
Research Article
Ann Materials Sci Eng. 2019; 4(1): 1036.
Studies of Metallic Hydride Clusters for Use in Hydrogen Storage
Darsey JA1,2*, Szwedo SM1,2 and Griffin WO1
¹Department of Chemistry, University of Arkansas at Little Rock, Little Rock
²Center of Molecular Design and Development, University of Arkansas at Little Rock, Little Rock
*Corresponding author: Darsey JA, Center for Molecular Design and Development, University of Arkansas at Little Rock, 2801 South University Ave., Little Rock, Arkansas, USA
Received: August 14, 2019; Accepted: August 27, 2019; Published: September 03, 2019
Abstract
It is well known that the outer orbitals of an atom or molecule are responsible for their major physical and chemical properties. We computed the energies of the Lowest Unoccupied Molecular Orbitals [LUMO] and the energies of the Highest Occupied Molecular Orbitals [HOMO], which were obtained using an ab initio molecular orbital program. These orbital energies were correlated with the properties, both physical and chemical, of the bulk materials made from these various atomic systems using Artificial Neural Network (ANN) modeling procedures. Some of the bulk properties which have been previously studied are 1st ionization potential, melting point and boiling point. Additional properties which will be discussed in this manuscript will be electron affinity and percent hydrogen storage. In addition, thermodynamic state functions can also be modeled such as enthalpy, entropy of the sorption process and the partial pressure of stored hydrogen, which will also be discussed in this paper. We will present results of the modeling studies of single atoms and small atomic clusters. We will present results that show several chemical and physical properties of the bulk materials which are modeled by our computational procedures. Gaussian 03 and 09 molecular modeling software was used to perform Density Functional Theory (DFT) single point energy calculations on the structurally optimized atomic and molecular systems. In the Gaussian program, the LanL2DZ basis set and the B3PW91 functional was used. The LanL2DZ basis set incorporates parameters that accounts for the relativistic effects of heavier elements. In addition, we will summarize results of several different types of metal hydride clusters used for the storage of hydrogen gas.
Keywords: Hydrogen storage; Artificial intelligence; Molecular modeling studies; Artificial neural network; Bulk properties; Metallic hydrides
Introduction
Hydrogen gas as a potential new fuel is undergoing increasing scientific attention as it is being seen as a new fuel source by transportation and other energy related industries [1]. What makes hydrogen so attractive as a fuel is that it produces zero-emissions when reacted with oxygen. It can be used in electrochemical cells such as fuel cells or directly as a fuel in an internal combustion engine. It has long been used as a fuel for the propulsion of spacecraft. Hydrogen is found in the first group and first period in the periodic table making it the lightest element. It is also the most abundant element in the universe and is the primary fuel source for our sun and all other stars. However, the most abundant source of hydrogen on earth is water. Therefore, having an inexpensive and reliable method for obtaining hydrogen from water would be necessary before a hydrogen economy could be realistically considered. In addition, a second necessary criteria before a hydrogen economy could be realized is that an economically feasible method to store hydrogen must be perfected. One class of materials that has been shown to be efficient in storing hydrogen gas at ambient temperatures and pressures is metal hydrides.
While there are several applications for metal hydrides, there are two main types: stationary and mobile [1]. Stationary metal hydrides are used as reducing agents (adding hydrogen) in chemical reactions, bases in organic synthesis, refrigeration, desiccants to remove water from solvents, hydrogen processing, thermal applications, hydroformylation reactions and hydrogen storage. Some of the specific uses for metal hydrides are in the areas of: switchable optics, battery storage (i.e. nickel metal hydride), heat pumps, thermal compression, and gas isotope separation.
The majority of the consumption of energy in the U.S. currently is fossil fuels [1]. Hydrogen is a more suitable candidate to replace fossil fuels if it can overcome several obstacles. One of these obstacles would be the development of a reliable, inexpensive hydrogen source. It would be costly to completely convert current infrastructure in order to move away from fossil fuel energy; however, it will be worth the effort. Hydrogen is a clean, efficient and almost inexhaustible fuel supply since water would be the primary source of the hydrogen fuel. A second obstacle is the safe storage of hydrogen in a form other than liquid or gas. It is the opinion of the authors that metal hydrides will help to alleviate this second obstacle.
