
Editorial
Austin J Lung Cancer Res. 2016; 1(2): 1008.
Nano-Enabled Approaches for Lung Cancer Therapy
Bharat Bhushan¹ and Gopinath P1,2*
¹Centre for Nanotechnology, Indian Institute of Technology Roorkee, India
²Department of Biotechnology, Indian Institute of Technology Roorkee, India
*Corresponding author: Gopinath P, Centre for Nanotechnology, Department of Biotechnology, Indian Institute of Technology Roorkee, Uttarakhand-247667, India
Received: April 11, 2015; Accepted: April 12, 2016; Published: April 15, 2016
Editorial
According to the GLOBOCAN estimates lung cancer is the most frequently diagnosed cancer (13% of total diagnosed cancers) and the leading cause of cancer-related deaths worldwide [1]. Lung cancer is classified into Non-Small-Cell Lung Carcinoma (NSCLC), which attributes to the 85% of the diagnosed cancer, while remaining 15 % cases are assigned as Small-Cell Lung Carcinoma (SCLC). Existing strategies relies on the type and stage of malignancy and often comprise a combination of conventional therapies. Since the late 1990s, chemotherapy regimens including the platinum-based drugs such as carboplatin and cisplatin either alone or in combination with other anticancer drugs emerged as a first-line treatment option for advanced-stage lung cancer. Due to the hydrophobic nature of the existing cancer chemotherapeutics, high doses have been administrated which results in severe side effects [2,3]. To mitigate many of these untoward effects, various strategies have been employed, which includes the tumor-targeted delivery of therapeutic molecules that enhanced the drug efficacy and reduced toxicity to normal tissues [4]. Moreover, various experimental therapies such as immunotherapy, gene therapy and Photodynamic Therapy (PDT) either alone or in combination with conventional therapy or surgery emerged as a potential tool to fight lung cancer. Photosensitizer, like porfimer sodium has been employed in the treatment of early as well as advanced lung carcinomas. However, the poor water solubility of these photosensitizers restricts their intravenous administration [5]. Moreover, the immunologically active agents harnessed in immunotherapy, either trigger the immune system or hinder the immune suppressing activities of the tumor by interrupting the tumorigenic cascades [6]. In recent past large number of cancer suppressing genes has been identified for gene therapy. However, the delivery of these therapeutic genes still remains a major challenge among the scientific community. Previously, viral vectors have been exploited to deliver gene-based therapeutics [7]; but viral vector induced host immune responses restrict their future implications [8,9]. In concern, to develop a safe and effective delivery system, nanotechnology provides a potential platform by overcoming the various limitations associated with traditional delivery systems. The nano-scale delivery systems revolutionized the cancer treatment by enhancing the therapeutic efficacy of anticancer agents [10]. Moreover, the possibilities of functional modifications of these nanoparticles enhance their therapeutic efficacy by attenuating their non-specific bio distribution. In addition, these nano-sized particles also offers several advantages when compared to the standard solvent based drug formulations, such as enhanced payload, protect therapeutic cargo molecules from biodegradation, prolonged circulation time, enhanced solubility and chemical stability, enhanced intratumoral accumulation and diminished side effects.
To date, many nano-enabled technologies have been developed for lung cancer therapy and few of them have been proved to be a clinical breakthrough [11]. Among them the most prominent ones are: liposomes, polymer and protein based nano-approaches as shown in Figure 1.
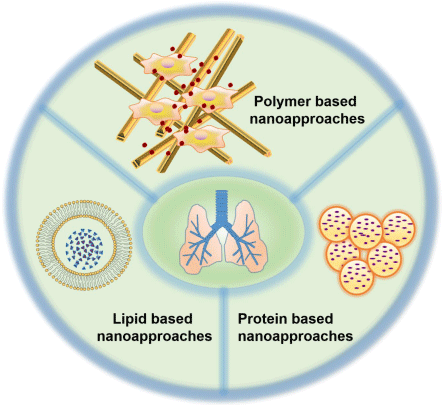
Figure 1: Schematic representation of different nanoapproaches for lung
cancer therapy.
