
Case Report
Austin J Med Oncol. 2015; 2(3): 1021.
A Prospective Phase III Open Label Study Comparing Low Dose Paclitaxel Versus Cisplatin as Weekly Regimen with Concurrent Radiation in Locally Advanced Head and Neck Cancer
Saugat S¹, Narayan S¹*, Singhal M¹, Kapoor A¹, Kumar N¹, Harsh K¹, Sharma N¹, Bothra R², Beniwal S² and Sharma A¹
¹Department of Radiation Oncology, S.P. Medical College and Associated Group of Hospital, India
²Department of Medical Oncology, S.P. Medical College and Associated Group of Hospital, India
*Corresponding author: Satya Narayan, Department of Radiation Oncology, S.P. Medical College, Room no 26, PBM Hospital Campus, Bikaner, Rajasthan, India
Received: February 02, 2015; Accepted: September 08, 2015; Published: September 15, 2015
Abstract
A prospective randomized study was done to determine the comparative effectiveness and safety of low dose weekly paclitaxel versus weekly cisplatin with concurrent radiation in the treatment of locally advanced head and neck cancer.
Material and Method: The prospective single centered study was conducted on 122 patients in the department of radiation oncology in a tertiary care hospital from October 2010 to November 2011. All patients were histopathologically proved squamous cell carcinoma. Previously untreated one hundred eight randomly selected and patients were divided into two groups by computer generated programme. The study group received injection Paclitaxel 20 mg/m² while control group received injection Cisplatin 30 mg/m². All patients had received 66-70 Gy concurrent radiation at the rate of 2 Gy/day, 5 #/week, in 6-7 weeks by cobalt-60 Theratron -780 E/780C Teletherapy units.
Result: Out of 122, 112 patients were men and 10 were women with a median age of 51 years range (30 - 65). 63 versus 59 patients were distributed on the basis of computer generated programme in Cisplatin and Paclitaxsel arm. There was no statistically significant difference in DFS in CDDP arm and Paclitaxel arm (Χ²= 0.072; p value 0.789) at the 24 months. There was no statistically significant difference in OS in CDDP arm and Paclitaxel arm (Χ²= 0.006; p value: 0.936) at the 24 months. The common adverse effect both in the CDDP arm and Paclitaxsel arm was oral mucosits. There was no significant difference in the incidence of mucositis and dermatitis between CDDP and Paclitaxsel arm.
Conclusion: Low dose Paclitaxel weekly schedule is comparable to Cisplatin in locally advanced head and neck squamous cell carcinoma both in form of survival and toxicity.
Keywords: Head and neck cancer; Low dose Paclitaxel; Cisplatin
Introduction
Head and neck squamous cell carcinoma is the most common cancer in India [1]. Locally advanced head and neck cancer is a great challenge for oncologists. In India 75% cases presents in advanced stage [2]. These cancers remain confined to the loco-regional site of origin in a significant proportion of patients and the most important cause of death is loco-regional recurrence [3]. Overall, 57.5% of global burden of head and neck cancer contributed by India only, for both sexes. In female accounted for 11-16% while in male accounted for 30% of all sites of cancers [3]. The maximum number of oral cancers among males was seen after the age of 55 years in India. Around 30% of all cancers in India are due to use of tobacco in the form of smoking and chewing. The most aggressive non-surgical treatment is the combination of chemotherapy and radiation [4]. However, advances in radiation therapy as well as combined modality treatment have made local control much more successful, even for advanced primary site disease. The treatment strategies are numerous for these patients, and advances in organ preservation with attention to quality of life remain the major focus of research investigations. Management of loco-regionally advanced head and neck squamous cell carcinoma (HNSCC) with curative intent has been the subject of intensive investigation during the last three decades and has evolved considerably over time with active research, volumes of literature, large randomized control trials and meta-analyses supporting evidence-based guidelines. Robust and mature data from various randomized studies and meta-analyses have shown the superiority of concurrent chemo-radiation schedules in loco-regional control (LRC) and overall survival [5].
Material and Method
Patients eligibility criteria includes age 18- 65 years, European Co-operative Oncology Group (ECOG) performance status =2, histologically proved advanced squamous cell carcinoma of the head and neck (Stage III /IV) and have not received prior malignancy oriented treatment (Table 1) [6]. The patients who gave informed patients consent and fully cooperative during study process were considered in study. The patients with distant metastases, concurrent malignancies, pregnant and lactating woman, associated severe co morbidities and history of previous treatment with any of the following modalities-surgery, radiotherapy, chemotherapy were excluded from study. One hundred eight randomly selected patients were divided into two groups study and control of patients each [7]. The criteria for putting a patient in group was done by computer generated programme and no particular patient is given a priority bias. The study group received injection Paclitaxel 20 mg/m² while control group received injection Cisplatin 30 mg/m². All patients were received 66-70 Gy concurrent radiation at the rate of 2 Gy/ day, 5 #/week, in 6-7 weeks by cobalt-60 Theratron -780 E/780C Teletherapy units.
Characteristic
CDDP (n=63)
No.of patients
%
Paclitaxsel (n=59)
No.of patients)
%
Male
47
74.6
49
83.0
Female
16
25.4
10
17.0
Age Mean
43.8
47.3
Age Range
21-65
30-65
ECOG
0-1
42
66.6
46
77.9
2
21
33.4
13
22.1
Site of lesion
Oral Cavity
27
42.8
23
38.9
Oro-Pharynx
26
41.2
28
47.4
Hypo-Pharynx
10
16.0
8
13.7
T stage
T1
-
-
-
-
T2
28
44.4
31
52.5
T3
20
31.7
19
32.2
T4
15
23.8
9
15.2
N stage
N0
9
14.2
5
8.5
N1
28
44.4
32
54.2
N2a
26
41.4
22
37.3
N3
-
-
-
-
TNM Stage
III
34
54.0
35
59.3
IVA
29
46.0
24
40.7
Table 1: Summary of the Patients characteristics.