The major impetus for this research was to determine new metal hydride materials for use in the storage of hydrogen. If we are to develop new sustainable energy sources, hydrogen must be at the top of this list. The source of hydrogen would be water, of which the earth has almost an inexhaustible supply. Hydrogen gas is known to have the largest energy content of any fuel that would be used in transportation. With an energy content of 120MJ/kg it is almost three times the energy content of gasoline which has an energy content of 45 MJ/kg [2,3]. The major problem in using hydrogen currently, is that it can only be used in two forms; liquid or gas. Liquid hydrogen requires temperatures to be maintained below -252.87°C (20.28 K). In the gaseous state, hydrogen would need to be stored in tanks pressurized to 10,000 psi. Neither the liquid nor gaseous state of hydrogen would make a viable and safe option for our current means of transportation. Metal hydrides provide a viable solution to this problem. For example, a metal hydride made from magnesium and nickel or one made from lanthanum and nickel can store twice as much hydrogen for the same energy content as can be stored in the gaseous form, and about twenty five percent more energy content than in the liquid form. In addition, if a tank of hydrogen in either the gaseous or liquid form ruptures a catastrophic explosion would result. If a tank containing hydrogen stored as a metal hydride ruptures, the only consequence would be the clean-up of the solid metal hydride material. No explosion would result from the rupture of a metal hydride containing tank.
Methods
The metal hydride clusters were initially modeled using HyperChem 5.01 molecular modeling software [4]. This program was used to construct and optimize where necessary, the atoms which made up the metal hydride nano-clusters. Spin multiplicity for the lowest ground state as determined from term symbols were used for geometry optimizations of these elements. Gaussian 03 [5] and Gaussian 09 [6] molecular modeling software was then used to perform Density Functional Theory (DFT) single point energy calculations on the structurally optimized clusters. In the Gaussian program, the LanL2DZ basis set and the B3PW91 functional was used. The LanL2DZ basis set incorporates parameters that accounts for the relativistic effects of heavier elements [7].
An artificial neural network (ANN) was used to predict the properties of the nanoclusters modelled in this study [8, 9]. A set of processing elements (or nodes) are used to construct a feed-forward, back propagation artificial neural network (see Figure 1) [8]. These nodes are interconnected in a network that can then identify patterns in the data. In a sense, the network learns from “experience” which distinguishes neural networks from traditional computing programs that simply follow instructions in a fixed sequential order. The ANN program extracts the functionality of the relationships buried within the weight space which the ANN uses to correlate the atomic orbitals and other input data, with the various chemical and physical properties of the nanocluster materials corresponding to the specific metal hydride. The input layer consists of twenty highest occupied molecular orbitals (HOMOs), twenty lowest unoccupied molecular orbitals (LUMOs), total energy, and dipole moment. The output node contains the physical or chemical property to be predicted in our modelling procedure. First, we present the above input data (LUMOs, HOMOs, etc…) from nanoclusters for which the chemical or physical properties are known. This input and output data constitute the training set for an artificial neural network. Next, we calculate the highest occupied molecular orbitals (HOMOs), lowest unoccupied molecular orbitals (LUMOs), total energy, and dipole moment for nanoclusters which have not previously had their properties measured. The trained ANN will use this input data to predict the specific property we are interested in from the list of properties below [10].
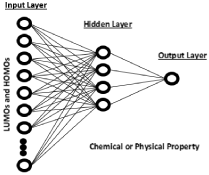
Figure 1: Artificial Neural Network Architecture.
After we completed the fully trained artificial neural network, we did a complete cross-validation. We took out approximately ten percent of the data from each property that was being modeled. We trained on the remaining ninety percent and used the remaining ten percent of the data that was removed to test the validity of the trained neural network. We repeated this process until every data point in a particular data set had its turn in a testing set. (Figure 1)
There were numerous physical and chemical properties that were modelled. Among the properties studied were [1-3,11]:
1. Negative log of pressure of stored hydrogen
2. Weight % hydrogen stored
3. Enthalpy
4. Entropy
5. Desorption temperature at 1 atm
6. Electron affinity
In the results section we will discuss several of the above properties.
