
Case Report
Austin J Lung Cancer Res. 2016; 1(1): 1005.
Pleural Malignant Mesothelioma with Micropapillary Pattern: A Case Report and Literature Review
Yang G¹*, Qin X², Zaheer S³ and Nepomuceno- Perez MC¹
¹Department of Pathology and Laboratory Medicine, Loma Linda University Medical Center, USA
²Department of Internal Medicine, Loma Linda University Medical Center, USA
³Department of Surgery/Cardiothoracic, Loma Linda University Medical Center, USA
*Corresponding author: Yang G, Department of Pathology and Laboratory Medicine, Loma Linda University Medical Center, 11234 Anderson St, Loma Linda, California 92354, USA
Received: November 13, 2015; Accepted: January 13, 2016; Published: February 11, 2016
Abstract
Recent studies show that invasive micropapillary pattern in pleural malignant mesothelioma, characterized by cellular tufts lacking central fibrovascular cores, can predict a more aggressive lymphatic spread, similarly seen in carcinomas in other organs with micropapillary pattern. Here, the authors report a rare case of pleural malignant mesothelioma with micropapillary pattern, widespread lymphovascular invasion, and regional nodal and pulmonary micro metastasis. The authors further describe the main histologic and immunehistochemical features, discuss possible mechanism(s), along with a brief literature review
Keywords: Malignant mesothelioma; Micropapillary; Pleura; Metastasis; Invasion; Immunohistochemistry
Case Presentation
A 56-year-old Hispanic female with uncertain asbestos exposure developed shortness of breath, cough and left chest pain. She was evaluated elsewhere and found to have left pleural effusion. After initial workup was non diagnostic, she underwent left Video Assisted Thoracic Surgery (VATS) and pleural biopsy. The pathology showed mesothelioma and she was referred to our institution for definitive care. Past medical history includes hypertension, diabetes mellitus and hyperlipidemia. Patient has a less than 1 pack year remote smoking history.
On presentation at our institution, she complained of left chest pain with a dry cough. Her physical examination was unremarkable with healing left chest incisions. Staging workup included chest Positron Emission Tomography / Computed Tomography Scan (PET/CT), Magnetic Resonance Imaging (MRI) of the chest, mediastinoscopy and laparoscopy. PET/CT showed hyper metabolic activity in the left pleural space and mild uptake in subcarinal, superior mediastinal and bilateral level IIa cervical lymph nodes (Figures 1&2). Chest (Figure 3) showed circumferential left pleural irregular thickening and loculated pleural fluid but no frank mediastinal, diaphragmatic or chest wall invasion. Mediastinoscopy showed no malignant involvement of #4R, #4L and #7 lymph node stations. Laparoscopy was negative for peritoneal involvement. She was clinical stage II. Results of pulmonary function tests showed FEV1 (Forced Expiratory Volume in 1 second) of 1.72 (68%) and DLCO (carbon monoxide diffusing capacity) of 66%. Her echocardiogram was normal at rest and after stress. She also underwent pulmonary stress test and had excellent VO2 max. Our histopathologic review of left pleural peel from the outside VATS confirmed epithelioid pleural malignant mesothelioma.
After extensive discussions about the risks, benefits and alternatives of different treatment options, she opted for tri-modality therapy. She was taken to the operating room for a left extra pleural pneumonectomy. We preserved the pericardium. The diaphragm was reconstructed with 2mm Gortex mesh. All the previous port sites were resected enbloc with the specimen. A complete Mediastinal lymph node dissection was performed. Her hospital course was unremarkable and she was discharged on the 5th post-operative day. Follow-up in the clinic after 2 weeks was unremarkable. She is scheduled to undergo adjuvant chemotherapy and radiation in the near future.
Gross & Microscopic pathology
The resected specimens consisted of left extra pleural pneumonectomy with diaphragm and chest wall, left 6th rib, level 5 and 7 lymph nodes. Gross examination showed multiple tan-white, firm, peripheral rind-like masses up to 6.5 cm in greatest dimension diffusely involving visceral and parietal pleura (Figure 4), with inferior and medial extension to diaphragmatic and mediastinal surfaces.