Result
Out of 122, 112 patients were men and 10 were women with a median age of 51 years range (21 - 65). The distribution of the disease according to the primary site was on follows Oral cavity (40.98%), Oropharynx (44.26%), Hypopharynx (14.76%). The commonest histopathology was moderately differentiated squamous cell carcinoma. 63 versus 59 patients were distributed on the basis of computer generated programme in Cisplatin and Paclitaxsel arm. At 24 months, in CDDP arm disease free survival (DFS) was 39.69% while 37.29% in Paclitexsal arm. The median DFS was 16.80 [95% CI; 15.276-18.406] months for CDDP arm versus 16.66 months [95% CI; 15.186-18.441] for Paclitaxsel arm respectively, with a hazard ratio (HR) 1.018 [95% CI; 0.647-1.601]. There was no statistically significant difference in DFS in CDDP arm and Paclitaxel arm (Χ²= 0.072; p value 0.789) at the 24 months (Figure 1). At 24 months, in CDDP arm overall survival (OS) was 58.74% while 57.63% in Paclitexsal arm. The OS throughout study was 21.40 months [95% CI; 20.526-22.364] for CDDP and 20.50 months [95% CI; 19.277- 21.808] for Paclitaxsel arm, with a HR 1.076 [95% CI; 0.622-1.864]. There was no statistically significant difference in OS in CDDP arm and Paclitaxel arm (Χ²= 0.006; p value: 0.936) at the 24 months (Figure 2). The common adverse effect both in the CDDP arm and Paclitaxsel arm was oral mucosits. These was no significant difference in the incidence of mucositis between CDDP and Paclitaxsel arm [Χ²=0.72; p value = 0.39]. Most of patients developing grade II or III mucositis needed dietary modification in the form of liquid diet. Most of patients developing severe mucositis were managed with intravenous fluids, steroids and analgesics with Ryles tube insertion. There was no significant dermatitis between CDDP and Paclitaxsel arm [Χ²=0.60 p value = 0.43]. Bone marrow toxicity was rare, as only one patient in the control group developed grade II anemia for which blood transfusion was administered.
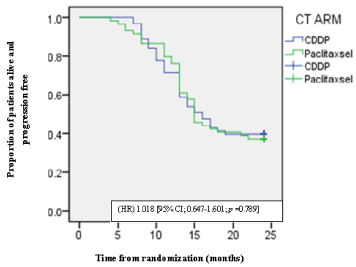
Figure 1: Kaplan meier plot of DFS.
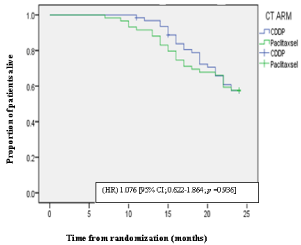
Figure 2: Kaplan meier plot of OS.
Discussion
Ideal management giving results in the shape of long disease free survival of cancer patients is the ultimate dream of an oncologist. Efforts are on to achieve as high as possible survival rates. It is realized that, there is no unique modality of treatment, which helps in achieving this objective. However multimodality treatment takes us a step of two closer to our target. This study intended to compare concomitant chemo- radiation using newer active agent Paclitaxel in low dose weekly schedule with Cisplatin added to conventional radiation in locally advanced head and neck cancer. Although some patients in the Paclitaxel arm sustained local toxicity, Mucositis with dysphagia, this was acceptable and comparable to the use of concurrent Cisplatin. No dose limiting systemic toxicity was encountered in this study. There was no statistically significant difference in DFS in CDDP arm and Paclitaxel arm (Χ²= 0.072; p value 0.789) and OS in CDDP arm and Paclitaxel arm (Χ²= 0.006; p value: 0.936) at the 24 months.
Comparing these results the Paclitaxel dose of 20mg/m²/wk was well tolerated with none of the patients requiring percutaneous gastrostomy. The mucosal toxicity observed during radiotherapy subsided with symptomatic treatment in the form of IV steroids and intravenous fluids. Looking at the results of these studies it can be concluded that the weekly administered of paclitaxel concurrent with radiation in carcinoma of head & neck is associated with an acceptable therapeutic profile and can be investigated further.
References
- Takiar R, Nadayil D, Nandakumar A. Projections of number of cancer cases in India (2010-2020) by cancer groups. Asian Pac J Cancer Prev. 2010; 11: 1045-49.
- https://rcfcare.org/admin/articles/doc/pg56to67.pdf
- Kulkarni MR. Head and neck cancer burden in india. Int J Head and Neck Surg. 2013; 4: 29-35.
- Adelstein DJ. Recent randomized trials of chemoradiation in the management of locally advanced head and neck cancer. Curr Opin Oncol. 1998; 10: 213-218.
- Pignon JP, le Maitre A, Maillard E, Bourhis J. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009; 92: 4-14.
- Joshi PS, Chaturvedi PS. Head and Neck Cancers in Developing Countries. Rambam Maimonides Med J Apr. 2014; 5: e0009.
- Haffty BG. Concurrent chemoradiation in the treatment of head and neck cancer. Hematol Oncol Clin North Am. 1999; 13: 719-742.
Citation: Saugat S, Narayan S, Singhal M, Kapoor A, Kumar N, Harsh K, et al. A Prospective Phase III Open Label Study Comparing Low Dose Paclitaxel Versus Cisplatin as Weekly Regimen with Concurrent Radiation in Locally Advanced Head and Neck Cancer. Austin J Med Oncol. 2015; 2(3): 1021. ISSN:2471-027X