
Research Article
Austin J Med Oncol. 2024; 11(1): 1079.
The Efficacy of Hepatic Transarterial Chemoembolization Combined with Microwave Ablation in the Treatment of Early- and Intermediate- Stage Hepatocellular Carcinoma
Jingyu Qian1#; Zhongqiang Qin1#; Yuyao Ge2#; Longfei Fan1; Guangdong Zhang3*; Ziyi Zhu3*
¹Department of Interventional Surgery, The First Affiliated Hospital of Bengbu Medical University, Bengbu, China
²Department of Plastic Surgery, The First Affiliated Hospital of Bengbu Medical University, Bengbu, China
³Department of Intervention, Pain and Vascular Surgery, Hefei Hospital Affiliated to Anhui Medical University, Hefei, China
*Corresponding author: Guangdong Zhang, Ziyi Zhu Department of Intervention, Pain and Vascular Surgery, Hefei Hospital Affiliated to Anhui Medical University, Hefei, China. Email: 1072339821@qq.com; 21541546@qq.com
#These authors have contributed equally to this article. The Efficacy of Hepatic Transarterial Chemoembolization Combined with Microwave Ablation in the Treatment of Early- and Intermediate- Stage Hepatocellular Carcinoma.
Received: July 17, 2024 Accepted: August 13, 2024 Published: August 20, 2024
Abstract
Objective: To evaluate the efficacy of the sequential treatment of hepatic Transarterial Chemoembolization (TACE) in combination with dermal Microwave Ablation (MWA) for patients with early- and intermediate-stage Hepatocellular Carcinoma (HCC).
Methods: We retrospectively extracted data of 108 patients with HCC who received TACE alone or TACE combined with MWA (TACE-MMW) from January 2017 to December 2018, and evaluated the short-term and long-term clinical outcomes. Subgroup analysis was also conducted based on tumor size and lesions. Propensity Score Matching (PSM) was used to reduce bias from concomitant confounding variables. The comparison of clinical efficacy between the TACE (n=26) and TACE-MMW (n=26) groups was conducted after PSM.
Results: The median survival for TACE (n=26) and TACE-MWA(n=26) group were 13 and 28 months, respectively. The survival rates of 1-, 2- and 3- years were 84.6%, 59.0%, 35.2% in TACE-MWA group and 57.7%, 30.3%, and 19.5% in TACE group. The short-term efficacy of the TACE-MWA is higher (61.5%) than the TACE group (30.8%) (P=0.026). Besides, the Disease Control Rate (DCR) in TACE-MWA and TACE group was 80.8% and 46.2%, respectively (P=0.01). Subgroup analysis suggested that TACE-MWA treatment is superior to TACE treatment alone in patients with tumor size larger than 5cm (P=0.038) and 2-4 metastatic lesions (P=0.034).
Conclusions: TACE sequential MWA treatment can effectively improve the survival rate of early- and intermediate- liver cancer and prolong the survival time.
Keywords: Hepatocellular carcinoma; Transarterial chemoembolization; Combined treatment; Survival; Efficacy
Introduction
Hepatocellular Carcinoma (HCC) is still one of the malignant tumors leading to high morbidity and mortality [1]. Several minimally-invasive transarterial and thermal ablative procedures have emerged as safe and alternative therapies for the treatment of HCC, which have been recommended by several guidelines for the clinical practice. The clinical outcomes of Microwave Ablation (MWA) are approximate to surgery and liver transplantation in HCC treatment [2-4]. Moreover, the MWA is more economical and reduced treatment times, is emerging the preferred treatment modality. TACE selectively delivering chemotherapeutic drugs into tumor-feeding arteries, leading to ischemic necrosis of the target tumor via cytotoxic and ischemiceffects. TACE significantly prolong the patient’s survival and is the current standard of therapy for HCC patients. Despite previous studies have verified TACE as an effective treatment for HCC, the clinical efficacy of TACE in combination with MWA are still less studied. This study included patients with BCLC Stage A and B using the PSM method, and then compared the clinical efficacy of TACE and TACE-MWA in HCC treatment.
