
Case Report
Austin J Med Oncol. 2015; 2(3): 1022.
Complete Response with weekly Paclitaxel and Cetuximab in a Patient with Pulmonary Metastasis of Laryngeal Cancer
David Gutiérrez¹*, Blanca Ludeña² and Guillermo Plaza³
¹Department of Medical oncology, Fuenlabrada University Hospital, Spain
²Department of Radiotherapy, Fuenlabrada University Hospital, Spain
³Department of Otorhinolaryngology, Fuenlabrada University Hospital, Spain
*Corresponding author: Gutiérrez Abad David, 1Department of Medical oncology, Fuenlabrada University Hospital, Madrid, Spain
Received: June 25, 2015; Accepted: September 05, 2015; Published: September 07, 2015
Abstract
We present a case of a 71 years old man with a metastatic squamous cell laryngeal carcinoma after local complete response with chemoradiotherapy and 24 months of disease free survival. Metastatic disease was treated with a combination of chemotherapy paclitaxel weekly schedule and the anti epidermal grouth factor inhibitor monoclonal antibody cetuximab.A very early and maintained complete response was achieved. We will discuss about the necessity of predictive and good prognosis biomarkers on laringeal tumours which can explain long survivors.
Keywords: Squamous Cell Laryngeal Carcinoma; Metastatic Disease; Cetuximab; Complete Response
Abbreviations
DL: Direct Laryngoscopy; LVC: Left Vocal Cord; CT: Computed Tomography; PA: Pathologic Anathomy; CRT: Chemoradiotherapy; RT: Radiotherapy; IMRT: Intensity-Modulated Radiation Therapy; PET: Positron Emission Tomography; CR: Complete Response; EGFR: Epidermal Growth Factor Receptor; PS: Performance Status
Case Presentation
A 71 years old male with a clinical history of:
-former smoker, 1 and a half packs until 5 months before diagnosis
-former drinker of 3 beers per day until 2 years before diagnosis
-Unknown allergies
-Cataract Surgery
-Construction worker
At the beginning of April 2011, the patient was reviewed by head and neck cancer service due to a 4-month progression of dysphonia. No hemoptoic sputum, no respiratory difficulties, nor cetuximab + paclitaxel, Fibroscopy: at the level of band and base of epiglottis granular tissue, LVC middle tertiary leukoplakia, thickened Left Vocal Cord. Normal Right Vocal Cord, Vocal Cords free and moveable. Remainder without clinical findings. Neck: Palpation negative.
Direct Laryngoscopy (DL) with biopsies of left posterior band, left 1/3 middle band, 1/3 middle LVC, 1/3 middle left ventricle union, 1/3 posterior squamous cell carcinoma moderately differentiated.
CT of the neck with contrast 11/25/2011: Transglottic tumor of the larynx, with band thickening, partial obliteration of the adipose tissue of the paraglottic space and caudal extension to the left vocal cord. No evidence of pathological adenopathy involving the lateral cervical nodes. Stage cT3 N0 larynx.
Left laser cordectomy was done 12/19/2011 for left laryngeal transglottic carcinoma, in the left ventricle band and left VC, extending to the arytenoids, without reaching the anterior commissure, was conducted. Amplified laser cordectomy PA Left transglottic laryngeal carcinoma, Stage pT2N0.
A bilateral modified radical cervical dissection with excision of areas I, II, and III, was done, with identification and conservation of both spinal, internal jugular, and carotids, absence of cervical adenopathy suspected. PA: “Right modified radical dissection”: 18 lymphatic nodes negative for malignancy. “Left modified radical dissection”: 26 lymphatic nodes negative for malignancy.
On 3/21/2012 DL with extraction combined with laser CO2 of the posterior tertiary band and LVC was conducted. Friable mucosa with neoplastic aspect of the left posterior tertiary band that still continues to have lesions in the posterior tertiary left vocal cord.