Results and Discussion
We began our calculations by modeling 225 pure metals and small metallic clusters, assuming some clusters would be trimmed as outliers to improve training. As stated, this includes 23 pure metals whose experimental value of percent weight of stored hydrogen is known. These 23 metal clusters were set aside in order to later use as a prediction set once the network has been trained. The ANN was trained to an error limit of 0.001. The outliers from those trainings were removed from the set leaving 198 from the original 225 metal clusters. The prediction with 198 metals is shown in Figure 2. The predicted values with greater than two standard deviations from the mean of the %weight hydrogen stored was the criteria for eliminating outliers. The network underwent cross validation for all 198 metal clusters and the results are seen in Figure 1 [11]. In addition, the unknown group was predicted using the correlations of the model. Their predicted value was compared with experimental values were available, which were found in the literature. The results are given in Table 1 [11] (Figure 2) (Table 1).
Metal Custer
ANN prediction of % weight H2
Known Cluster
Measured value
YNi
1.15
1.58
ZrMn
1.63
PrAg
1.55
PrGa
0.05
SmMg
0.27
YCu
0.75
LaAl
1.08
(LaAlH2.6)
1.54
GdCu
1.20
(GdCuH2.0)
0.90
DyAl
1.07
GdAl
1.32
TiCo2
1.72
LaZn
1.63
MgNi2
3.86
TiAg
0.77
SmMn2
1.64
(SmMn2H2)
0.76
DyAg
1.13
LaAg
0.25
2.23
CeAg
1.35
ScRu2
1.08
TiFe2
1.07
LaAl2
0.51
LaPt2
0.96
PrMg
0.41
Table 1: Prediction of unknown metal hydrides along with a limited number of experimental values.
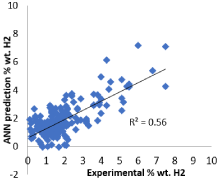
Figure 2: Correlation plot of experimental % weight hydrogen vs. ANN
predicted % weight H2.
Figure 3 is a correlation plot of the experimental -log of the pressure vs. the neural network prediction of -log of the pressure at 25°C (using units in atmospheres). The plot shows a very strong correlation between the experimental and the predicted -log of the pressure with a correlation of R2 equal to 0.99 [9,11].
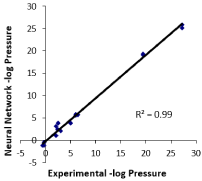
Figure 3: Correlation plot of experimental -log pressure vs. NN predicted
-log pressure.
Figure 4 shows the correlation of twelve pure metal hydride clusters from the neural network prediction of percent weight hydrogen to experimental values of percent weight hydrogen for the metal hydrides with the highest values measured [11].
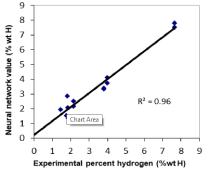
Figure 4: Correlation plot of experimental % weight hydrogen vs. ANN
predicted % weight hydrogen.
Figure 5 shows the correlation of the neural network experimental enthalpy vs. neural network predicted enthalpy. The units are kilajoules/mole. The correlation is very good with a value of R2 =0.96 [9,11].
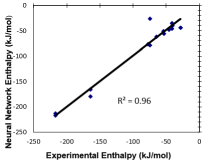
Figure 5: Correlation plot of experimental enthapy vs. ANN predicted
enthalpy.
Figure 6 shows a correlation between experimental entropy and predicted entropy using ANN. The units are in kilajoules/mole. The correlation for this plot is very good with an R2 value of 0.89 [9,11].
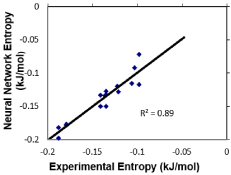
Figure 6: Correlation plot of experimental entropy vs. ANN predicted entropy.
Figure 7 shows the correlation of the neural network prediction of temperature at one atmosphere of pressure. The unit of temperature is Kelvin and the unit of pressure is in atmospheres. The correlation is very strong with an R2 value of 0.94 [9,11].
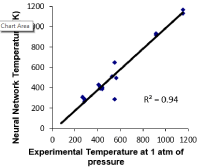
Figure 7: Correlation plot of experimental temperature at 1 atm of pressure
vs. ANN predicted temperature at 1 atm of pressure.
As can be seen in (Figure 8), the electron affinities for 9 two atom clusters was plotted with a correlation R2 =0.76. This correlation was obtained using the leave one out cross validation procedure. That is training occurred by eliminating one data point at a time and training on the remaining data points. Each data point took its turn in not being included in the training [9,12].