Materials and Methods
Clinical Information
This retrospective study included 108 patients with stage A and B HCC who admitted to our hospital from January 2017 to December 2018. The follow-up was finished in May 2020. The following inclusion criteria: (1) Diagnosed as HCC according to the hepatocellular carcinoma diagnosis and treatment guidelines; (2) The patients refuse surgical treatment or are not suitable for surgical treatment; (3) Child-Pugh grade A or B; (4) Patients receiving TACE or TACE sequential MWA treatment; (5) The number of tumor lesions =4. Exclusion criteria included: (1) Performance Status (PS) score> 2 points; (2) vascular invasion; (3) extrahepatic metastasis; (4) Child-Pugh classification C grade. To minimize the potential bias, we performed a PSM design with 1:1 matching. A total of 52 patients containing TACE group (n=26) and TACE+MWA group (n=26) were included for analysis after PSM (Figure 1). The biodemographic data of the two groups of patients are listed in Table 1. This prospective study was approved by the ethics committee of the First Affiliated Hospital of Bengbu Medical College. Written informed consent were signed for all participants.
Variables
TACE+MWA
(n=26)TACE
(n=82)P-Value
TACE after PSM
(n=26)P-Value
Gender/n
1.000
1.000
Male
22
69
22
Female
4
13
4
Age/years
56.5±10.0
60.5±10.0
0.080
58.9±10.0
0.385
Tumor size/n
0.002
0.165
<5cm
16
23
11
=5cm
10
59
15
Lesions/n
0.625
1.000
Single
16
46
16
Multiple
10
36
10
AFP/n
0.448
0.158
<400/ng/ml
18
50
13
=400/ng/ml
8
32
13
Child-Pugh/n
0.739
A
22
72
21
1.000
B
4
10
5
Albumin /g/L
38.6±4.6
38.8±5.0
0.861
38.2±5.2
0.789
PT/s
13.6±4.6
13.0±1.6
0.527
13.0±1.3
0.528
ALT/U/L
41.7±33.6
49.9±41.1
0.357
34.5±20.0
0.352
AST/U/L
45.2±25.1
63.6±50.2
0.015
49.3±32.9
0.617
TBIL/umol/L
CRE/umol/L
INR
MELD-Na17.6±9.3
62.7±2.8
1.1±0.1
7.3±1.217.8±11.1
64.5±3.4
1.1±0.1
7.4±1.40.941
0.008
0.900
0.66219.5±11.7
0.521
AFP: Alpha-Fetoprotein; PT: Prothrombin Time; ALT: Alanine Aminotransferase; AST: Aspartate Aminotransferase; TBIL: Total Bilirubin; TACE: Transarterial Chemoembolization; MWA: Microwave Ablation; PSM: Propensity Score Matching; CRE: Creatinine; INR: Prothrombin Time-International Normalized Ratio.
Table 1: Basic information of patients after PSM matching.
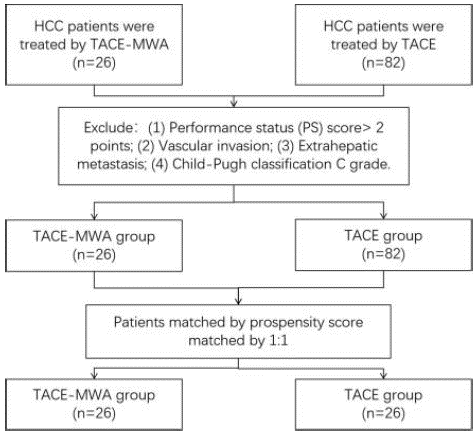
Figure 1: Flowchart of patient selection.
Preparation
Patients underwent routine abdomen CT/MRI scan. The size and number of tumor lesions were measured before assessing the presence of vascular invasion as well as extrahepatic metastasis. Besides, blood biochemical, coagulation, Alpha-Fetoprotein (AFP), liver function, and immunological indexes were examined. Chest radiographs and electrocardiograms were performed to assess the patient’s cardiopulmonary function. Head MRI or bone ECT were checked to exclude extrahepatic metastasis depending on the patient’s symptoms.
TACE
The Seldinger puncture was used to introduce a 5F catheter sheath at the strongest pulsation point in the right femoral artery area. Tumor blood supply was assessed under hepatic angiography using microcatheter before injecting 4 mg Raltitrexed. Liquefied iodized oil combined with 30 mg Epirubicin emulsion was injected to embolize tumor blood vessels. To strengthen the embolization, gelatin sponge particles were added. Those patients who suffered from poor blood supply or iodized oil deposition were embolized supplemented with a small number of polyvinyl alcohol particles.
