
Research Article
Ann Materials Sci Eng. 2021; 5(2): 1043.
Effect of B Incorporation on the SiOC Precursor Fabrication and High-Temperature Oxidation Behavior of SiBOC Ceramics
Sun Z1, Xu H2, Yuan F3, Zhou S4* and Zhou H5
1Yantai Vocational College, Yantai, PR China
2Shandong Industrial Ceramic Research & Design Institute Co., Ltd., Zibo, PR China
3Aerospace Research Institute of Materials & Processing Technology, Beijing, PR China
4National Key Laboratory of Science and Technology on Advanced Composites in Special Environments, and Center for Composite Materials and Structures, Harbin Institute of Technology, Harbin, PR China
5School of Materials Science and Engineering, Harbin University of Science and Technology, Harbin, PR China
*Corresponding author: Shanbao Zhou, National Key Laboratory of Science and Technology on Advanced Composites in Special Environments and Center for Composite Materials and Structures, Harbin Institute of Technology, Harbin 150040, PR China
Received: August 31, 2021; Accepted: September 29, 2021; Published: October 06, 2021
Abstract
The SiBOC precursor ceramics were fabricated by polymer-derived method SiBOC pre-cursors with different B/Si molar ratios were synthesized by controlling monomer content. The effects of different process conditions on SiBOC ceramics and the oxidation resistance of SiBOC ceramics at the high temperature (1400OC) were studied. The SiBOC precursor’s bonds of Si-O-C, Si-O-Si, and Si-O-B were detected by FTIR. The precursor is converted to a rigid ceramic material that has an 85.3wt% ceramic yield of SiBOC ceramics with B/ Si=0.4 by TG-DSC. The results of SEM analysis show that the SiBOC ceramics surface bulges and folds increase with the increase of boric acid content. With the increase of pyrolysis temperature and pyrolysis time, the size and number of pores on the surface of SiBOC ceramics also increase. Static oxidation tests show that SiBOC ceramics with B/Si=0.4 have better oxidation resistance under other conditions being the same.
Keywords: Polymer-derived ceramics; SiBOC ceramics; Sol-gel method; Oxidation resistance
Introduction
SiOC precursor ceramics have a wide range of raw materials, low price, large output, simple, preparation and designable molecular structure. However, its high-temperature resistance is still insufficient and carbothermal reduction begins at about 1300oC, which limits its wider application [1,2]. Besides, it was found that the content, pyrolysis atmosphere, particle size, and apparent morphology of the samples had a significant influence on the high temperature stability of SiOC ceramics [3]. The high-temperature stability of SiOC precursor ceramics can be improved by doping B, N, and Zr, and therein the improvement of element B is significant [4-7]. This kind of ceramic material prepared by using an organic polymer as a ceramic precursor is usually called polymer-derived ceramics (PDCs). The method of preparing ceramics by the polymer-derived method has been widely used in the preparation of silicon-based precursor ceramics. In the precursor of SiBOC, boron atoms are introduced into the SiOC gel network in the form of Si-O-B bonds, and the corresponding SiBOC ceramics are obtained through pyrolysis. SiBOC ceramics are composed of silicon carbide (SiCxO4-x) and boron carbide (BCyO3-y) mixed units [8]. The microstructure of SiBOC ceramics with different B/Si ratios has been studied by many researchers. In the temperature range of 1000-1500oC, the introduction of boron is conducive to the graphitization of free carbon, reducing the reaction activity of free carbon, thus inhibiting the carbothermal reduction reaction [9-12]. Also, the introduction of boron leads to a higher ceramic yield of 89% as well as the enhanced crystallization of both β-SiC and free carbon [13]. During crystallization, SiCxO4-x and BCyO3-y units were consumed and boron was mainly pre-sent in trigonal borosilicate BO3 sites at 1500oC [11,14,15].
To obtain SiBOC gel precursor, Schiavon et al add to Methyltriethoxysilane (MTES) a proper amount of boric acid with different B/Si molar ratios and gelation time. The weight loss showed that the addition of boron to the Si-O-C glass slightly increase the high-temperature stability of the ternary oxycarbide glass [14]. Riedel et al found that the polymer-to-ceramic transformation process which can avoid cristobalite formation and the degradation of oxidation resistance of the material [8].
