
Research Article
Ann Materials Sci Eng. 2021; 5(2): 1044.
Nano-Sized Double Perovskite Oxide as Bifunctional Oxygen Electrocatalysts
Li T¹, Sun H²* and Shi Q³*
¹Institute of Automotive and Traffic Engineering, Yancheng Polytechnic College, Yancheng, China
²Department of Materials Science and Engineering, Korea Advanced Institute of Science and Technology (KAIST), Daejeon, Republic of Korea
³School of Material Science and Engineering, Yancheng Institute of Technology, Yancheng, China
*Corresponding author: Hainan Sun, Department of Materials Science and Engineering, Korea Advanced Institute of Science and Technology (KAIST), Daejeon, 34141, Republic of Korea
Qingle Shi, School of Material Science and Engineering, Yancheng Institute of Technology, Yancheng 224051, Jiangsu, P.R. China
Received: September 28, 2021; Accepted: October 26, 2021; Published: November 02, 2021
Abstract
Double perovskite oxide La2Co0.5Fe0.5MnO6-d is synthesized by the moltensalt- assisted method with Nano-sized particles (≈55 nm). The as-obtained La2Co0.5Fe0.5MnO6-d exhibits obviously enhanced electro catalytic activities towards oxygen evolution reaction and oxygen reduction reaction compared to the counterpart La2CoMnO6-d. This work provides a simple method to synthesize Nano-sized double perovskite oxide as superior electro catalysts for energy conversion.
Keywords: Nano-sized; Molten-salt-assisted method; Double perovskite; OER and ORR
Introduction
The Oxygen Evolution Reaction (OER) and Oxygen Reduction Reaction (ORR) are two important reactions involved in the applications of rechargeable metal-air batteries and fuel cells [1,2]. Many efforts have been devoted to developing cost-effective and highperformance electro catalysts for OER and ORR as an alternative to the benchmark noble metal catalysts [3-8]. Among them, perovskite oxide, especially double perovskite oxide, has been extensively studied due to their earth-abundant nature and intrinsically high catalytic activity [9-11]. The flexible compositional structure of perovskite oxide makes its wide potential to be tuned as superior catalysts [12- 15].
Besides, Nano-sized perovskite oxide catalysts can expose abundant active sites, which can make full use of the advantage of high intrinsic activity perovskite oxide [16-20]. Compared with the conventional solid-state reaction, the molten-salt-assisted method is an effective method to synthesize controllable small powder, which is ascribed to the salt melt shows a high ion-diffusion rate and strong dissolving capability [21,22].
In this work, we synthesize Nano-sized double perovskite oxides La2Co0.5Fe0.5MnO6-d by the molten-salt-assisted method with Nanosized particles (≈55 nm). The as-obtained La2Co0.5Fe0.5MnO6-d exhibits obviously enhanced electro catalytic activity for oxygen evolution reaction and oxygen reduction reaction in alkaline solution compared with the counterpart La2CoMnO6-d. The improved electro catalytic activities are contributed from the enhanced surface active sites, modulated electronic structure, and fast charge transfer resistance.
Experimental Section
Materials synthesis
La2CoMnO6-d and La2Co0.5Fe0.5MnO6-d were synthesized by the molten-salt method. La(NO3)3•6H2O, Co(NO3)2•6H2O(Fe(NO3)3•9H2O), NaNO3, and KNO3 mixed in a molar ratio of 2 : 1 : 1 : 60 : 60 were used to prepare the precursor. The precursor was then placed in a covered nickel crucible and heated to 700 oC for 5 h. After cooling down to room temperature naturally, the products were repeatedly washed with distilled water. Finally, the remaining precipitate was dried overnight.
Characterizations
The crystalline structure of the as-obtained powders was analyzed with Cu Ka radiation (λ = 1.54 Å) powder X-Ray Diffraction (XRD). To investigate the morphology of the particles, Scanning Electron Microscopy (SEM) was carried out. XPS measurement was conducted to analyze surface species and chemical states of the samples, using a Thermo ESCALAB 250 with Al Ka (hλ = 1486.69 eV) X-ray source. The Brunauer-Emmett-Teller specific surface areas and corresponding Barrett-Joyner-Halenda (BJH) pore size distribution of the powders was studied by nitrogen adsorption-desorption measurements.