Liposomes based nano-approaches
Liposome is one of the most successful delivery system with several FDA-approved nanoformulations in the market. Liposomes are closed spherical structures composed of one or more lipid bilayers made up of amphiphilic phospholipids and cholesterol that allows the storage of hydrophobic drugs, while the aqueous core are responsible for holding hydrophilic drugs [12]. The liposome enhanced the solubility of hydrophobic anticancer agents, extends their circulation time and enhanced their chance of intratumoral uptake.
Stealth TMPEGylated liposomal doxorubicin (Doxil or Caelyx), was among the FDA approved nanoparticles for cancer therapy. In phase I/ II study, Doxil in combination with other conventional therapy and supportive growth factors revealed significant results in Small Cell Lung Cancer (SCLC) and NSCLC patients [13-17]. Similarly, PEGylated liposomal formulation of irinotecan (PEP02, or MM-398) offer higher drug load, prolonged circulation and stability as compared to standard irinotecan [18]. In preclinical models, compared to standard irinotecan, MM-398 exhibited higher antitumor efficacy and lower toxicity in squamous cell lung cancer and small cell lung cancer models [19]. PEGylated liposomal cisplatin (LipoplatinTM), originally designed by Regulon, Inc. Mountain View, CA, USA has been extensively examined in NSCLC treatment in Europe. LipoplatinTM exhibited higher antitumor efficacy, higher intratumoral concentrations and lesser toxicity as compared to standard cisplatin [20]. Apart from it, the liposomal aerosolized formulations have been extensively explored for lung cancer therapy. The liposomal formulation of 9-nitrocamptothecin shows higher efficacy, negligible side effects compared to free drug [21]. Similarly, liposomal aerosolized formulation of anticancer drug, cisplatin (Sustained Release Lipid Inhalation Targeting, SLIT) showed higher accumulation in lungs and comparable cytotoxicity to free cisplatin both in human tumor cell line (NCI-H460) and Sprague-Dawley rats, respectively [22].
Polymers based nano-approaches
Polymeric nanoparticles are solid colloidal systems consists of polymer matrix made up of natural polymers such as chitosan, heparin etc. or synthetic polymers such as Polyethylene Oxide (PEO), Polycaprolactam (PCL), poly (D,L-lactide-co-glycolic) acid (PLGA) etc. to which various therapeutic, imaging and targeting moieties are either covalently linked or encapsulated within [11].
Genexol-PM (Cynviloq) is a paclitaxel loaded polymeric micelle formulation consist of a monomethoxy polyethylene glycol-poly (D, L-lactic acid (mPEG-PDLLA) amphiphilic dib lock copolymer, originally designed by Sam yang Co, Seoul, Korea. Preclinical studies showed that the Genexol-PM either alone and in combination with other therapeutic drugs have higher antitumor efficacy, enhanced biodistribution, higher maximum tolerated dose and lesser toxicity issues as compared to other standard solvents based paclitaxel formulations and has been approved for NSCLC treatment in South Korea [23-25]. NC-6004 is another polymer based nano-approach comprised of hydrophilic PEG outer shell and contains a coordinate complex of polyglutamate [P(Glu)] with cisplatin in the inner core [26]. This polymeric nanoformulation offers extreme stability in distilled water, prolonged plasma circulation, lesser toxicity, enhanced intratumoral accumulation and has higher drug holding capacity as compared to standard cisplatin and other cisplatin-containing polymeric micelles [27,28].
Recently, polymeric nanofiber based scaffolds have been extensively exploited for the controlled and sustained delivery of various therapeutic molecules either alone or in combination with other bioactive molecules [29,30]. Moreover, an alternative approach has been investigated by developing a composite core shell nanofibrous scaffold that can efficiently transfect suicide gene in nonsmall lung cancer cells (A549) and simultaneously deliver prodrug, 5-Fluorocytosine (5-FC), which has been metabolically converted into toxic intermediates by the Cytosine Deaminase-Uracil Phosphoribosyltransferase (CD::UPRT) suicide gene expressing A549 cells [31].