In this paper, MTES and ethanol were alcoholized in the presence of Hydrochloric Acid (HCl) catalyst. Boric acid was used to provide B element to modify the SiOC precursor ceramic system to synthesize SiBOC precursor. A new structure of SiBOC ceramics was obtained by subsequent pyrolysis of the dry precursor at a high temperature. Then the synthesis mechanism of SiBOC precursor starting from gel to ceramic was investigated. The effect of different B/Si on the yield of SiBOC precursor ceramics, the influence of different pyrolysis temperatures and time on the morphology, and related properties of SiBOC ceramics at high temperatures were studied.
Experimental
Materials synthesis and processing
The commercially available methyltriethoxysilane (MTES, CH3Si(OCH2CH3)3, Macklin Co. Ltd., Shanghai, China), boric acid (B(OH)3, Tianjin Zhiyuan Chemical Reagent Co., Ltd., Tianjin, China), anhydrous ethanol (CH3CH2OH, Harbin Chemical Reagent Factory Co. Ltd., Harbin, China) and hydrochloric acid (HCl, Harbin Chemical Reagent Factory Co. Ltd., Harbin, China) were used to synthesize SiBOC precursors. First, the appropriate mass of ethanol (4.0g) was added into MTES (50mmol), and then boric acid with a certain proportion corresponding to different B/Si (i.e., 0.4, 0.5, 0.6 and 0.7) was added into MTES, which was stirred evenly. Under magnetic stirring, an appropriate amount of hydrochloric acid catalyst was dropped until boric acid was completely dissolved to form a homogeneous solution. The SiBOC precursor sol was reacted at 40oC for 24h, and then at 50oC for 24h. The obtained SiBOC precursor xerogel was dried at 80oC for 12h. Finally, SiBOC xerogels were pyrolyzed in an argon-protected tubular furnace, so that SiBOC ceramics were pre-pared by controlling the pyrolysis temperature and the pyrolysis time under no pressure conditions.
Characterization and oxidation test
Fourier Transform Infrared Spectroscopy (FTIR) of EQUINOX-55 (produced by Brooke spectrometers, Germany) was used to qualitatively analyze the composition and bonding of precursor. The phase composition of the high-temperature pyrolyzed SiBOC ceramics was measured by the D/Max-2200 X-ray diffractometer (produced by Nippon Neoku Co. Ltd., Japan) at a scan speed of 5°/ min in the 2-theta range of 10-90°. To observe the uniformity, micro surface, and fracture morphology of SiBOC ceramics, and to study the oxidation resistance of SiBOC ceramics, Scanning Electron Microscopy (SEM) was performed on FEI Sirion200 (produced by Philip, Netherlands). Thermogravimetry differential thermal analysis (TG-DSC, Netzsch star 449c, Germany) was used to analyze the mass change and heat absorption, and release of the precursor. The test was carried out in an air atmosphere, and the temperature range was Rt- 1400oC. According to the DSC curve, it can be analyzed whether some components in the SiBOC ceramics are oxidized at high temperatures.
Results and Discussion
Synthesis mechanism of SiBOC polymer precursors
The infrared spectra of different B/Si in the process of synthesized SiBOC ceramic precursors are shown in Figure 1. The absorption bonds of pure MTES are mainly in the range of 1000-1200 cm-1, which is related to the Si-O-Si bond [16]. At the same time, the presence of the absorption peak at 890cm-1, related to Si-O-B= borosiloxane bridges [17] has been observed for all samples.