Electrochemical measurement
10 mg of the as-synthesized powder, 10 mg carbon black, and 100 μL of 5 wt% Nafion solution were mixed with 1000 μL of ethanol under continuous ultra-sonication. Then, 10 μL of the prepared ink was transferred onto a polished glassy carbon electrode (0.196 cm2) and dried naturally. The electrochemical tests were conducted by a CHI760 electrochemical station. A carbon rod and Ag/AgCl were used as counter and reference electrodes, respectively. The obtained potentials were calibrated to the reversible Hydrogen Electrode (RHE) by the following equation: ERHE = EAg/AgCl + 0.197 V + 0.0591 pH. OER and ORR were conducted in O2-saturated 1.0 M KOH and 0.1 M KOH solution at 1600 rpm at a sweep rate of 5 mV s-1, respectively. The Electrochemical Impedance Spectra (EIS) were collected at 0.7 V vs. Ag/AgCl with frequencies ranging from 100 kHz to 0.1 Hz. Cyclic Voltammograms (CV) were conducted to calculate the Cdl. The voltage in a non-faradic current region ranging from 0.2 to 0.3 V vs. Ag/AgCl with different scan rates (20, 40, 60, 80, 100 and 120 mV s-1) was selected to conduct.
Result and Discussion
The molten-salt-assisted method was used to synthesis the Nanosized perovskite LCM and LCFM, which were firstly characterized by XRD and SEM measurements. The crystal structure was confirmed by the XRD technique. The diffraction peaks of LCF and LCFM can be well indexed to the pure perovskite structure without observed impurities phase appeared. Notably, with the incorporation of Fe ions, the diffraction peaks shift to a low angle direction, indicating the lattice expands (Figure 1a). The SEM image of LCFM is shown in (Figure 1b) to detect the morphological property, in which the LCFM shows nanoparticle morphology with a uniform size distribution. Based on the careful observation and statistics, the size distribution pattern was plotted, and the average size of LCFM is approximately 55 nm (Figure 1c). The specific surface area of LCFM calculated from the Brunauer-Emmett-Teller method was measured to be 67 m² g-1 (Figure 1d). Notably, perovskite oxides synthesized by conventional methods, such as the sol-gel method and solid-state reaction method, show low specific surface area (typically low than 10 m² g-1). The specific surface area of LCFM reported in this work even exhibits clear advantages over powders prepared by electrospinning and template-assisted method, which ensures a large amount of active species exposed (Figure 1e).
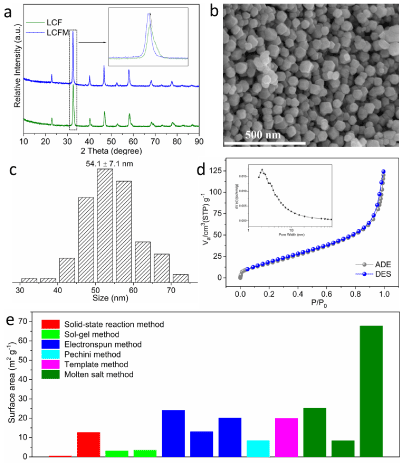
Figure 1: (a) XRD patterns of LCF and LCFM. (b) SEM image of LCFM. (c) Size distribution of LCFM. (d) N2 adsorption-desorption isotherm and pore size
distribution (inset) patterns of LCFM. (e) Comparison of specific area of various materials synthesized by different kinds of methods.
We first evaluated the OER activities of LCM and LCFM. As shown in (Figure 2a), the polarization curves show that the LCFM catalyst exhibits enhanced OER activity with a low over potential of 400 mV at 10 mA cm-2, significantly better than the LCM catalyst of 490 mV. Besides, LCFM delivers current densities of 3.5 and 27.1 mA cm-2 at potentials of 1.6 and 1.7 V, which are 7.1 and 4.0 times larger than that of LCM catalyst, highlighting the significantly improved OER activities (Figure 2b). Similar to OER activities, ORR activities of LCFM also show much improvement compared with that of LCM. Specifically, the limited current density for LCFM reaches 6 mA cm- 2, which is comparable to that of benchmark material Pt/C, making LCFM a high active material for ORR. The enhanced ORR activities can be also reflected by the low over potential. Besides, LCFM delivers current densities of -1.4 and -5.87 mA cm-2 at potentials of 0.4 and 0.7 V, which are 1.3 and 1.2 times larger than that of LCM catalyst, highlighting the significantly improved ORR activities (Figure 2d).
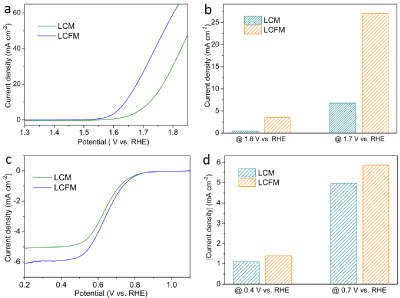
Figure 2: (a) LSV curves of LCM and LCFM for OER. (b) Comparisons of the OER current densities at various potentials. (c) LSV curves of LCM and LCFM for
ORR. (d) Comparisons of the ORR current densities at various potentials.
