Short Communication
Ann J Materials Sci Eng. 2014;1(4): 5.
Use of Aerospace Fasteners in Mechanical and Structural Applications
Melhem GN1,2*, Bandyopadhyay S1 and Sorrell CC1
1School of Materials Science and Engineering, University of New South Wales, Australia
2Perfect Engineering Pty. Ltd, Australia
*Corresponding author: Melhem GN, School of Materials Science and Engineering, University of New South Wales, Sydney, NSW 2052, Australia
Received: September 16, 2014; Accepted: November 15, 2014; Published: November 20, 2014
Abstract
The intention of the present short review is to introduce to the non-specialist reader the feasibility of the use of alternative materials not generally considered by engineers working in mechanical and structural applications. That is, the use of specialised aerospace rivets in the more general area of construction is considered. To this end, the text briefly overviews the different types of fasteners used in the construction industry and their common mechanisms of failure. The most common types of fasteners used for conventional mechanical and structural applications are all-steel rivets and pop rivets consisting of aluminium shank and mandrel of a higher strength alloy. In contrast, the aerospace industry makes universal use of pop rivets consisting of high-strength aluminium alloys, the design and installation of which are illustrated. These more specialised rivets are suitable for implementation because the aluminium alloys used exhibit superior mechanical properties and corrosion resistance compared to those of other rivets. For comparison, the mechanical properties of the aluminium alloys used in both conventional and aerospace rivets are surveyed in tabular form.
Since environmental failure by galvanic corrosion owing to exposure to seaspray is very common, the factors that affect galvanic corrosion are discussed. A relatively comprehensive graphic survey of the galvanic series for corrosion of metals and alloys in seawater, drawn from a variety of sources, is provided. While this provides a well known ranking of the susceptibility to corrosion, this version of the series is uncommon in that it illustrates the series generically for alloys and it differentiates the metals and alloys into four ranges of corrosion resistance rather than as a continuous series. More specifically, since the susceptibility to corrosion of chemically similar alloys can be subtly shaded and hence difficult to rank, the galvanic series for corrosion in seawater of an extended range of aluminium alloys also is provided. Finally, an example of the successful 10-year performance of aluminium alloy aerospace rivets for the rectification of the failure of a major rooftop structure, which failed rapidly owing to steel shank-aluminium alloy workpiece corrosion from seaspray, is briefly mentioned.
Fasteners
In the construction industry, which regularly involves mechanical and structural applications, metallic materials are utilised heavily. One major area of application is mechanical fasteners or connections, which include rivets, bolts/nuts, lock bolts, and pins [1,2]. More broadly, fasteners are categorised generally as follows:
Threaded fasteners
Bolt/nut systems are designed with threads, which allow this fastener to be removed without damage to the system.
Rivets
Rivets are permanent fasteners that constrain the joint with a head (factory-head) and an expanded tail (shop-head or buck-tail) on the opposite end of the shank.
Blind fasteners
Blind fasteners are those that are installed and can be accessed on one side of the joint only, such as pop rivets.
Pin fasteners
Pin fasteners typically are of a single elongated piece (solid or tubular), although they may include a malleable collar.
Special-purpose fasteners
Specialised fasteners often are designed for quick removal and replacement and may include studs, latches, slotted springs, and retaining rings.
Fasteners for composites
In the joining of composites, specialised design considerations often are required for joints subject to high stresses, tight tolerance requirements, thermal expansion mismatch, galvanic corrosion, and/ or leakage.
Failure
Failure of fastening systems usually is from static loading (overload in tension, bending, shear, or torsion), dynamic fatigue (from cyclic loading or repeated impact), or corrosion (galvanic, chemical, or stress) [1]. Typical locations of failure of the most common mechanical fasteners are directly beneath the head(s) of rivets, at the thread-shank transition (in bolts), at the first inner thread (in nuts), and at microstructural imperfections. Alternatively, failure of the plates or sheets being joined regularly is by dynamic fatigue [3].
Although it usually is straightforward to design for static and dynamic loads in mechanical and structural systems, the potential effects of corrosion are more difficult to predict. This is partly because they depend on factors that are intrinsic to the product, such as the chemical composition and the microstructure, which is dependent on the processing. It also is because they depend on factors that are extrinsic to the product, particularly the environmental conditions to which it is exposed. Consequently, these effects often can be overlooked when specifying materials for such applications.