Protein based nano-approaches
Proteins have numerous unique features that make them an ideal drug delivery vehicle. The major advantages associated with protein nanoparticles includes their biocompatibility, biodegradability, ability to interact with large number of hydrophobic molecules and enhance the solubility and bioavailability by their controlled release.
Among various protein nanoparticles, albumin nanoparticles based approaches have been widely explored [32,33]. The albumin bound nano-formulation of anticancer drug paclitaxel also known as Abraxane was the first FDA approved nanomedicine for treatment of advanced/metastatic NSCLC. In preclinical trials, the Abraxane either alone or in combination with other therapeutic agents shows higher therapeutic efficiency and lesser side effects as compared to the organic solvent based paclitaxel due to higher drug loading capacity and enhanced bioavailability [34-36]. Recently, a self assembled inhalable albumin nanoparticles conjugated with doxorubicin and octyl aldehyde and adsorbed with apoptotic TRAIL (Tumor Necrosis Factor (TNF)-related apoptosis-inducing ligand) protein has been fabricated for treating drug-resistance lung cancer [37]. Similarly, a biotinylated-EGF-modified cisplatin-loaded –Gelatin Nanoparticles (GNP) has been developed that exhibit enhanced anti-tumor activity and was less toxic than free cisplatin, in vivo. Moreover, aerosol delivery of these targeted nanodrug carrier leads to enhanced cisplatin accumulation in cancerous lungs [38]. Moreover, the gelatin nanoparticles have been extensively exploited for the delivery of hydrophilic and hydrophobic anti-cancer drugs such as resveratrol [39], curcumin [40] etc. for lung cancer therapy. In sum, these exciting nano-enabled approaches will serve as a foundation for the future lung cancer therapy.
Acknowledgement
Our sincere thanks to Science and Engineering Research Board (No. SR/FT/LS-57/2012), and Department of Biotechnology (No.BT/ PR6804/GBD/27/486/2012), Government of India, for the financial support.
References
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015; 65: 87-108.
- Kumar A, Sahoo SK, Padhee K, Kochar PPS, Satapathy A, Pathak N. âReview on solubility enhancement techniques for hydrophobic drugs,â Int J Compr Pharm. 2011; 3: 1–7.
- Bhushan B, Dubey P, Uday Kumar S, Sachdev A, Matai I, Gopinath P. Bionanotherapeutics: Niclosamide Encapsulated Albumin Nanoparticles as a Novel Drug Delivery System for Cancer Therapy. RSC Adv. 2015; 5: 12078- 12086.
- Bhushan B, Gopinath P. Tumor-targeted folate-decorated albumin-stabilised silver nanoparticles induce apoptosis at low concentration in human breast cancer cells. RSC Adv. 2015; 5: 86242-86253.
- Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, et al. Photodynamic therapy of cancer: an update. CA Cancer J Clin. 2011; 61: 250-281.
- Dougan M, Dranoff G. Immune therapy for cancer. Annu Rev Immunol. 2009; 27: 83-117.
- Giacca M, Zacchigna S. Virus-mediated gene delivery for human gene therapy. J Control Release. 2012; 161: 377-388.
- Nayak S, Herzog RW. Progress and prospects: immune responses to viral vectors. Gene Ther. 2010; 17: 295-304.
- Bhushan B, Kumar SU, Matai I, Sachdev A, Dubey P, Gopinath P. Ferritin nanocages: a novel platform for biomedical applications. J Biomed Nanotechnol. 2014; 10: 2950-2976.
- Wang AZ, Langer R, Farokhzad OC. Nanoparticle delivery of cancer drugs. Annu Rev Med. 2012; 63: 185-198.
- Uday Kumar S, Bhushan B, Dubey P, Matai I, Sachdev A, Gopinath P. Emerging Applications of Nanoparticles for Lung Cancer Diagnosis and Therapy. Int Nano Lett. 2013; 3: 45-53.