In order to obtain more insights into the nature of LCFM towards alkaline OER and ORR, the electronic structures of these two materials are studied. As can be seen from the X-Ray Photoelectron Spectroscopy (XPS) in (Figure 3a), compared to LCM, an additional Fe peak is found in the survey spectra of LCFM, suggesting the successful introduction of Fe into LCM (Figure 3b). The low intensity of Fe peak is attributed to the low content of Fe in the LCFM (Figure 3c). Shows the Co 2p XPS spectra of LCM and LCFM. It was found that two pronounced peaks at binding energies around 795.0 eV (Co 2p1/2) and 780.0 eV (Co 2p3/2) and two satellite peaks (sat) at 789.5 and 804.2 eV. The XPS spectrum of LCFM exhibits higher binding energy values of the two main peaks and lower intensities of the satellites, suggesting that the electronic states of surface Co ions are modulated by the doping effects. More specifically, the valence state of surface Co ions is partially oxidized to a higher valence state. The improved activity of LCFM is ascribed to a favorable electronic structure of Co ions.18 as can be seen from O 1s XPS spectra (Figure 3d), the peaks around 531.5 and 529.4 eV correspond to OH- groups and lattice oxygen, respectively. Notably, LCFM has more lattice oxygen on its surface than that of LCM, which was previously confirmed as active sites for the electrochemical process. Additionally, the active oxygen species are closely related to the surface oxygen vacancies. The enhanced concentration of lattice oxygen can be also verified by its slightly decreased valence state of Mn for LCFM compared with that of LCM (Figure 4). The electrochemical impedance spectroscopy of LCM and LCFM were measured to evaluate the kinetics property. The charge transfer resistance, revealing by the semicircles in (Figure 3e), of LCFM is much smaller than that of LCM. In addition, the electrochemical Double-Layer Capacitance (Cdl) can reflect the electrochemically effective surface area, which can be found in (Figure 3f). A series of linear fitting was obtained based on the cycle voltammetry curves (Figure 5). LCFM possessed enhanced ECSA compared with that of LCM, indicating that the incorporation of Fe cations to LCM could create more exposed surface active sites, which improves the overall catalytic activity.
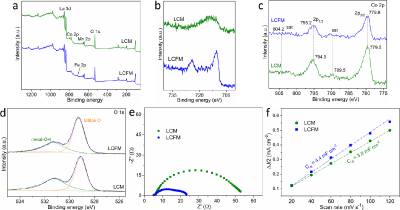
Figure 3: XPS spectra of LCM and LCFM. (a) Survey, (b) Fe 2p, (c) Co 2p, and (d) O 1s. (e) EIS curves of LCM and LCFM. (f) The electrochemical double-layer
capacitance of LCM and LCFM.
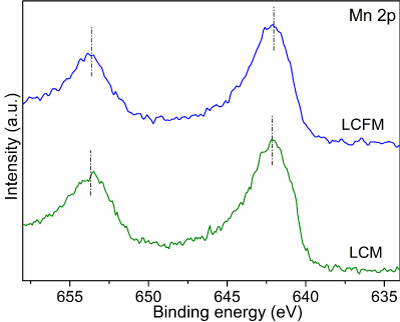
Figure 4: Mn 2p XPS spectra of LCM and LCFM.
Figure 5: CVs at the potential range from 0.2 to 0.3 V vs. Ag/AgCl with scan rate of 20, 40, 60, 80, 100 and 120 mV s-1 of various catalysts.
Conclusions
A simple molten-salt method was used to design Nano-sized double perovskite oxides. The as-synthesized La2Co0.5Fe0.5MnO6-d shows the high specific surface area and uniform morphology with nanoparticle size. The improved OER and ORR electro catalytic activities are contributed from the enhanced surface active sites, modulated electronic structure, and fast charge transfer resistance.
Acknowledgements
The authors are grateful for the financial support of the project funded by Yancheng Polytechnic College school-level scientific research (ygy2004). Jiangsu Province Educational Science “Thirteenth Five-Year Plan” 2020 Project (D/2020/03/37).
References
- Seh ZW, Kibsgaard J, Dickens CF, Chorkendorff I, Norskov JK, Jaramillo TF. Combining theory and experiment in electrocatalysis: Insights into materials design. Science. 2017; 355: 4998.
- Li M, Bi X, Wang R, Li Y, Jiang G, Li L, et al. Relating Catalysis between Fuel Cell and Metal-Air Batteries. Matter. 2020; 2: 32-49.