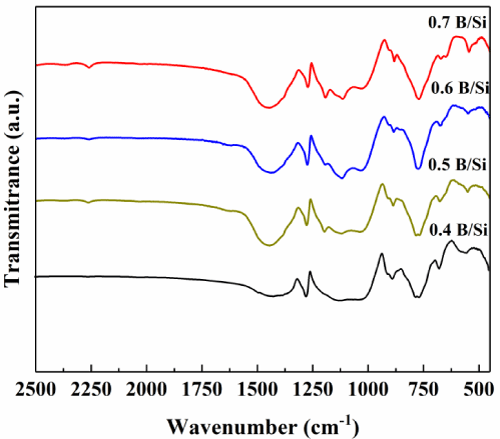
Figure 1: FT-IR spectra of SiBOC precursor gel with different B/Si.
It can also be seen from the Figure 1 that the infrared spectra of several different B/Si are similar except that the intensity of B-O absorption peaks are different, which comes from different addition of boric acid. Two Si-O-B vibration absorption peaks can be seen between 887cm-1 and 1024cm-1, and 1363cm-1 represents B-O bond. The formation of Si-O-B bond indicates that boric acid reacts with MTES, and B element successfully enters Si-O framework with the form of Si-O-B bond in the molecular chain of MTES [18-21]. A large number of B-OH groups are transformed into Si-O-B bonds, and then Si-O-B bonds are formed. The introduction of B element into the SiOC precursor can improve the ceramic yield of the SiBOC ceramic precursor and the high temperature performance of the ceramic materials.
In the mixed solution, B-OH of boric acid reacts with Si-OEt of MTES to form Si-OH and Si-O-B, as shown in formula (1) (2). When the catalyst HCl is added to the reaction, the dissolution rate of boric acid is accelerated by adjusting pH, so the reaction is obviously accelerated. The formed Si-OH will continue to react with B-OH of boric acid to form Si-O-B and water, as shown in formula (3). Moreover, the excess Si-OH will react to form Si-O-Si and water, as shown in formula (4). The unreacted Si-OEt reacts with water to remove Et-OH and continue to generate Si-OH, as shown in formula (5). The formation of Et-OH will further promote the dissolution of boric acid, and the excess Si-OEt and Si-OH will continue to undergo double decomposition reaction to form Si-O-Si and Et-OH, as shown in formula (6).
Effects on microstructure of SiBOC ceramics
It can be seen from Figure 2 that the XRD patterns of B/Si=0.4, B/ Si=0.5 and B/Si=0.6 are amorphous, and only the amorphous steamed bread peak of B-Si polymer network structure with 20=22° as the center is found, indicating that the SiBOC ceramics are amorphous.
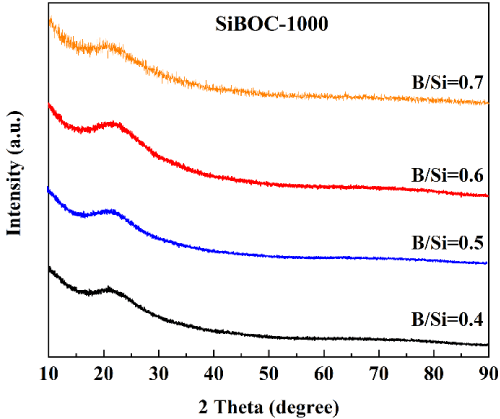
Figure 2: XRD patterns of SiBOC ceramics with different B/Si at 10000C.
At the same pyrolysis temperature of 1000oC and pyrolysis time of 1h, the SiBOC ceramics with B/Si of 0.4, 0.5, 0.6 and 0.7 were scanned respectively. The scanning electron microscope is shown in Figure 3. From Figure 3, we can find that the micro morphology of the products obtained by pyrolysis at different B/Si is different, showing different morphology. The results show that the surface of B/Si=0.4 SiBOC ceramics is smooth with good density. With the increase of boric acid content, the amount of white inorganic matter on the surface increases. The surface of the other three molar ratios is covered with many granular protrusions and folds, which may be that boric acid does not react and completely accumulates on the surface. At the same time, it also shows that the yield of SiBOC ceramics with B/Si=0.4 is higher and the density is better.