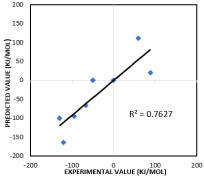
Figure 8: Correlation plot of experimental value of electron affinity and ANN
predicted value of electron affinity.
Table 2 displays examples of experimental data which characterizes the specified metal and metal clusters shown in the first column. This data was used for training all five predictions shown in the above table. The convergence limit for error was set at 0.001 for all training. Log pressure at 25°C, temperature at one atmosphere of pressure, weight percent hydrogen, enthalpy and entropy were all trained successfully [9,11].
Metal
Pressure at 25°C (atm)
Temperature for 1 atm
Weight %H
ΔH (kJ/mol)
ΔS (kJ/mol·K)
TiFe
4.1
-8
1.86
-28.1
-0.106
Ti
4E-20
643
3.98
-164
-0.179
TiCo
0.004
135
1.45
-54
-0.135
Zr
6.4E-28
881
2.16
-217
-0.188
ZrCr2
0.0029
166
1.82
-45.2
-0.103
ZrMn2
0.001
167
1.77
-53.2
-0.121
ZrNi
0.0000004
292
1.85
-76.85
-0.136
Mg
0.000001
279
7.66
-74.5
-0.135
Mg2Ni
0.00001
255
3.6
-64.5
-0.122
V
2.1
12
3.81
-40.1
-0.1407
Pd
0.0082
147
0.72
-41
-0.0976
Table 2: Experimental data for pressure, temperature for 1 atm, weight percent hydrogen, enthalpy and entropy.
Summary
This manuscript summarizes the results of modeling studies concerning the prediction of numerous physical properties of small metallic hydride nanoclusters. These properties include; negative log pressure, weight percent hydrogen stored, enthalpy, entropy, temperature at 1 atm pressure and electron affinity. The result of these predicted values was excellent ranging in R2 values from a high of 0.99 to a low of 0.56. Considering the diversity of properties predicted, the authors feel these predictions made in these studies were very good. The input for all neural network simulations were the same; that is for each nanocluster the same lowest unoccupied molecular orbitals, LUMOs, highest occupied molecular orbitals, HOMOs, total energy and dipole moment were used. The only different data for each new training set was the specific physical property being modeled, i.e. enthalpy, electron affinity, etc…
Conflicts of Interest
The authors declare no conflict of interests.
References
- The Hydrogen Economy: Opportunities, Costs, Barriers, and R&D Needs. Washington, DC: The National Academies Press. National Research Council and National Academy of Engineering 2004.
- “Fossil and Alternative Fuels – Energy Content” Engineering ToolBox. 2008.
- Overview of Storage Development DOE Hydrogen Program. Office of Energy Efficiency & Renewable Energy. 2000.
- HyperChem(TM) Professional 5.01, Hypercube, Inc., 1115 NW 4th Street, Gainesville, Florida 32601, USA. 2013; 3: 61-70.
- Gaussian, Revision C, Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, et al. Gaussian, Inc., Wallingford CT, 2004.
- Gaussian, Revision A, Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, et al. Gaussian, Inc., Wallingford CT, 2016.
- Kohn W, Sham LJ. “Self-consistent equations including exchange and correlation effects”. Physical Review. 1965; 140: A1133–A1138.
- Darsey JA, Mitchell NC, Buzatu D. Artificial Intelligence modeling of materials’ bulk chemical and physical properties. STAR. 2006; 44: 1-7.
- Griffin WO, Darsey JA. Bulk Metallic System Modeling of Metal Hydride Dimer and Trimer Nanoclusters. J. Comput. Theor. Nanosci. 2010; 7: 1-6.
- Griffin WO, Darsey JA. Artificial neural network predication indicators of density functional theory metal hydride models. Int. J. Hydrogen Energy. 2013; 38: 11920-11929.
- Griffin WO. A DFT Computational Model of Metal Hydrides. Ph.D., University of Arkansas at Little Rock, Little Rock, Arkansas 72204. 2012.
- Mitchell NC. A Systematic Approach for Developing Feed-Forward/Back- Propagating Neural Network Models for Predicting Bulk /chemical and Physical Properties of Transition Metals. Ph.D., University of Arkansas at Little Rock, Little Rock, Arkansas. 2007.