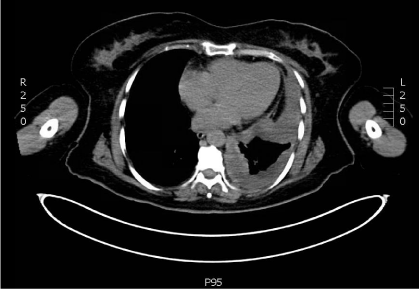
Figure 1: CT scan shows circumferential and irregularly nodular pleural
thickening encasing the left lung.
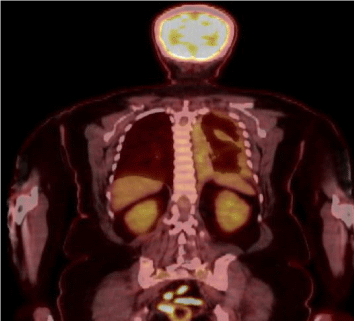
Figure 2: PET scan shows diffuse left pleural thickening and loculated pleural
fluid with hypermetabolic activity circumferentially in the left pleural space.
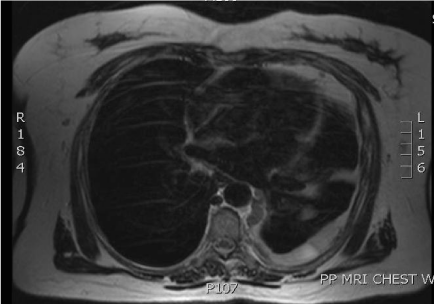
Figure 3: MRI scan shows circumferential left pleural thickening and loculated
pleural fluid.
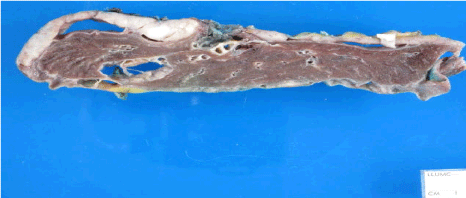
Figure 4: Cross – section of gross specimen shows multiple tan white, firm,
peripheral rind-like masses in parietal and visceral pleura.
Histologic examination revealed the following; 1) diffuse malignant mesothelioma, involving parietal and visceral pleurae (Figure 5); 2) epithelioid subtype with mixed morphologic variants including micropapillary pattern with atypical small epithelioid cells forming free-floating cell tufts lacking fibro vascular cores, comprising at least 10% of the tumor (Figure 6); 3) extensive pleural lymphovascular invasion; 4) multiple regional nodal metastasis (Figure 7); and 5) pulmonary micro metastasis (Figure 8). Background lung shows emphysema with no asbestos bodies seen.
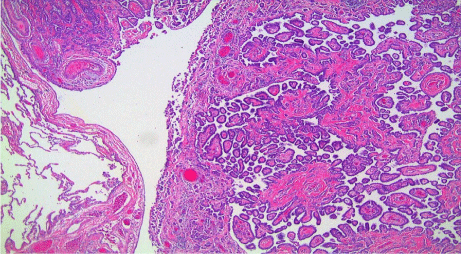
Figure 5: Pleural malignant mesothelioma, involving visceral (upper-left
corner) and parietal (center and right half) pleura (hematoxylin & eosin; x40).
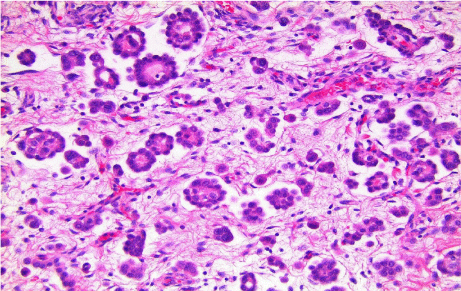
Figure 6: PMM; epithelioid subtype, micropapillary growth pattern
(hematoxylin & eosin stain; x200).
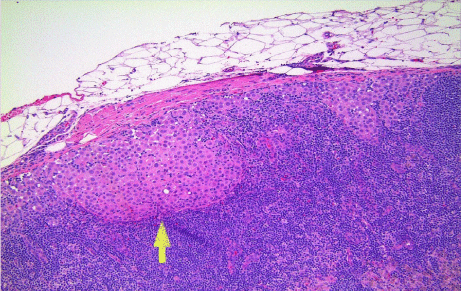
Figure 7: Regional nodal metastasis; at arrow tip (hematoxylin & eosin stain;
x100).