MWA
The 32-row spiral CT (Toshiba, Japan) and MRI (Achieva, Philips Healthcare, Netherlands) examination of the upper abdomen was conducted at two weeks after TACE. The puncture path, length, and number of microwave ablation needle (Kangyou KY-2450B-1, Nanjing, China) were determined according to the CT image combined with the MRI/CT data. During NWA treatment, microwave ablation instrument with microwave frequency 2450MHz (Kangyou KY2000, Nanjing, China) was used. After disinfecting, we gradually adjusted the direction and depth of the ablation needle under the guidance of CT. After the ablation needle injection, we turned on the water circulation and set the treatment power, time, and point according to tumor size. Ablation range was evaluated through CT examination. In general, the ablation range is 1cm larger than the tumor lesion. After the ablation, the puncture tract was burned.
Patients Follow-up
The patient’s radiographic data and laboratory examinations were collected 1 month after surgery to assess tumor status and active tumor lesions. Patients with stable conditions were followed up each 1-2 months. Follow-up data including laboratory and imaging examinations were recorded to evaluate the short-term efficacy. The mean follow-up was 21 months (range 4-65month) in the TACE group compared with 29 months (range 9-46month) in the TACE-MWA group. No patients were lost follow-up.
Outcome
According to the modified solid tumor evaluation criteria (mRECIST), Complete Response (CR), Partial Response (PR), Stable Disease (SD), disease Progression (PD), Objective effective Rate (ORR) = CR + PR, Disease Control Rate (DCR) = CR + PR + SD were recorded. Survival time was calculated from the date of TACE surgery in the TACE group and MWA surgery in the TACE+MWA group until death or the end of follow-up in May 2020. Survival time was calculated from the date of TACE surgery in the TACE group and MWA surgery in the TACE+MWA group until death or the end of follow-up in May 2020.
Statistical Analysis
Continuous data were presented by mean ± Standard Deviation (SD) or rate. t-test or Chi-square test was used to compare the difference between the two groups. Survival was analyzed using the Kaplan-Meier method, and log-rank tests were used to compare survival distributions. PSM was conducted to adjust for baseline differences (e. g. age, sex, tumor size, number of lesions, albumin, and total bilirubin). Statistically significant was defined as P<0.05. SPSS software version 19.0 was used for statistical analysis.
Results
Short-Term Efficacy
Short-term clinical effect of TACE group after PSM at 1 month after procedure was with CR: 3.8% (1/26), PR: 26.9% (7/26), SD: 15.4% (4/26), ORR: 30.8% (8/26), DCR: 46.2% (12/26). For TACE+MWA group, the CR: 26.9% (7/26), PR: 34.6% (9/26), SD: 19.2% (5/26), ORR: 61.5% (16/26), and DCR: 80.8% (21/26). The differences of ORR (P=0.026) and DCR (P=0.010) between two groups were statistically significant.
Survival Analysis
The median survival of TACE and TACE+MWA group calculated by Kaplan-Meier method was 13 months and 28 months, respectively. The 1-, 2-, and 3-year survival rates were 57.7%, 30.3%, 19.5% for TACE group, and 84.6%, 59.0%, 35.2% for ACE+MWA group. The survival curves of the two groups are shown in Figure 2, and the difference between two groups is statistically significant (P=0.008).
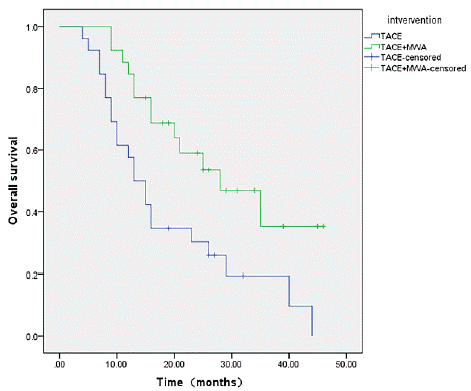
Figure 2: Cumulative Overall Survival (OS) rate curves for patients who underwent trans arterial chemoembolization (TACE) or TACE combined with microwave ablation (TACE-MWA) after propensity score matching (n=26 for each group).
