoking cessation trial. Biol Psychiatry. 2007; 62: 635-641.
- Fujita T, Urban TJ, Leabman MK, Fujita K, Giacomini KM. Transport of drugs in the kidney by the human organic cation transporter, OCT2 and its genetic variants. J Pharm Sci. 2006; 95: 25-36.
- Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, et al. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005; 48: 3474-3477.