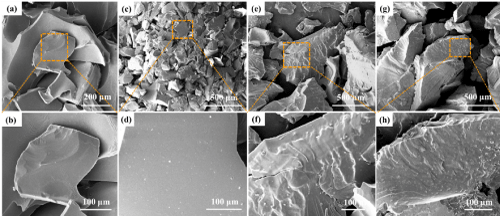
Figure 3: SEM micrographs of SiBOC ceramics with different B/Si: (a, b) 0.4, (c, d) 0.5, (e, f) 0.6, (g, h) 0.7.
Figure 4 shows the SEM diagram of B/Si=0.4 precursor of SiBOC ceramics pyrolysis for 1h at 1000oC, 1200oC, and 1400oC. With the increase of pyrolysis temperature, the surface of the precursor of SiBOC ceramics has different pore sizes, which is about 10-20 μm. The reason for the pore is that the gas and water evaporation will be produced with the increase of pyrolysis temperature, and the poles will be formed on the surface of the original SiBOC ceramic precursor. It can be found that the surface of the ceramics is flat by SEM when the pyrolysis temperature is 1000oC. Cracks and white bumps appeared on the surface at 1200oC. The surface of the SiBOC ceramics has different sizes of pores and pores at 1400oC, and the apparent density of SiBOC ceramics is poor at this temperature.

Figure 4: SEM micrographs of SiBOC ceramics with B/Si=0.4 at different pyrolysis temperatures for 1h: (a) 10000C, (b) 12000C, (c) 14000C.
Figure 5 shows SEM of B/Si=0.4 precursor of SiBOC ceramics pyrolysis for 1h, 2h, and 3h at 1000oC. It can be seen clearly from Figure 5 that with the increase of pyrolysis time, the amount of white folds is increasing. The surface of the sample in Figure 5a is flat without cracks and pores, the sample in Figure 5b has just begun to fold, and the overall performance is good, a large number of folds appear on the surface of SiBOC ceramics obtained from Figure 5c, so the longer the pyrolysis time, the higher the inorganic transition degree of ceramic precursor.

Figure 5: SEM micrographs of different pyrolysis time of SiBOC ceramic precursor with B/Si=0.40 at 10000C: (a) 1h, (b) 2h, (c) 3h.
Effects on the yield of SiBOC ceramics
Table 1 takes the experimental data of pyrolysis temperature of 1000oC, pyrolysis time of 1h, B/Si of 0.4, 0.5, 0.6, and 0.7 as an example to analyze the influence of different B/Si on ceramic yield. According to the results, the yield of SiBOC ceramics with B/Si=0.4 is the highest when the pyrolysis temperature and pyrolysis time are the same, that is, the ratio makes the cross-linking effect of B element in MTES the best.
Simple
Mass before pyrolysis (g)
Mass after pyrolysis (g)
Ceramic Yield (%)
B/Si 0.4
1.454
1.24
85.3
B/Si 0.5
1.536
1.161
75.6
B/Si 0.6
1.4
0.983
70.2
B/Si 0.7
1.5
0.918
61.2
Table 1: Variation trend of ceramic yield caused by different B/Si.
The TG-DSC curves of SiBOC ceramics pyrolysis at 1000°C for 1h with different molar ratios of B/Si are shown in Figure 6. The weight loss of B/Si=0.6 is more obvious than that of B/Si=0.4 at 1000oC. The high-temperature stability of B/Si=0.4 is very good, and the weight loss is very small with the residual amount of the final sample is as high as 85.3%.
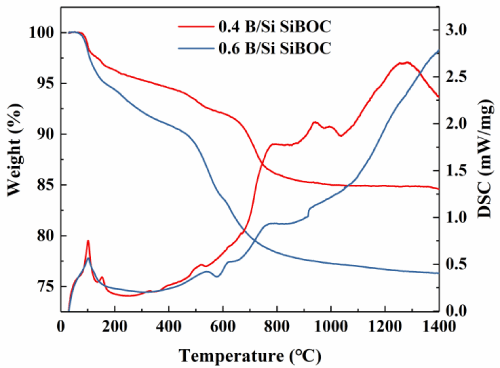
Figure 6: TG-DSC curves of SiBOC ceramics prepared by different B/Si.