Most conventional threaded fasteners are fabricated from various grades of alloy steel, which may have protective coatings, such as zinc, tin, cadmium, or aluminium [1]. In contrast, pop rivets are constructed from alloys in all-steel, all-aluminium, and aluminium shank/steel mandrel configurations. In most construction applications, the joined plates and sheets also consist of alloys of steel and aluminium. Therefore, the potential for galvanic corrosion resulting from the opposition of dissimilar metals is clear [4].
The principles of galvanic corrosion are well known [5-8]. Since many mechanical and structural applications are exposed to rainwater, condensed humidity, seawater, and seaspray, the metals used in these systems are subject to anodic corrosion by electrochemical reactions during which at least one of the metals is altered from the metallic to the non-metallic state. In terms of galvanic corrosion, there are five general issues of consideration:
Galvanic series
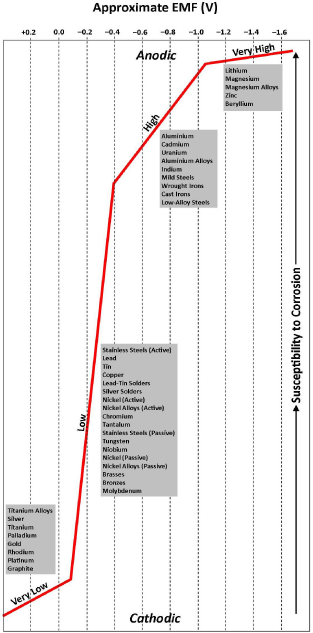
In galvanic reactions, dissimilar metals act as cathode and anode while the water acts as electrolyte. This configuration is sufficient to establish an electrical circuit involving a potential (voltage) difference between the electrodes and associated current (amperage) flow. The electromotive force (EMF) series [5], which ranks the potential for corrosion between pure bimetallic couples in water in terms of electrochemical cell voltages, is well known. Another, perhaps more practical, variant is the galvanic series, which provides the same ranking for commercial metals and alloys in seawater, which is a more conductive electrolyte than water. This is shown in Figure 1. These data, which are drawn from a range of sources, can be used to determine the probable location of corrosion (i.e., oxidation). For example, if structural steel members (i.e., mild steel) are fastened with a zinc-plated bolt and nut, their relative vertical locations in Figure 1 indicate that the former acts as the cathode (higher in Figure 1 → decelerated corrosion) and the latter acts as the anode (lower in Figure 1 → accelerated corrosion). The greater the separation of the two in Figure 1, the more severe the corrosion. Since electrons are conducted to the anode and cause reaction, then the zinc-plated bolt and nut will corrode (where the contact surface areas of both electrodes are identical) and hence form zinc oxide (ZnO).
Figure 1: Generalised galvanic series in seawater [9-13,22,28].
Similar EMF ranges
Since commercial metals and alloys exhibit a range of EMF values, rather than specific consistent values, when two metals in contact have EMF ranges that overlap, it becomes uncertain which acts as cathode and which acts as anode.
Surface area effect
When the surface areas of the electrodes are dissimilar, then an area effect becomes important. That is, when the anode is small relative to the cathode, then the concentration of electrons being conducted to the anode becomes high, which enhances reaction. Therefore, it is desirable to ensure that the anodic metal surface area is large compared to that of the cathodic metal surface area. The greater the difference in areas, the more severe the corrosion. So, when a coating is pitted or scratched and the underlying metal is exposed, the latter becomes an electrode of very small surface area. In this case, it is essential for the underlying metal to be cathodic relative to the anodic surface coating. Figure 1 shows that zinc-plated bolts and nuts, the coatings of which can be damaged relatively easily, meet this criterion.
Biocorrosion
Marine slimes or biofilms often form, which can facilitate bacterial corrosion [14]. As the films grow, bacteria can release corrosive species or establish conditions conducive to corrosion, both of which can increase corrosion rates significantly.
Contact resistance
Consideration of the effect of the galvanic series on the probability of corrosion relies on the assumption of two dissimilar metals in contact and subsequent active corrosion [15]. However, the resultant electrical circuit may be broken through the formation of an electrically insulating layer consisting of an oxide or another corrosion product [16], which typically results in passive corrosion [15]. This has the effect of hindering further corrosion and so reducing its severity and/or rate.
Rivets
There are five main types of conventional rivets, which are bifurcated or split, compression, full tubular, solid, and semitubular [1]. All of these rivets require access to both sides of the joint and, with the exception of compression rivets, they require the use of a bucking bar, which is a specially shaped metal piece that expands and work hardens the tail upon impact to the head (bucking or upsetting). Compression rivets have two heads and they form the join from radial compressive stress and deformation.