To further study whether tumor size and number of lesions have effect on prognosis with different treatment methods, we performed subgroup analysis. The results are shown as follows: (1) The survival time of the TACE+MWA group was significantly higher than that of the TACE group when the tumor size< 5 cm (P=0.038). However, the survival time between the two groups was not statistically significant when the tumor size= 5 cm (Figure 3). (2) When the number of lesions is single, the survival time of the two groups of patients is not significantly different (P=0.114), while the survival time of the TACE+MWA group is significantly longer than that of TACE alone when multiple tumor lesions exist (P=0.034) (Figure 4).
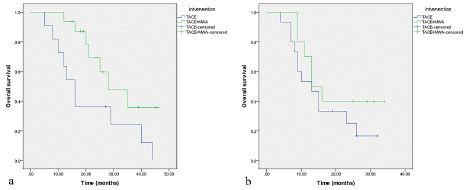
Figure 3: Survival curves for patients with different tumor sizes between TACE group (n=26) and TACE+MWA group after PSM (n=26). a) tumor size=5cm; b) tumor size<5cm.
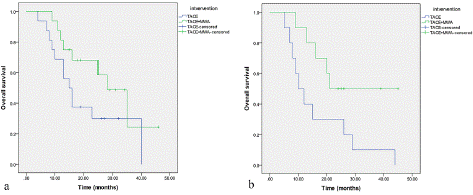
Figure 4: Survival curves of patients with different numbers of lesions between TACE group (n=26) and TACE+MWA group after PSM (n=26). a) single tumor. b) multiple tumors.
Complications
No deaths and serious complications (e. g. liver failure, abdominal bleeding, hepatic encephalopathy) were observed during follow-up. Patients in both groups had vomiting, pain, fever, and other complications that lasted for 2-3 days. After symptomatic treatment. complications were disappeared. However, there was a transient increase in transaminase and bilirubin at 1 week after surgery. No significant difference was observed between two groups for the transaminase and bilirubin at 1 month after surgery (Table 2).
Parameters
TACE (n=26)
TACE+MWA (n=26)
t
P
ALT (U/L)
41.1±44.3
44.7±44.0
-0.292
0.771
AST (U/L)
55.8±67.2
48.3±43.9
-0.592
0.554
TBI (umol/L)
18.4±11.6
20.9±17.5
0.472
0.639
ALT: Alanine Aminotransferase; AST: Aspartate Aminotransferase; TBIL: Total Bilirubin; TACE: Transarterial Chemoembolization; MWA: Microwave Ablation.
Table 2: Liver function at 1 month after procedurea.
Discussion
As a kind of malignant tumor with no obvious symptoms at early stage, most Hepatocellular carcinoma (HCC) patients have no chance of surgery once diagnosed. Although most patients were treated with TACE [5,6], the effect of TACE treatment has obvious limitations, such as complete necrosis rate is low [7]. The reasons may be addressed as following: (1) New blood vessels or collateral circulation are likely to form [8]; (2) Most portal vein lacks blood supply besides the hepatic artery. (3) Poor blood vessel is difficult to completely embolize. (4) Poor iodized oil deposits. MWA emits electromagnetic waves and forms a sustained high temperature within a short time, leading to killing tumor cells through coagulative necrosis. After ablation, it can effectively reduce the immunosuppressive effect [9]. MWA produces higher temperature, larger range, and shorter time than Radiofrequency Ablation (RFA).
This retrospective study contained a total of 108 HCC patients. To eliminate the baseline confounders such as tumor size, 56 patients were excluded through PRS analysis. The median survival time and 1-, 2-, and 3- survival rates of TACE+MWA group were significantly improved compared with TACE group. The median survival time of the TACE group and TACE+MWA group were 13 months and 28 months, respectively. The 1-, 2-, and 3-year survival rates were 57.7%, 30.3%, 19.5% in TACE group and 84.6%, 59.0%, 35.2% in TACE+MWA group, respectively. Our data showed that patients with combined treatment have a longer survival time but without serious complications and liver damage. Our study also demonstrated that TACE-MWA has a synergistic effect. TACE-MWA has some advantages including but not limited to: (1) TACE reduces tumor blood flow leading to the cooling of hepatic artery blood flow during ablation process [10]. (2) TACE marks the location of the tumor, especially in the subfocals that are difficult to find where the iodized oil deposits. (3) After the TACE treatment, the ischemic water, inflammatory, and iodized oil enhance the temperature inside the tumor when they encounter electromagnetic waves [11]. (4) MWA can be used as a supplementary treatment in patients with distorted blood vessels or difficult to select anatomical locations. (5) The thermal damage of MWA leads to the sensitivity of tumor cells to chemotherapy drugs [12]. (6) TACE-MWA treatment can reduce the frequency of TACE treatment helping to reduce the side effects of repeated TACE treatment.