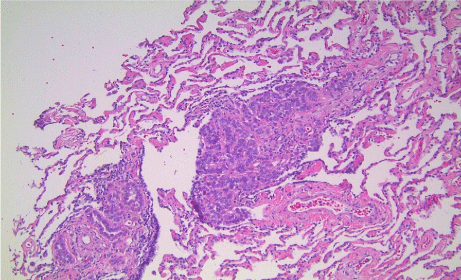
Figure 8: Pulmonary micrometastasis (hematoxylin and eosin; x 100).
The tumor cells were positive for Calretinin and D2-40 the latter also staining lymphatic endothelium. The tumor cells were negative for thyroid transcription factor -1 and MOC31. In addition, the micro papillae showed positivity for MUC1 (Figure 9) and EMA stains; and decreased to focally negative staining for CD44 (Figure 10). Pathologic staging was pT1b N1 M1 (Stage 4).
Figure 9: Positive staining in micropapillae (MUC1 stain; x200).
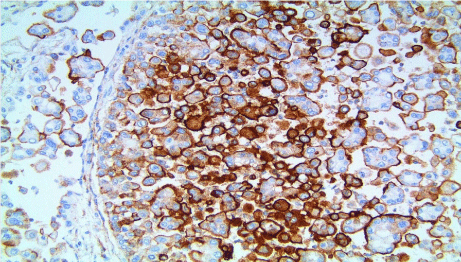
Figure 10: Decreased or negative staining (CD 44 stain; x200).
Discussion
Malignant Mesothelioma (MM) is an uncommon and aggressive neoplasm that develops from mesothelial cells lining serosal surfaces of the pleura, peritoneum, pericardium, and tunica vaginalis. Synonyms of MM include âdiffuse malignant mesotheliomaâ and âmesotheliomaâ [1].
Among all the types of MM, Pleural Malignant Mesothelioma (PMM) is the most common and comprises approximately 80% of all mesothelioma diagnoses [2] PMMs are more commonly seen in patients over 60 years of age, but the age distribution is wide and occasionally affects children. In North America, PMMs in males outnumber those in females by approximately 9:1, but the ratio is lower in other countries such as the UK, France and Australia. [1].
In the United States, PMM occurs in approximately 2,500 persons per year [3]. The worldwide incidence of PMM is expected to increase over the next decade with a peak in the year of 2020, particularly in Europe, Asia, and Australia [4]. The clinical course is of progressive shortness of breath and unrelenting chest wall pain. Moreover, this cancer is uniformly fatal, with survival from few weeks to a few years. To date, current treatments are not only onerous but of marginal clinical benefits [5]. Because of the rising worldwide incidence, and invariable fatal outcome, early and accurate diagnosis of PMM is crucial.
The International Mesothelioma Interest Group (IMIG) suggests that the diagnosis of MM should always be based on the results obtained from an adequate biopsy in the context of appropriate clinical, radiologic, and surgical findings [6]. Based on its histological features, MM can be broadly divided into 3 subtypes: epithelioid, sarcomatoid, or mixed (biphasic) [6]. As the most frequent histologic subtype, epithelioid MM is found in approximately 50% of cases, and has the best prognosis; whereas sarcomatoid subtype is seen in 16% and is more aggressive [7].
Epithelioid MMs are composed of polygonal, oval, or cuboidal cells; with secondary morphologic variants such as: tubulopapillary, micropapillary, trabecular, acinar, adenomatoid, solid, clear cell, etc. Among these, the micropapillary variant is characterized by cellular tufts lacking central fibrovascular cores [6]. To the best of our knowledge, there are only 2 articles specifically mentioning micropapillary pattern. In the study of Mogi et al, a micropapillary component in 2 of 34 PMM cases (5.9%) was identified, and these two micropapillary-pattern-positive cases showed significantly increased lymphatic invasion, pulmonary metastasis, and a trend toward increased lymph node involvement compared with micropapillarypattern- negative PMMs, similar to micropapillary –pattern-positive carcinomas in other organs [8]. It was reported by Kadota et al. that 20 cases (9%) out of 232 patients with epithelioid diffuse malignant pleural mesothelioma were micropapillary-predominant and showed a significant association with lymphatic invasion. That study also suggested that micropapillary pattern PMM had shorter overall survival compared to tubulopapillary and trabecular patterns [9].