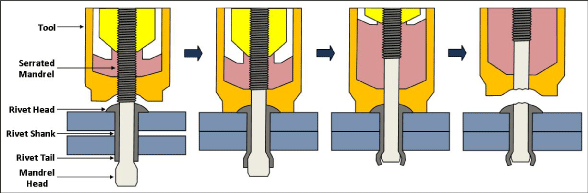
In contrast, blind rivets require access to only the head side of the join. There are four main types of blind rivets, which are chemically expanded, drive-pin, pull-mandrel, and threaded [1]. Of these, the most commonly used and the most convenient are known as the pop rivet, which consists of a tubular shank (sleeve) and contains an interior mandrel (pin). When the mandrel is drawn into the rivet shank with the appropriate tool, the mandrel causes the exposed shank tail to expand, after which the mandrel snaps off, leaving the mandrel head (or head + part of the mandrel) locked into the tail (or tail + shank). The design and installation of an open-end break mandrel is illustrated in Figure 2 [17].
Figure 2: Schematic of operation of Huck Magna-Lok® [17] rivet system.
There are two main considerations concerning the failure of rivets in the environment:
Corrosion
Since the mandrels are made from aluminium alloy, low-carbon steel, stainless steel, copper, and Monel (63Ni31Cu) alloy, the potential for galvanic corrosion with what typically is an aluminium alloy tubular shank within the rivet itself is clear. Consequently, two common problems in mechanical and structural applications exposed to the environment near the ocean are shank-workpiece corrosion in the joint and mandrel-shank corrosion in the rivet.
Mechanical properties
Stresses deriving from periodic wind loading, cyclic thermal expansion/contraction, and continuous static loading can be significant since the mechanical strengths of the aluminium alloys typically used as the shank are relatively low, as shown in Table 1.
Aluminium
Alloy Grade
Temper
Tensile
Strength
(MPa)
Yield
Strength
(MPa)
Shear
Strength
(MPa)
Fatigue
Strength
(MPa)
5050
O
145
55
105
83
H32
170
145
115
90
H38
220
200
138
97
5052
O
195
90
125
110
H32
230
195
140
115
H38
290
255
165
140
5056
O
290
152
179
138
H18
434
407
234
152
H38
414
345
221
152
Table 1: Mechanical Data for Aluminium Alloys Commonly Used in Pop Rivet Shanks [18].
Aluminium in Aerospace Applications
History
Aluminium metal was first isolated by Hans Christian Ørsted in Denmark in 1825 [19] and a commercial process for its manufacture was developed simultaneously by Charles Martin Hall in America and Paul Héroult in France in 1886 [20]. Aluminium in aerospace applications goes back to the earliest days of successful flight, where the crankcase of the engine used in the Wright Brothers’ inaugural flight of 1903 was fabricated using an aluminium alloy [21]. The first mass-produced aeroplane to make extensive use of aluminium was the Bréguet 14 bomber of 1916 [22]. The first all-aluminium aircraft was produced in the following year in the form of the Junkers J7 fighter [23]. In 1936, aluminium rivets were used in aircraft construction for the first time in both the US by Cherry Aerospace [24] and the UK by Aviation Developments (now Avdel) [25].
Applications of Aerospace Rivets in Mechanical and Structural Applications
The use of rivets in aerospace construction is well established [26-29]. This high-performance application requires superior performance in terms of corrosion resistance and mechanical stability. However, the use of aerospace rivets in more conventional mechanical and structural applications has remained very limited probably owing to lack of familiarity and higher costs. Consequently, the main purpose of the present work is to introduce to the reader the potential to use these more specialised rivets in conventional applications for which they may not have been considered. Table 2 gives some of the mechanical properties of aluminium alloys that are used commonly in aerospace rivets.
Aluminium
Alloy Grade
Temper
Tensile
Strength
(MPa)
Yield
Strength
(MPa)
Shear
Strength
(MPa)
Fatigue
Strength
(MPa)
References
1100
O
90
34
62
34
14
H14
124
117
76
48
H18
165
152
90
62
2017
T4
427
276
262
124
30
2024
T3
483
345
283
138
30
2117
T4
296
165
193
97
31
2219
T851
455
352
285
103
30
5056
O
290
152
179
138
14
H18
434
407
234
152
H38
414
345
221
152
7050
T7451
524
469
303
240
30,32
7075
T6
572
503
331
159
29
Table 2: Mechanical Data for Aluminium Alloys Commonly Used in Aerospace Rivets.