Direct Laryngoscopy with laser CO2 extraction, in various fragments at the left band level and in one third of the left posterior vocal cord. PA: left tertiary posterior band. Invasive squamous cell carcinoma moderately differentiated.
Since April 2012, the patient continued with dysphonia and more coughing. No hemoptysis, without dyspnea at rest. No weight loss.
CT of larynx with contrast 06/01/2012: Tumor persistent. Left transglottic tumor with thickening of the middle tertiary and posterior left vocal cord band and obliteration of adipose tissue in left paraglottic. It has extended slightly to the arytenoepiglottic folds. The left arytenoid is sclerotic. Small cuff of left subglottic soft tissue. No significant andenopathy.
A new DL and Biopsies was completed in which ulcerated lesions were observed affecting the medial and anterior aspect of the arytenoids, 1/3 of the left posterior band, left ventricle, in addition to 1/3 posterior and medial LVC. Affecting inferiorly the subglottis at the left posterior level.
PA: left anterior aspect of the arytenoids squamous cell carcinoma moderately differentiated.
Left 1/3 posterior band LVC squamous cell carcinoma and middle 1/3 LVC moderate focal epithelial dysplasia.
Based on the extension of the lesion and the two previous laser results, a new CO2 laser is not indicated. A diagnosis of left transglottic squamous cell carcinoma of larynx after the ineffectiveness of the partial laser surgery is explained to the patient.
Direct Laryngoscopy - Posterior zone of the cord and left ventricle band with an overgrowth in comparison to the previous evaluation, paretic. CT scan of the neck, thorax, and abdomen is conducted and the final TNM stage was cT3N0M0.
The option of radical surgery via laryngectomy or chemoradiotherapy for preserving the organ is offered, and the patient elected conservative treatment with CRT.
Intensity-Modulated Radiation Therapy (IMRT) was planned using fractioned integrated boost. The treatment is administered in 35 sessions, in 5 fractions weekly. During each session a dosis of 1.66 Gy is given, until reaching 58.1 Gy, to the PTV that corresponds to the primary tumor located in the larynx and the bilateral cervical lymph node (II- IV bilateral and VI). Simultaneously, 2 Gy per session is administered until reaching a total of 70 Gy to the primary tumor including safety measures.
Radiotherapy treatment was initiated 07/02/2012 concomitant with cisplatin 100 mg/m2 every 21 days receiving 3 cycles every 21 days of cisplatin, and completing RT 08/20/2012 with good tolerance. After completing treatment patient with grade 1 dermatitis in the neck and grade 2 mucositis that produced clinical symptomology of odynophagia and dysphagia, although tolerating solid and bland foods.
Follow up with neck and thoracic CT was made. After 24 months CRT was finished, PET-CT verified recurrent lung metastasis.
CT OF THORAX WITH CONTRAST 05/14/2014: Diagnosis: Multiple lung metastasis (Figures 1,3 and 5).
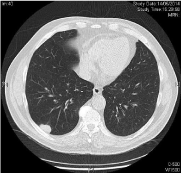
Figure 1:
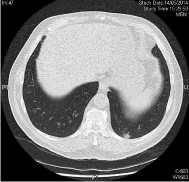
Figure 3:
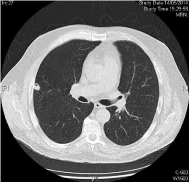
Figure 5:
A lung node Biopsy was conducted 06/13/2014: positive Cytology. Squamous Cell Carcinoma.
Chemotherapy was started 6/23/2014, taxol 80 mg/m2 days 1, 8, and 15 every 28 days + cetuximab 250 mg/m2 days 1,8,15, and 21 every 28 days (initial dosis of day 1 cetuximab 400 mg/m2) with very early grade 3 cutaneous rash with dosis reduction of cetuximab 20% and very early complete response (CR) after 3 cycles. After 6 cycles of cetuximab + paclitaxel, CR still present and the treatment was suspended. CR still ongoing for more than 8 months demonstrated in CT of thorax with contrast 04/17/2015: other clinical findings include complete tumoral response. No recurrences in neck (Figures 2,4, and 6).