- Wang H, Zhou M, Choudhury P, Luo H. Perovskite oxides as bifunctional oxygen electrocatalysts for oxygen evolution/reduction reactions-A mini review. Appl. Mater. Today. 2019; 16: 56-71.
- Liu X, Gong M, Deng S, Zhao T, Shen T, Zhang J. et al. Transforming Damage into Benefit: Corrosion Engineering Enabled Electrocatalysts for Water Splitting. Adv. Funct. Mater. 2021; 31: 2009032.
- Zhao J, Zhang JJ, Li ZY, Bu XH. Recent Progress on NiFe-Based Electrocatalysts for the Oxygen Evolution Reaction. Small 2020; 16: 2003916.
- Chen RR, Chen G, Ren X, Ge J, Ong SJH, Xi S, et al. SmCo5 with a reconstructed oxyhydroxide surface for spin selective water oxidation under elevated temperature. Angew. Chem., Int. Ed. 2021.
- Chen G, Hu Z, Zhu Y, Gu B, Zhong Y, Lin HJ, et al. A Universal Strategy to Design Superior Water-Splitting Electrocatalysts Based on Fast In Situ Reconstruction of Amorphous Nanofilm Precursors. Adv. Mater. 2018; 30: 1804333.
- Zeng K, Zheng X, Li C, Yan J, Tian JH, Jin C, et al. Recent Advances in Non- Noble Bifunctional Oxygen Electrocatalysts toward Large-Scale Production. Adv. Funct. Mater. 2020; 30: 2000503.
- Sun H, Dai J, Zhou W, Shao Z. Emerging Strategies for Developing High- Performance Perovskite-Based Materials for Electrochemical Water Splitting. Energy Fuels. 2020; 34: 10547-10567.
- Xu X, Zhong Y, Shao Z. Double Perovskites in Catalysis, Electrocatalysis, and Photo (electro) catalysis. Trend. Chem. 2019; 1: 410-424.
- Yin WJ, Weng B, Ge J, Sun Q, Li Z, Yan Y. Oxide perovskites, double perovskites and derivatives for electrocatalysis, photocatalysis, and photovoltaics. Energy Environ. Sci. 2019; 12: 442-462.
- Sun C, Alonso JA, Bian J. Recent Advances in Perovskite-Type Oxides for Energy Conversion and Storage Applications. Adv. Energy Mater. 2020; 11: 2000459.
- Sun H, Xu X, Hu Z, Tjeng LH, Zhao J, Zhang Q, et al. boosting the oxygen evolution reaction activity of a perovskite through introducing multi-element synergy and building an ordered structure. J. Mater. Chem. A 2019; 7: 9924- 9932.
- Sun H, Song S, Xu X, Dai J, Yu J, Zhou W, et al. Recent Progress on Structurally Ordered Materials for Electrocatalysis. Adv. Energy Mater. 2021; 11: 2101937.
- Song HJ, Yoon H, Ju B, Kim DW. Highly Efficient Perovskite-Based Electrocatalysts for Water Oxidation in Acidic Environments: A Mini Review. Adv. Energy Mater. 2020; 11: 2002428.
- Chen H, Liang X, Liu Y, Ai X, Asefa T, Zou X. Active Site Engineering in Porous Electrocatalysts. Adv. Mater. 2020; 32: 2002435.
- Li Y, Sun Y, Qin Y, Zhang W, Wang L, Luo M, et al. Recent Advances on Water-Splitting Electrocatalysis Mediated by Noble-Metal-Based Nanostructured Materials. Adv. Energy Mater. 2020; 10: 1903120.
- Xu X, Wang W, Zhou W, Shao Z. Recent Advances in Novel Nano structuring Methods of Perovskite Electro catalysts for Energy-Related Applications. Small Methods 2018; 2: 1800071.
- Meng Z, Xu J, Yu P, Hu X, Wu Y, Zhang Q, et al. Double perovskite La2CoMnO6 hollow spheres prepared by template impregnation for highperformance super capacitors. Chem. Eng. J. 2020; 400: 125966.
- Shi J, Gan H, Wang C, Shen Q. Fe-doping effect on magnetic properties of La2CoMnO6 ceramics prepared by Plasma Activated Sintering. J. Eur. Ceram. Soc. 2021; 41: 6516-6522.
- Song S, Zhou J, Su X, Wang Y, Li J, Zhang L, et al. Operando X-ray spectroscopic tracking of self-reconstruction for anchored nanoparticles as high-performance electro catalysts towards oxygen evolution. Energy Environ. Sci. 2018; 11: 2945-2953.
- Sun H, Xu X, Song Y, Zhou W, Shao Z. Designing High-Valence Metal Sites for Electrochemical Water Splitting. Adv. Funct. Mater. 2021; 31: 2009779.