- Gopinath P, Uday Kumar S, Matai I, Bhushan B, Malwal D, Sachdev A, et al. Cancer Nanotheranostics. Springer briefs in applied science and technology. 2015.
- Samantas E, Kalofonos H, Linardou H, Nicolaides C, Mylonakis N, Fountzilas G, et al. Phase II study of pegylated liposomal doxorubicin: inactive in recurrent small-cell lung cancer. A Hellenic Cooperative Oncology Group Study. Ann Oncol. 2000; 11: 1395-1397.
- Leighl NB, Goss GD, Lopez PG, Burkes RL, Dancey JE, Rahim YH, et al. Phase II study of pegylated liposomal doxorubicin HCl (Caelyx) in combination with cyclophosphamide and vincristine as second-line treatment of patients with small cell lung cancer. Lung Cancer. 2006; 52: 327-332.
- Tsoutsou PG, Froudarakis ME, Bouros D, Koukourakis MI. Hypofractionated/ accelerated radiotherapy with cytoprotection (HypoARC) combined with vinorelbine and liposomal doxorubicin for locally advanced non-small cell lung cancer (NSCLC). Anticancer Res. 2008; 28: 1349-1354.
- Patlakas G, Bouros D, Tsantekidou-Pozova S, Koukourakis MI. Triplet chemotherapy with docetaxel, gemcitabine and liposomal doxorubicin, supported with subcutaneous amifostine and hemopoietic growth factors, in advanced non-small cell lung cancer. Anticancer Res. 2005; 25: 1427-1431.
- Koukourakis MI, Romanidis K, Froudarakis M, Kyrgias G, Koukourakis GV, Retalis G, et al. Concurrent administration of Docetaxel and Stealth liposomal doxorubicin with radiotherapy in non-small cell lung cancer: excellent tolerance using subcutaneous amifostine for cytoprotection. Br J Cancer. 2002; 87: 385-392.
- Drummond DC, Noble CO, Guo Z, Hong K, Park JW, Kirpotin DB. Development of a highly active nanoliposomal irinotecan using a novel intraliposomal stabilization strategy. Cancer Res. 2006; 66: 3271-3277.
- Chan DC, Kalra A, Zhang Z, Paz N, Kirpotin D, Drummond D, et al. Abstract 4626: Evaluating the pharmacodynamics and pharmacokinetic effects of MM- 398, a nanoliposomal irinotecan (nal-IRI) in subcutaneous xenograft tumor models of human squamous cell carcinoma and small cell lung cancers. Cancer Res. 2014; 74: 4626.
- Arienti C, Tesei A, Ravaioli A, Ratta M, Carloni S, Mangianti S, et al. Activity of lipoplatin in tumor and in normal cells in vitro. Anticancer Drugs. 2008; 19: 983-990.
- Knight V, Kleinerman ES, Waldrep JC, Giovanella BC, Gilbert BE, Koshkina NV. 9-nitrocamptothecin liposome aerosol treatment of human cancer subcutaneous xenografts and pulmonary cancer metastases in mice. Ann N Y Acad Sci. 2000; 922: 151–163.
- Wittgen BP, Kunst PW, van der Born K, van Wijk AW, Perkins W, Pilkiewicz FG, et al. Phase I study of aerosolized SLIT cisplatin in the treatment of patients with carcinoma of the lung. Clin Cancer Res. 2007; 13: 2414-2421.
- Kim TY, Kim DW, Chung JY, Shin SG, Kim SC, Heo DS, et al. Phase I and pharmacokinetic study of Genexol-PM, a cremophor-free, polymeric micelleformulated paclitaxel, in patients with advanced malignancies. Clin Cancer Res. 2004; 10: 3708-3716.
- Kim DW, Kim SY, Kim HK, Kim SW, Shin SW, Kim JS, et al. Multicenter phase II trial of Genexol-PM, a novel Cremophor-free, polymeric micelle formulation of paclitaxel, with cisplatin in patients with advanced non-smallcell lung cancer. Ann Oncol. 2007; 18: 2009-2014.