There are two obvious weight loss exothermic stages in the two curves. The first temperature range of weight loss is 100-150oC, which is mainly the oxidative decomposition of small molecules such as methyl and the volatilization of ethanol and water, the second temperature range of weight loss is 500-700oC, which is mainly the oxidative decomposition of residual ethoxy. When the temperature reaches 700oC, there is no obvious weight loss, and the process of inorganic is completed. In the second decomposition stage, the weight loss is as high as 52.5%. After the introduction of the Boron element, the decomposition temperature is increased by about 200oC, and the residual mass is improved. The results show that the introduction of B-O with higher bond energy is beneficial to enhance the thermal stability of SiBOC ceramics, and the formed Si-O-B network structure encapsulates small molecules such as methyl to prevent their oxidative decomposition and improve their oxidation resistance.
Effects on oxidation resistance of SiBOC ceramics
The SiBOC ceramic precursors with the B/Si of 0.4 were put into an alumina crucible with graphite paper at 1000oC, 1200oC, and 1400oC respectively for 1h. The samples were oxidized in a tubular furnace with the temperature rising from room temperature to 1400oC for 10min. The surface morphology and internal structure of the oxidized samples were observed by SEM to analyze the effect of pyrolysis temperature on oxidation resistance.
Figure 7a shows that there are no holes on the surface of SiBOC ceramics after oxidation after pyrolysis at 1000oC. As shown in Figure 7b, the SiBOC ceramic surface is very dense. Figure 7c shows that the surface of SiBOC ceramics with pyrolysis temperature of 1200oC begins to appear holes after oxidation. Under the lens of 100μm, the holes are mainly attached to the surface with small sizes. As shown in Figure 7e, large numbers of pores appear after oxidation of SiBOC ceramics with pyrolysis temperature of 1400oC, and some even form channels inside the ceramics. When the pyrolysis temperature is 1400oC, the carbonaceous material in the ceramic matrix reacts with oxygen to produce gas and water, and the water continuously evaporates to form channels, resulting in poor physical properties.
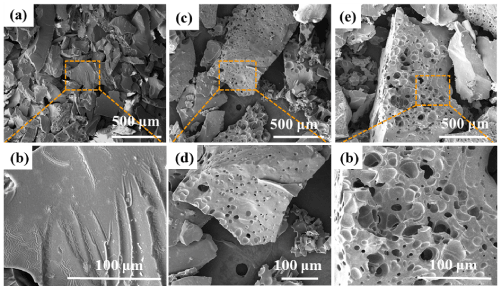
Figure 7: SEM of oxidized SiBOC ceramics at different pyrolysis temperatures: (a, b) 10000C, (c, d) 12000C, (e, f) 14000C.
The samples of SiBOC ceramic precursor with a B/Si of 0.4 with pyrolysis time of 1h, 2h, 3h, and pyrolysis temperature of 1000oC were put in alumina crucible with graphite paper. In a tube furnace with the temperature rising from room temperature to 1400oC, the samples were oxidized in the air for 10 minutes. The surface morphology and internal structure of the samples were observed by SEM after oxidation to analyze the effect of pyrolysis temperature on oxidation resistance. According to Figure 8, with the extension of pyrolysis time, the oxidized sample has surface pores and internal pores. In Figure 8a, the surface of SiBOC ceramics with pyrolysis time of 1h remains flat after oxidation, but there are white particles attached to the ceramic surface, which may be that boric acid does not react completely and accumulates on the ceramic surface. In Figure 8b, holes and surface depressions begin to appear on the surface and inside of SiBOC ceramics with a pyrolysis time of 2h after oxidation. In Figure 8c, after oxidation, a large number of holes and pores appear on the surface and inside of SiBOC ceramics with a pyrolysis time of 3h, and some even form channels inside the ceramics. This is because when the pyrolysis time is 3h, the carbonaceous material in the ceramic matrix reacts with oxygen, generating gas and water, and water continuously evaporates to form channels, resulting in poor physical properties.
