
Mini Review
Austin J Med Oncol. 2016; 3(1): 1028.
Targeting ErbB-2/Her-2+ Breast Cancer with HSP90 Inhibitors
Saini N* and Yang X*
Department of Biological and Biomedical Sciences, North Carolina Central University, USA
*Corresponding author: Nipun Saini and Xiaohe Yang, Department of Biological and Biomedical Sciences, Julius L. Chambers Biomedical/Biotechnology Research Institute, North Carolina Central University, USA
Received: September 15, 2016; Accepted: October 13, 2016; Published: October 14, 2016
Abstract
ErbB-2/Her-2 positive breast cancer constitutes 20-30% of invasive breast cancer and is associated with poor prognosis and survival. The inability of anti- Her-2 drugs to improve the overall response rate and reoccurrence in Her-2 positive breast cancer patients presents a major challenge to clinical oncology. Heat shock protein 90 (HSP90) inhibitors have shown great potential in overcoming the side effects and resistance associated with current anti-Her- 2-therapies. In this review, we provide a brief overview of the therapies against Her-2 positive breast cancer and the various HSP90 inhibitors currently under investigation as a potential therapeutic for targeting Her-2-mediated breast cancer.
Keywords: Breast cancer; ErbB-2/Her-2 positive; Heat Shock Proteins (HSP); HSP90 inhibitors; Cancer therapy
Abbreviations
17-AAG: 17-Allylamino-17-Demethoxy-Geldanamycin; ATP: Adenosine Triphosphate; CBR: Clinical Benefit Rate; CR: Complete Response; CTD: C-Terminal Domain of HSP90 EGFR: Epidermal Growth Factor Receptors; ER: Estrogen Receptor; FDA: Food and Drug administration; Her-2+: Her-2 positive; HSF1: Heat Shock Factor 1; HSP: Heat Shock Proteins; HSP90: Heat Shock Protein 90; MAPK: Mitogen-Activated Protein Kinase; MBC: Metastatic Breast Cancer; MD: Middle Domain Of HSP90; Mtor: Mammalian Target Of Rapamycin; NCSLC: Non-Small Cell Lung Cancer; NG: Nano-Gel; NP: Nano-Particle; NTD: N-Terminal/Amino Terminal Domain of HSP90; ORR: Overall Response Rate; OS: Overall Survival; PFS: Progression Free Survival; PR: Partial Response; PI3K- Phosphatidylinositol-4, 5-Bisphosphate 3-Kinase; PK: Pharmacokinetic; PR: Progesterone Receptor; RR: Response Rate; RTK: Receptor Tyrosine Kinase; SCLC: Small Cell Lung Cancer; T-DM1: Trastuzumab Emtansine; TNBC: Triple Negative Breast Cancer; VEGFR: Vascular Endothelial Growth Factor Receptor
Introduction
ErbB-2/Her-2 is a member of the epidermal growth factor family, which also includes erbB-1/EGFR, erbB-3 and erbB-4. Tight regulation of these Receptor Tyrosine Kinases (RTKs) is critical for the growth and development of mammary tissues [1]. Dysregulation of Her-2 and its family members play a critical role in breast cancer development. Her-2 amplification and overexpression has been detected in approximately 20-30% of invasive breast cancer and is associated with higher tumor grade, metastasis, poor prognosis and resistance to chemotherapeutics [2]. Although the application of Her- 2-targeting agents has improved clinical outcomes for responsive patients, effective treatment of refractory Her-2-overexpressing breast cancers remains a significant challenge in clinical oncology.
Her-2 is an orphan receptor with no ligand. Its activation mainly depends on the dimerization with other erbB family receptors upon the binding of their cognate ligands. The dimerization triggers an array of intracellular downstream signaling pathways involved in cell proliferation, differentiation, survival, angiogenesis and invasion [3,4]. Major pathways downstream of Her-2 activation include the Phosphatidylinositol-4, 5-Bisphosphate 3-Kinase (PI3K), Mammalian Target of Rapamycin (mTOR), Mitogen-Activated Protein Kinase (MAPK), Src kinases and Signal Transducer and Activator of Transcription (STAT) transcription factors. Hyperactivation of these pathways promotes tumorigenesis by enhancing cell proliferation, differentiation, angiogenesis and other pro-cancer events, which provides a survival advantage to the Her-2-overexpressing tumor cells [1]. Importantly, Her-2 has been established as a therapeutic target that leads to the development of numerous agents for Her-2 positive (Her-2+) breast cancer treatment.