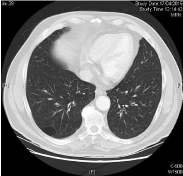
Figure 2:
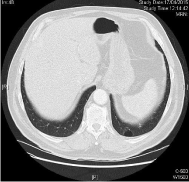
Figure 4:
Discussion
Squamous Cell Carcinoma of the larynx is the last stage of a carcinogenic process that includes multiple stages from normal histology, hyperplasia, various grades of dysplasia, carcinoma in situ, and finally infiltrative carcinoma. In this carcinogenic process genetic changes occur, loss of heterozygosity (LOH) of specific chromosomes (3p14, 9p21, 17p13, 8p, 11q, 13q, 14q, 6p, 4q27 and 10q23) and amplification, deletion, up-regulation or down-regulation of some oncogenes or suppressor genes which includes the EGFR, p53, Rb, p65, cyclooxygenase 2 (COX-2), p16, Cycline D1 and PTEN [1].
Carcinoma of the head and neck is the sixth most frequent cancer in the world and there has been a significant increase in the global incidence for 2 decades. The unimodal treatment with surgery or radiotherapy is generally recommended for 40% of patients with tumors in Stages I or II. In patients with tumors of the larynx, the combined treatment option of CRT with cisplatin 100 mg/m2 every 21 days in 3 cycles is a good option for larynx preservation. Nevertheless, there is a risk of local recurrences and/or regional and distant metastasis of 20-30% [2].
Combined treatment cetuximab and chemotherapy in recurrent/ metastatic head and neck cancer has been demonstrated effective with improvement in overall survival and progression free survival in various studies. The standard treatment in this patients is the combination of cisplatin, 5 FU ic, and cetuximab [3,4]. Weekly schedule paclitaxel and cetuximab has also demonstrated efficacy in this context with 22% (10/46 pacs) of complete response rates and acceptable toxicity [5,6]. Although, this treatment is not the standard in patients platinum-sensitive, there are studies with data suggesting that the most adequate treatment should be based on different patients and tumour criteria, Charlson Index, ECOG, PS, weight loss, primary tumor location, cellular differentiation [2].
Cetuximab is the first antiEGFR monoclonal antibody effective in head and neck cancer. Now, the genetic and molecular tumour profile should be included for better survival and response predictions as prognostic and predictive factors. These will help to select patients with better outcome and high probability of complete response rates and better survival with less toxicity and less costs [2,5].
References
- Haddad RI, Shin DM. Recent advances in head and neck cancer. N Engl J Med. 2008; 359: 1143–1154.
- Vermorken JB, Specenier P. Optimal treatment for recurrent/metastatic head and neck cancer. Annals of Oncology 2010; 21: vii252–261.
- Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, et al. Platinum-based chemotherapy plus cetuximab in head and neck cáncer. N Engl J Med. 2008; 359: 1116-27.
- Burtness B, Goldwasser MA, Flood W, Mattar B, Forastiere AA. Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: an Eastern Cooperative Oncology Group study. J Clin Oncol. 2005; 23: 8646-54.
- Péron J, Ceruse P, Lavergne E, Buiret G, Pham BN, Chabaud S, et al. Paclitaxel and cetuximab combination efficiency after the failure of a platinum-based chemotherapy in recurrent/metastatic head and neck squamous cell carcinoma. Anticancer Drugs. 2012; 23: 996-1001.
- Hitt R, Irigoyen A, Cortes-Funes H, Grau JJ, García-Sáenz JA, Cruz-Hernandez JJ, et al. Phase II study of the combination of cetuximab and weekly paclitaxel in the first-line treatment of patients with recurrent and/or metastatic squamous cell carcinoma of head and neck. Ann Oncol. 2012; 23: 1016-22.