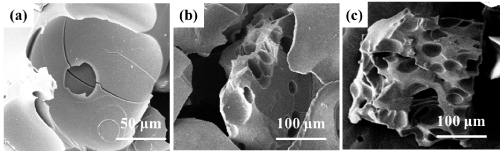
Figure 8: SEM of SiBOC ceramics after oxidation at different pyrolysis time: (a) 1h, (b) 2h, (c) 3h.
From the analysis results above, the surface and internal structure of the oxidized samples are relatively good when the B/Si ratio is 0.4, the pyrolysis temperature is 1000oC, the pyrolysis time is 1h, and the oxidation is carried out in the air in a tube furnace at 1400oC for 10min. Therefore, the samples with this parameter were analyzed by TG-DSC, and the results are shown in Figure 9. The weight change is less than 1%, and the curve is fluctuating, which indicates that the weight of SiBOC ceramics under this parameter is in a dynamic balance. There are obvious endothermic and exothermic peaks in the DSC curve, which indicates that the sample not only absorbs oxygen for a redox reaction, but also releases small molecule gases and water, such as CO and CO2, so its weight is in dynamic equilibrium.
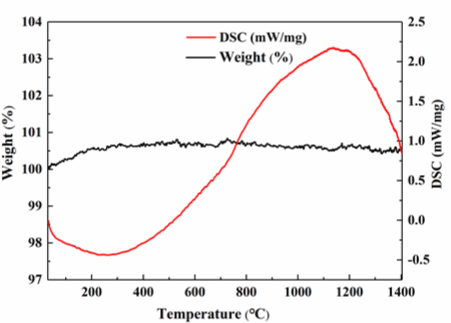
Figure 9: TG-DSC curves of the oxidation experiment of SiBOC ceramics
with pyrolysis temperature of 10000C, pyrolysis time of 1h and B/Si=0.4.
Conclusion
In this study, a novel SiBOC multicomponent macromolecule is successfully fabricated as a pre-cursor which is prepared via a solgel route for polymer-derived ceramic. The alcoholysis reaction of MTES and ethanol under the action of hydrochloric acid catalyst was carried out. It was concluded that the highest ceramic yield was 85.3% when the B/Si was 0.4. Si-O-B bond appears in the infrared spectrum of SiBOC ceramic precursor, which indicates that B is connected to the Si-O frame-work. According to the XRD pattern of SiBOC ceramics, the B-Si polymer network structure is an amorphous steamed bread peak. SEM micrographs show that with the increase of boric acid content, there are many granular protrusions and folds on the surface. In addition, with the increase of pyrolysis temperature and the extension of pyrolysis time, the surface defects and holes appear on the sur-face of SiBOC ceramics after oxidation. This is because the carbon-containing materials in the ceramic matrix react with oxygen to produce gas and water, and the water continuously evaporates to form a channel. When the B/Si is 0.4, the pyrolysis temperature is 1000oC and the pyrolysis time is 1h, the microstructure of SiBOC ceramics shows the least defects and the best oxidation resistance. The study in this paper is of great significance to the synthesis mechanism of SiBOC precursor, the preparation of high ceramic yield precursor ceramics, and the application of oxidation resistance of SiOC based precursor ceramics.
Acknowledgment
This work is supported by National Natural Science Foundation of China (No. 51772061) and Horizontal Subject of Yantai Vocational College (HX2020017).
References
- Li Y, Wu H, Liu X, Huang Z. Microstructures and Properties of Porous Liquid- Phase-Sintered SiC Ceramic by Hot Press Sintering. Mater. 2019; 12: 639.
- Stabler C, Reitz A, Stein P, Albert B, Riedel R, Ionescu E. Thermal Properties of SiOC Glasses and Glass Ceramics at Elevated Temperatures. Mater. 2018; 11: 279.
- Awin WE, Lale A, Kumar KCNH, Demirci BU, Bernard S, Kumar R. Novel Precursor-Derived Meso-/Macroporous TiO2/SiOC Nanocomposites with Highly Stable Anatase Nanophase Providing Visible Light Photocatalytic Activity and Superior Adsorption of Organic Dyes. Mater. 2018; 11: 362.