Previous studies have revealed that [13-15], the prognosis of hepatocellular carcinoma is related to the size and tumor lesions. Subgroup analysis based on the size of the tumors showed that patients with tumor size < 5 cm benefited from the combined treatment group (P<0.05). The median survival time was 28 months and 16 months, respectively. Leung [16] et al. has reported that the initial tumor size in TACE-MWA treatment is a major risk factor for tumor prognosis. Chen et al. [17] analyzed 244 patients with hepatocellular carcinoma whose tumor size was <5 cm. Consistent with our study, the complete ablation rate in the TACE-MWA treatment group reached 92.1% but only 46.3% in the TACE group. In addition, a subgroup analysis was also conducted based on the number of tumor lesions. The result indicated that survival time between the two groups has a significant difference when the number of tumor lesions was 2-4 (P<0.05). The survival time of the combined treatment group was longer, and the median of the TACE-NWA group was 21 months compared with 10 months in the TACE group. Yamakado et al. [18] reported that the prognosis of simple TACE is greatly affected by the number of lesions. Zheng et al. [19] analyzed 276 patients with liver cancer and their subgroup results also showed that 3 or fewer tumor lesions benefited significantly from combined treatment. This study did not include hepatocellular carcinoma with tumor lesions >4. Subgroup analysis showed that patients with single tumor lesions showed no difference between two groups (P=0.114), but the median survival time of the combined treatment group was longer than that of TACE treatment alone. However, no statistically significant difference was probably due to the small number of cases. According to our experience, hypovascular tumors are more likely to benefit from TACE-MWA therapy particularly tumors located in safe locations, such as lesions away from the intestine, gallbladder, and blood vessels. In addition, for patients with BCLC stage A and stage B HCC receiving liver transplantation, TACE-MWA treatment can significantly prolong Progression-Free Survival (PFS), and prolonged PFS increases the probability of patients receiving liver transplantation
Moreover, significant differences in the short-term efficacy between two groups of patients was observed. The ORR of the TACE + MWA group and the TACE group were 61.5% and 30.8%, and the DCR was 80.8% and 46.2%, respectively. The combined treatment group was superior to the TACE group. The difference between transaminase and total bilirubin at one month after procedure between two groups was not significant indicating that the combined treatment would not lead to more liver complications. However, this study has some limitations. First, this study has a small number of single-center cases with shorted follow-up time; Second, tumor blood supply was not calculated, which probably affected our conclusions; Third, the long-term efficacy was evaluated by survival analysis, while the recurrence of the tumor still needs to be further observed.
Conclusions
This comparative study suggests that TACE-MWA is significantly better than TACE treatment in early and midterm HCC. The short-term effect is significantly improved and effectively prolongs the survival. Especially, when the tumor diameter is less than 5 cm or has multiple lesions, the benefit is greater. In conclusion, TACE-MWA combination therapy is safe and does not aggravate the damage to liver function compared with TACE treatment.
Author Statements
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author Contributions
JQ, ZQ, and YG contributed equally to the work. GZ and ZZ contributed to the conception and design. JQ, ZQ, YG, and LF are responsible for the provision of the study materials and data collection. JQ and ZQ contributed to the data analysis and interpretation. JQ and YG contributed to the manuscript writing. All authors read and approved the final manuscript.
Funding
This work was supported by the Natural Science Research Project of Anhui Educational Committee (KJ2021A0804) and General Project of Anhui Provincial Education and Teaching Research (2021jysm0956).
Acknowledgments
Thanks for the cooperation of all the patients.
References
- Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012; 379: 1245-1255.
- Itoh S, Ikeda Y, Kawanaka H,Okuyama T, Kawasaki K, Eguchi D, et al. Efficacy of surgical microwave therapy in patients with unresectable hepatocellular carcinoma. Ann Surg Oncol. 2011; 18: 3650-3656.