The most successful aerospace rivet probably is the Huck Magna- Lok® [17], which is manufactured by Alcoa Fastening Systems. In light of the previous comments concerning the importance of corrosion and mechanical properties, it is clear that there are advantages in the use of these all-aluminium rivets, which consist of aluminium alloy grades 5056 shank and 7075 mandrel, both of which are coated with zinc chromate.
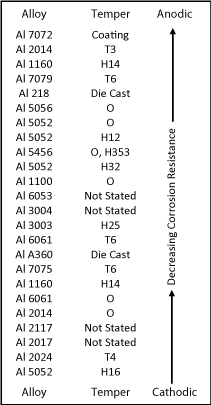
Following installation, the partially retained mandrel, as shown in Figure 2, carries the majority of the mechanical load, so the optimal mechanical properties of alloy grade 7075 are a major advantage. A second major advantage is the resistance to corrosion in the rivet and, in the case of joining of aluminium sheet, at the shank-workpiece interface, where the chemical similarities of the aluminium alloys minimize the chemical differences and hence the EMF differences. Although Figure 1 is simplified in terms of metal and alloy groups for the benefit of the non-specialist reader, a more comprehensive list of aluminium alloys in the galvanic series in seawater is as shown in Figure 3. A qualitative assessment of the corrosion resistance of a more comprehensive range of aluminium alloys is available elsewhere [36].
Figure 3: Galvanic series for some aluminium alloys in seawater [33-35].
Finally, it is noted that Huck Magna-Lok® [17] rivets have been used by the authors in a major construction rectification of an aluminium louvre system installed on the roof of a high-rise building located ~2 km from the Pacific Ocean [37]. The louvre system failed principally owing to galvanic corrosion initiated by the use of steel rivets that had been used to join the louvre mullions and profiles, which were constructed from aluminium grades 6063(T6) and 6060 (T5), respectively. The original louvre system was replaced with mullions and profiles of aluminium grades 6351 (T6) and 6063 (T6). The mechanical properties of these aluminium alloys are contrasted in Table 3. While the original system failed within 1 year of installation, the rectification using all-aluminium rivets has performed without corrosion for nearly 10 years.
Aluminium
Alloy Grade
Temper
Tensile
Strength
(MPa)
Yield
Strength
(MPa)
Shear
Strength
(MPa)
Fatigue
Strength
(MPa)
References
6060
T5
220
185
140
90
38,39
6063
T6
241
214
152
69
30
6351
T6
310
283
200
90
31
Table 3: Mechanical Data for Aluminium Alloys Used in Louvre System.
References
- Jensen WJ, “Failures of Mechanical Fasteners”. In: Metals Handbook, 9th Edn. Failure Analysis and Prevention. American Society for Metals, Metals Park, OH. 1986; 11: 529-549.
- Popov EP. Mechanics of Materials, Second Edition. Prentice/Hall International, Inc., London. 1978.
- Forrest PG. Fatigue of Metals. Pergamon Press, Oxford. 1962.
- Evans UR. “An Outline of Corrosion Mechanisms, Including the Electrochemical Theory”. In: Uhlig HH, editor. The Corrosion Handbook. John Wiley & Sons, London, 1948; 3-11.
- Marek MI, Natalie CA, Piron DL. “Thermodynamics of Aqueous Corrosion”, In: Metals Handbook, Ninth Edition. Corrosion. American Society for Metals, Metals Park, OH. 1987; 13: 18-28.
- Shoe smith DW. “Kinetics of Aqueous Corrosion”, In: Metals Handbook, Ninth Edition. Corrosion. American Society for Metals, Metals Park, OH, 1987; 13: 29-36.
- Silverman DC, Puyear RB. “Effects of Environmental Variables on Aqueous Corrosion”. In: Metals Handbook, Ninth Edition. Corrosion. American Society for Metals, Metals Park, OH. 1987; 13: 37-44.
- Shoesmith DW. “Effects of Metallurgical Variables on Aqueous Corrosion”. In: Metals Handbook, Ninth Edition. Corrosion. American Society for Metals, Metals Park, OH. 1987; 13: 45-49.
- Uhlig’s Corrosion Handbook, 3rd Edition. Revie RW, editor. John Wiley & Sons, Inc., Hoboken, NJ. 2011.
- Anonymous. “Corrosion Characteristics of Carbon and Alloy Steels”. In: Metals Handbook, Desk Edition. Edited by Boyer HE, Gall TL. American Society for Metals, Metals Park, OH, 1985; 4-89 – 4-94.