- Ahn HK, Jung M, Sym SJ, Shin DB, Kang SM, Kyung SY, et al. A phase II trial of Cremorphor EL-free paclitaxel (Genexol-PM) and gemcitabine in patients with advanced non-small cell lung cancer. Cancer Chemother Pharmacol. 2014; 74: 277-282.
- Nishiyama N, Okazaki S, Cabral H, Miyamoto M, Kato Y, Sugiyama Y, et al. Novel cisplatin-incorporated polymeric micelles can eradicate solid tumors in mice. Cancer Res. 2003; 63: 8977-8983.
- Uchino H, Matsumura Y, Negishi T, Koizumi F, Hayashi T, Honda T, et al. Cisplatin incorporating polymeric micelles (NC-6004) can reduce nephrotoxicity and neurotoxicity of cisplatin in rats. Br J Cancer. 2005; 93: 678-687.
- Baba M, Matsumoto Y, Kashio A, Cabral H, Nishiyama N, Kataoka K, et al. Micellization of cisplatin (NC-6004) reduces its ototoxicity in guinea pigs. J Control Release. 2012; 157: 112-117.
- Uday Kumar S, Matai I, Dubey P, Bhushan B, Sachdev A, Gopinath P. Differentially cross-linkable core-shell nanofibers for tunable delivery of anticancer drugs: synthesis, characterization and its anticancer efficacy. RSC Adv. 2014; 4: 38263 - 38272.
- Kumar SU, Gopinath P. Controlled delivery of bPEI-niclosamide complexes by PEO nanofibers and evaluation of its anti-neoplastic potentials. Colloids Surf B Biointerfaces. 2015; 131: 170-181.
- Sukumar UK, Packirisamy G. Bioactive Core-Shell Nanofiber Hybrid Scaffold for Efficient Suicide Gene Transfection and Subsequent Time Resolved Delivery of Prodrug for Anticancer Therapy. ACS Appl Mater Interfaces. 2015; 7: 18717-18731.
- Elzoghby AO, Samy WM, Elgindy NA. Albumin-based nanoparticles as potential controlled release drug delivery systems. J Control Release. 2012; 157: 168-182.
- Bhushan B, Gopinath P. Antioxidant nanozyme: A facile synthesis and evaluation ofreactive oxygen species scavenging potential of nanoceria encapsulated albumin nanoparticles. J Mater Chem B. 2015; 3: 4843-4852.
- Desai N, Trieu V, Yao Z, Louie L, Ci S, Yang A, et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clin Cancer Res. 2006; 12: 1317-1324.
- Gardner ER, Dahut WL, Scripture CD, Jones J, Aragon-Ching JB, Desai N, et al. Randomized crossover pharmacokinetic study of solvent-based paclitaxel and nab-paclitaxel. Clin Cancer Res. 2008; 14: 4200-4205.
- Socinski MA, Bondarenko I, Karaseva NA, Makhson AM, Vynnychenko I, Okamoto I, et al. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. J Clin Oncol. 2012; 30: 2055-2062.
- Choi SH, Byeon HJ, Choi JS, Thao L, Kim I, Lee ES, et al. Inhalable selfassembled albumin nanoparticles for treating drug-resistant lung cancer. J Control Release. 2015; 197: 199-207.
- Tseng CL, Su WY, Yen KC, Yang KC, Lin FH. The use of biotinylated-EGFmodified gelatin nanoparticle carrier to enhance cisplatin accumulation in cancerous lungs via inhalation. Biomaterials. 2009; 30: 3476-3485.
- Karthikeyan S, Rajendra Prasad N, Ganamani A, Balamurugan E. Anticancer activity of resveratrol-loaded gelatin nanoparticles on NCI-H460 non-small cell lung cancer cells. Biomed Prev Nutr. 2013; 3: 64–73.
- Cao F, Ding B, Sun M, Guo C, Zhang L, Zhai G. Lung-targeted delivery system of curcumin loaded gelatin microspheres. Drug Deliv. 2011; 18: 545- 554.