- Liang P, Yu J, Yu XL, Wang XH, Wei Q, Yu SY, et al. Percutaneous cooled-tip microwave ablation under ultrasound guidance for primary liver cancer: a multicentre analysis of 1363 treatment-naive lesions in 1007 patients in China. Gut. 2012; 61: 1100-1101.
- Wang Z, Liu M, Zhang DZ, Wu SS, Hong ZX, He GB, et al. Microwave ablation versus laparoscopic resection as first-line therapy for solitary 3-5-cm HCC. Hepatology. 2022; 76: 66-77.
- Bruix J, Sherman M. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011; 53: 1020-1022.
- Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016; 10: 1-98.
- Liapi E, Geschwind JF. Chemoembolization for primary and metastatic liver cancer. Cancer J. 2010; 16: 156-162.
- Liu YS, Ou MC, Tsai YS, Lin XZ, Wang CK, Tsai HM. Transarterial chemoembolization using gelatin sponges or microspheres plus lipiodol-doxorubicin versus doxorubicin-loaded beads for the treatment of hepatocellular carcinoma. Korean J Radiol. 2015; 16: 125-132.
- den Brok MH, Sutmuller RP, Nierkens S, Bennink EJ, Frielink C, Toonen LW, et al. Efficient loading of dendritic cells following cryo and radiofrequency ablation in combination with immune modulation induces anti-tumour immunity. Br J Cancer. 2006; 95: 896-905.
- Stewart CL, Warner S, Ito K, Raoof M, Wu GX, Kessler J, et al. Combined hepatic arterial embolization and hepatic ablation for unresectable colorectal metastases to the liver. Am Surg. 2012; 78: 1243-1248.
- Ni JY, Kong J, Sun HL, Chen YT, Luo JH, Wang WD, et al. Prognostic Factors for Survival After Transarterial Chemoembolization Combined with Sorafenib in the Treatment of BCLC Stage B and C Hepatocellular Carcinomas. Acad Radiol. 2018; 25: 423-429.
- Goldberg SN, Saldinger PF, Gazelle GS, Huertas JC, Stuart KE, Jacobs T, et al. Percutaneous tumor ablation: increased necrosis with combined radio-frequency ablation and intratumoral doxorubicin injection in a rat breast tumor model. Radiology. 2001; 220: 420-427.
- Salvalaggio PR, Felga GE, Alves JA, Meirelles RF Jr, Almeida MD, de Rezende MB. Response to transarterial chemoembolization in candidates with hepatocellular carcinoma within Milan criteria does not predict post-transplant disease-free survival. Transplant Proc. 2014; 46: 1799-1802.
- Sun AX, Cheng ZL, Wu PP, Sheng YH, Qu XJ, Lu W, et al. Clinical outcome of medium-sized hepatocellular carcinoma treated with microwave ablation. World J Gastroenterol. 2015; 21: 2997-3004.
- Jin YJ, Lee JW. Therapeutic priorities for solitary large hepatocellular carcinoma in a hepatitis B virus endemic area; an analysis of a nationwide cancer registry database. J Surg Oncol. 2017; 115: 407-416.
- Leung U, Kuk D, D’Angelica MI, Kingham TP, Allen PJ, DeMatteo RP, et al. Long-term outcomes following microwave ablation for liver malignancies. Br J Surg. 2015; 102: 85-91.
- Chen QF, Jia ZY, Yang ZQ, Fan WL, Shi HB. Transarterial Chemoembolization Monotherapy Versus Combined Transarterial Chemoembolization-Microwave Ablation Therapy for Hepatocellular Carcinoma Tumors =5 cm: A Propensity Analysis at a Single Center. Cardiovasc Intervent Radiol. 2017; 40: 1748-1755.
- Yamakado K, Miyayama S, Hirota S, Mizunuma K, Nakamura K, Inaba Y, et al. Subgrouping of intermediate-stage (BCLC stage B) hepatocellular carcinoma based on tumor number and size and Child-Pugh grade correlated with prognosis after transarterial chemoembolization. Jpn J Radiol. 2014; 32: 260-265.
- Zheng L, Li HL, Guo CY, Luo SX. Comparison of the Efficacy and Prognostic Factors of Transarterial Chemoembolization Plus Microwave Ablation versus Transarterial Chemoembolization Alone in Patients with a Large Solitary or Multinodular Hepatocellular Carcinomas. Korean J Radiol. 2018; 19: 237-246.