- An JD. Master’s thesis. Harbin Institute of Technology. 2016.
- Marchewka J, Jelen P, Rutkowska I, Bezkosty P, Sitarz M. Chemical Structure and Microstructure Characterization of Ladder-Like Silsesquioxanes Derived Porous Silicon Oxycarbide Materials. Mater. 2021; 14: 1340.
- Saha A, Raj R. Crystallization Maps for SiCO Amorphous Ceramics. J. Am. Ceram. Soc. 2007; 90: 578-583.
- Iwase Y, Fuchigami T, Horie Y, Daiko Y, Honda S, Iwamoto Y. Formation and Thermal Behaviors of Ternary Silicon Oxycarbides derived from Silsesquioxane Derivatives.Mater. 2019; 12: 1721.
- Eckel ZC, Zhou C, Martin JH, Jacobsen AJ, Carter WB, Schaedler TA. Additive manufacturing of polymer-derived ceramics. Sci. 2016; 351: 58-62.
- Marchewka J, Jelen P, Rutkowska I, Bezkosty P, Sitarz M. Chemical Structure and Microstructure Characterization of Ladder-Like Silsesquioxanes Derived Porous Silicon Oxycarbide Materials. Mater. 2021; 14: 1340.
- Cano-Crespo R, Rivero-Antúnez P, Gómez-García D, Moreno R, Domínguez- Rodríguez A. The Possible Detriment of Oxygen in Creep of Alumina and Zirconia Ceramic Composites Reinforced with Graphene. Mater. 2021; 14: 984.
- Lun H, Zeng Y, Xiong X, Zhao L, Li D, Ye Z, et al. The Effect of SiC Content on Microstructure and Microwave Heating Rate of h-BN/SiC Ceramics Fabricated by Spark Plasma Sintering. Mater. 2019; 12: 1909.
- Soraru GD, Babonneau F, Maurina S, Vicens J. Sol-gel synthesis of SiBOC glasses. J. Non-Cryst. Solids. 1998; 224: 173-183.
- Zhang X, Liu C, Hong C, Han J, Han W, Du S. Sol-gel-derived SiBOC ceramics with highly graphitized free carbon. Ceram. Int. 2015; 41: 15292- 15296.
- Schiavon MA, Gervais C, Babonneau F, Soraru GD. Crystallization Behavior of Novel Silicon Boron Oxycarbide Glasses. J. Am. Ceram. Soc. 2004; 87: 203-208.
- Schiavon MA, Armelin NA, Yoshida IVP. Novel Poly(borosiloxane) Precursors to Amorphous SiBCO Ceramics. Mater. Chem. Phys. 2008; 112: 1047-1054.
- Lyu Y, Tang H, Zhao GD. Effect of Hf and B incorporation on the SiOC precursor architecture and high-temperature oxidation behavior of SiHfBOC ceramics. J. Eur. Ceram. Soc. 2020; 40: 324-332.
- Alosime EM, Alsuhybani MS, Almeataq MS. The Oxidation Behavior of ZrB2- SiC Ceramic Composites Fabricated by Plasma Spray Process. Mater. 2021; 14: 392.
- Liu L, Ma Z, Yan Z, Zhu S, Gao L. The ZrO2 Formation in ZrB2/SiC Composite Irradiated by Laser. Mater. 2015; 8: 8745-8750.
- Lu C, Wang G, Wang K, Guo D, Bai M, Wang Y. Modified Porous SiO2- Supported Cu3(BTC)2 Membrane with High Performance of Gas Separation. Mater. 2018; 11: 1207.
- Sreejith KJ, Prabhakaran PV, Laly KP. Vinyl-functionalized Poly(borosiloxane) as Precursor for SiC/SiBOC Nanocomposite. Ceram. Int. 2016; 41: 15285- 15293.
- Wu ML, Ren CZ, Xu HZ, Zhou CL. Fabrication of a bionic microstructure on a C/SiC brake lining surface: Positive applications of surface defects for surface wetting control. Appl Surf Sci. 2018; 440: 669-679.