The underlying mechanism of this micropapillary patternassociated lymphatic invasion is unclear. However, it is suggested that two cell surface glycoproteins, namely CD44 and MUC1 may play important roles in this phenomenon [8]. CD44, which is important in epithelial cell adhesion, is reported as with absent or decreased cell surface expression in micropapillary pattern [10]. In contrast, MUC1, which inhibits cell-stroma interaction, is over-expressed in stromal-facing cell surfaces in invasive micropapillary carcinomas of the breast [11]. This MUC1 over expression can cause reversal of cell polarity and thereby contribute to lymphatic tumor spread [12]. In the present case report, we have similar observations, both histologically and immunohistochemically, regarding this micropapillary pattern.
In most industrialized countries, greater than 90% of PMMs in men are related to prior asbestos exposure. However, in women in North America only about 20% tumors are caused by asbestos [1]. IMIG considers the positive or negative history of asbestos exposure as not useful in making a diagnosis of mesothelioma [6].
In conclusion, we present a rare case of a pleural malignant mesothelioma with micropapillary pattern. It is suggested that the histologic diagnosis of PMM with this pattern should be based on characteristic histomorphology complimented by immunohistochemistry, when appropriate. Recognition of this micropapillary pattern is important due to its association with lymphovascular invasion and metastasis, poorer prognosis and shorter overall survival.
References
- Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC. International Agency for Research on Cancer. Pathology and genetics of tumours of the lung, pleura, thymus and heart/edited by William D. Travis, et al. 2004.
- Maziak DE, Gagliardi A, Haynes AE, Mackay JA, Evans WK. Cancer Care Ontario Program in Evidence-based Care Lung Cancer Disease Site Group . Surgical management of malignant pleural mesothelioma: a systematic review and evidence summary. Lung Cancer. 2005; 48: 157-169.
- Ismail-Khan R, Robinson LA, Williams CC, Garrett CR, Bepler G, Simon GR. Malignant pleural mesothelioma: a comprehensive review. Cancer Control. 2006; 13: 255-263.
- McAleer MF, Mehran RJ, Tsao A. Mesothelioma. In Lung Cancer. Humana Press. 2010; 435-465.
- Treasure T, Sedrakyan A. Pleural mesothelioma: little evidence, still time to do trials. Lancet. 2004; 364: 1183-1185.
- Husain AN, Colby T, Ordonez N, Krausz T, Attanoos R, Beasley MB, et al. Guidelines for pathologic diagnosis of malignant mesothelioma: 2012 update of the consensus statement from the International Mesothelioma Interest Group. Archives of Pathology and Laboratory Medicine. 2013: 137: 647-667.
- Ismail-Khan R, Robinson LA, Williams CC, Garrett CR, Bepler G, Simon GR. Malignant pleural mesothelioma: a comprehensive review. Cancer Control. 2006; 13: 255-263.
- Mogi A, Nabeshima K, Hamasaki M, Uesugi N, Tamura K, Iwasaki A, et al. Pleural malignant mesothelioma with invasive micropapillary component and its association with pulmonary metastasis. Pathol Int. 2009; 59: 874-879.
- Kadota K, Suzuki K, Sima CS, Rusch VW, Adusumilli PS, Travis WD. Pleomorphic epithelioid diffuse malignant pleural mesothelioma: a clinicopathological review and conceptual proposal to reclassify as biphasic or sarcomatoid mesothelioma. J Thorac Oncol. 2011; 6: 896-904.
- Gong Y, Sun X, Huo L, Wiley EL, Rao MS. Expression of cell adhesion molecules, CD44s and E-cadherin, and microvessel density in invasive micropapillary carcinoma of the breast. Histopathology. 2005; 46: 24-30.
- August C, August K, Schroeder S, Bahn H, Hinze R, Baba HA, et al. CGH and CD 44/MIB-1 immunohistochemistry are helpful to distinguish metastasized from nonmetastasized sporadic pheochromocytomas. Mod Pathol. 2004; 17: 1119-1128.
- Acs G, Esposito NN, Rakosy Z, Laronga C, Zhang, PJ. Invasive ductal carcinomas of the breast showing partial reversed cell polarity are associated with lymphatic tumor spread and may represent part of a spectrum of invasive micropapillary carcinoma. The American journal of surgical pathology. 2010: 34: 1637-1646.