- Anonymous, “Failures from Various Mechanisms and Related Environmental Factors”. In: Metals Handbook, Desk Edition. Boyer HE, Gall TL, editors. American Society for Metals, Metals Park, OH. 1985; 32-8 – 32-32.
- Cardarelli F. Materials Handbook: A Concise Desktop Reference, 2nd Edition. Springer, London. 2008.
- U.S. Air Force and U.S. Navy. Technical Manual: Corrosion and Corrosion Control, Volume I: Corrosion Program and Corrosion Theory, TO Engineering Handbook Series for Aircraft Repair. General Manual for Structural Repair, NAVAIR 01-1A-509-1, TO 1-1-689-1. 2005.
- Videla HA. Manual of Biocorrosion. CRC Press, Boca Raton, FL. 1996.
- Ghali E. Corrosion Resistance of Aluminum and Magnesium Alloys: Understanding, Performance, and Testing. John Wiley & Sons, Inc., Hoboken, NJ. 2010.
- Mark HF. Encyclopedia of Polymer Science and Technology, Concise 3rd Edition. John Wiley & Sons, Inc., Hoboken, NJ. 2007.
- Alcoa, Alcoa Fastening Systems, Huck Magna-Lok®.
- ASM Committee on Aluminum and Aluminum Alloys, “Properties of Wrought Aluminums and Aluminum Alloys”. In: Metals Handbook, 9th Edtn. Properties and Selection: Nonferrous Alloys and Pure Metals. American Society for Metals, Metals Park, OH. 1979; 2: 63-139.
- Hasan H. Understanding the Elements of the Periodic Table: Aluminum. The Rosen Publishing Group, Inc., New York. 2007.
- Hall-Héroult Centennial: First Century of Aluminum Process Technology 1886-1986. Peterson WS, Miller RE, editors. The Minerals, Metals & Materials Society, Warrendale, PA. 2002.
- Smithsonian National Air and Space Museum. The Wright Brothers / The Invenention of the Aerial Age / Inventing a Flying Machine.
- Morrow Jr JH. The Great War in the Air: Military Aviation from 1909 to 1921. Smithsonian Institute Press, Washington, D.C. 1993.
- Norman A. The Great Air War. The Macmillan Company, New York. 1968.
- Cherry Aerospace. History of Cherry Aerospace.
- Salmenkova EA, Politov DV. [On the 75th anniversary of the birth of Yurii Petrovich Altukhov (1936-2006)]. Genetika. 2011; 47: 1429-1437.
- U.S. Department of Defense, Department of Defense Handbook, Metallic Materials and Elements for Aerospace Vehicle Structures, MIL-JDBL-5J. 2003.
- U.S. Department of Transportation, Federal Aviation Administration, Advisory Circular AC 43.13-1B – Acceptable Methods, Techniques, and Practices – Aircraft Inspection and Repair. 1998.
- U.S. Air Force, U.S. Navy. Technical Manual: Engineering Handbook Series for Aircraft Repair. General Manual for Structural Repair. NAVAIR 01-1A-1, TO 1-1A-1. 2006.
- Biel EW, Humrichouser GL, Ervin BA. Aviation Structural Mechanic (H & S) 3 & 2, NAVEDTRA 12338. 1993.
- ASM Aerospace Specification Metals Inc.
- MatWeb. Material Property Data.
- AZO Materials, Aluminium / Aluminum 7050 Alloy (UNSW A97050).
- U.S. Air Force, Military Standard: Dissimilar Metals, MIL-STD-889B, 1993.
- The Engineering Toolbox.
- Barrett R. The Engineer’s Companion: Fastener Design Manual, Part One.
- Kaufman JK (Revision), “Corrosion of Aluminium and Aluminium Alloys”. In: ASM Handbook. Corrosion: Materials. Cramer SD, Covino BS, Jr, Editors. ASM International, Materials Park, Ohio. 2005; 13B: 95-124.
- Melhem GN. Design Variables for Steel and Aluminium in High-Rise Rooftops. University of New South Wales. 2008.
- ThyssenKrupp, ThyssenKrupp Materials (UK) Ltd, Aluminium Alloy 6060.
- L.’tHoen-Velterop, den Bakker AJ, Edwards SP, Ubels LC. Static and Dynamic Mechanical Properties of Longitudinal Weld Seams in Industrial AA6060, AA6082 and AA7020 Aluminium Extrusions. Nationaal Lucht- en Ruimtevaartlaboratorium, Report No. NLR-TP-2007-783. 2008.