The purpose of this review is to provide a brief overview of different therapies targeting Her-2 positive breast cancer and to discuss the potential of currently investigated HSP90 inhibitors in Her-2+ breast cancer for utilization in other cancer models.
Current Her-2-targeting therapies
Multiple strategies have been exploited for the treatment of Her-2+ breast cancer by targeting and interfering with the functions and downstream signaling of Her-2 molecule [5]. Trastuzumab (Herceptin) is an FDA approved antibody-based drug that targets Her-2, resulting in inhibition of downstream signaling and cell cycle arrest [6]. Initial studies of trastuzumab as a monotherapy in metastatic breast cancer patients were not effective with only 10- 15% patients yielding Partial Response (PR) and a rare Complete Response (CR). Also, the development of cardiomyopathy in elderly patients after the treatment with trastuzumab emerged as a matter of concern [7]. However, the combination of trastuzumab with other chemotherapeutic agents such as vinorelbine, docetaxel, and gemcitabine, has shown an improved Response Rate (RR) and Overall Survival (OS) in Her2+ metastatic patients and is routinely being used as a treatment option [7]. Lapatinib, a small molecule dual inhibitor targeting the kinase domain of EGFR and Her-2, is approved for the treatment of metastatic breast cancer [8]. The toxicity profile of this molecule is limited to skin rash and diarrhea being the most frequent side effects [7,9]. The single agent activity of lapatinib is modest in patients with trastuzumab-resistant tumors, but the combination of lapatinib with capecitabine and taxanes has shown significant improvements in RR, Progression Free Survival (PFS) and OS as compared to the single drug therapies in Her-2+ patients [7,10,11]. Recently, pertuzumab, another novel antibody drug targeting Her- 2 to prevent its activation through interruption of Her-2/Her-3 dimer formation, has also been approved for the treatment of Her- 2+ metastatic breast cancer [12]. In combination with trastuzumab and docetaxel, pertuzumab has yielded further improved Overall Response Rate (ORR) and survival, yet tumor recurrence is still evident and needs further clinical investigation [13,14]. Moreover, an antibody conjugate of trastuzumab with a cytotoxic agent, emtansine (T-DM1), has shown targeted inhibition of tumor cells with improved therapeutic potential and minimal systemic toxicity in preclinical studies [15,16], even in Her-2+ metastatic breast cancer patients previously treated with other drugs [17,18]. Meanwhile, more Her- 2-targeting agents are being investigated for the treatment of Her-2- overexpressing breast cancer.
Currently employed therapies for Her-2+ breast cancer have partially improved the general outcomes of the disease by enhancing the ORR and PFS; nevertheless, resistance and relapse are still common. Resistance is associated with the activation of compensatory signaling pathways downstream of RTKs [7,19]. Mutations or alterations in the drug binding site or kinase activity site of the Her- 2 gene may also contribute to the resistance to anti-Her-2 therapy. Thus, in-depth understanding of Her-2 signaling and development of novel targeted therapies are necessary for further improvement of the overall survival in Her-2+ patients.
HSP90 and cancer
Heat shock proteins (HSPs) are a family of proteins that are expressed in response to various cellular stresses. These molecules allow organisms to thrive and adapt to diverse conditions including drugs and environmental stressors [20]. Heat shock protein 90 (HSP90), in particular, is essential for the correct folding, assembly and proteolytic turnover of various client proteins that are pivotal regulatory proteins involved in cell growth, differentiation and survival. HSP90 consists of four structural domains- an N-Terminal Domain (NTD), a charged linker that connects the NTD and Middle Domain (MD), a middle domain and a C-Terminal Domain (CTD) [21]. The NTD of HSP90 is the binding site for ATP and connects to the MD with a charged linker. This domain modulates ATPase activity of HSP90 by binding to gamma-phosphate of ATP and acts as recognition and binding site for co-chaperones and client proteins. Finally, the CTD of HSP90 helps in the homodimerization of HSP90 to obtain active conformation [21]. HSP90 consists of a wide array of client proteins that includes proteins such as Her-2, Epidermal Growth Factor Receptor (EGFR), c-Raf, Src, B-Raf, Akt and Met [22]. Importantly, HSP90 is over expressed in various solid tumors and malignancies. Cancer cells utilize the essential chaperone features of HSP90 for stabilizing mutated or dysregulated onco-proteins, which promote malignant transformation [23]. The overexpression of HSPs in cancers has been attributed to the constitutive activation of Heat Shock Factor 1 (HSF1), the central regulator of HSPs involved in their synthesis and transcription [23,24]. In non-cancerous cells, the overexpression of HSP90 negatively regulates HSF1 expression and restricts HSP90 upregulation. In contrast, the upregulation of HSF1 occurs concomitantly with the upregulation of HSP90 in cancer cells. This anomaly is suggested to be due to cancer-mediated alterations in the post-translational modifications in HSP90 and its signaling [25]. Thus, cancer cells become dependent on chaperones, such as HSP90, for their survival and expansion of the oncogenic proteome.
HSP90 and Her-2-associated cancer development
ErbB-2/Her-2 is the most sensitive client protein of HSP90 and was the first protein shown to be degraded by ansamycins, inhibitors of HSP90 [26]. More HSP90 client proteins that are associated with breast cancer biology include Vascular Endothelial Growth Factor Receptor (VEGFR), Estrogen Receptor (ER) and Progesterone Receptor (PR) [27]. The overexpression of HSP90 has been correlated with high Her-2 and ER levels, lymph node status, size of tumors and decreased survival in breast cancer [28,29]. HSP90-mediated deregulation of these critical factors may contribute to the poor prognosis breast cancer [28]. HSP90 in association with a cochaperone, Cdc37, is required for the stability and maturation of Her- 2. Studies have shown that the inactivation of HSP90 or degradation of the HSP90-Cdc37 complex by inhibitors leads to ubiquitindependent proteasomal degradation of Her-2 [26,30,31]. HSP90 is also known to regulate Her-2 activity by interacting with a specific loop in its kinase domain [30,32]. This interaction sequesters Her-2 homodimers and prevents Her-2 dimerization with other erbB family receptors, thus restricting its catalytic activity. When ligands bind to other erbB receptors, HSP90 is dissociated due to steric hindrance and heterodimerization activates Her-2-mediated signaling pathways, such as MAPK and PI3K pathways [33]. Another study has reported an upregulation of Src-dependent kinase activity in the presence of mutant Her-2 that was unable to bind HSP90, further indicating the role of HSP90 in the activation and signaling of Her-2 [34]. In addition, surface HSP90 has been shown to interact with the extracellular domain of Her-2, activating heregulin-mediated Her- 2 pathway, leading to cytoskeletal rearrangement and contributing to cancer cell motility and invasion [32,33]. Taken together, these reports indicate that the functional status of HSP90 is critical for tight regulation of Her-2 and its family members. Alterations of HSP90 would have profound impact on Her-2 activity and its oncogenic potential. Indeed, targeting HSP90 for the treatment of Her-2+ breast cancer is an attractive target for anti-cancer therapy.
Targeting HSP90 in Her-2 positive breast cancer
Various N-terminal domain-targeting HSP90 inhibitors are being investigated for their potential role in anti-cancer therapy. Geldanamycin, a benzoquinone ansamycin antibiotic, was one of the first generation HSP90 inhibitors that showed effective inhibition of Her-2+ breast cancer cells [30]. It acts by inhibiting the interaction between HSP90 and Her-2, and induction of CHIP-mediated Her-2 ubiquitinylation and proteasomal degradation [28,30,35]. Tanespimycin [17-allylamino-17-demethoxy-geldanamycin (17- AAG)], a geldanamycin derivative, has shown great potency against Her-2+ breast cancer by promoting Her-2 ubiquitinylation, lysosomal degradation and inhibition of cell growth by inducing cell cycle arrest and apoptosis in preclinical models [36-39]. It is also effective against the mutant form of Her-2 (p95-Her-2) which showed resistance to trastuzumab treatment [40]. In clinical studies, combination of 17- AAG plus trastuzumab exhibited 22% ORR and 59% clinical benefit rate in Her-2+ trastuzumab-refractory breast cancer patients [41]. Despite its success, the clinical use of this inhibitor is discontinued owing to its poor solubility and formulation issues [42]. Retaspimycin (IPI-504), a derivative of tanespimycin, is another effective HSP90 inhibitor. In preclinical studies on Her-2+ trastuzumab-resistant cell and xenograft models, IPI-504 significantly reduced the levels of Her- 2, Akt, MAPK and phosphorylated Akt and MAPK, thereby silencing the pathways partially responsible for trastuzumab resistance [43,44]. Modest clinical activity was displayed by retaspimycin plus trastuzumab treatment in phase II trials of Her-2+ metastatic breast cancer patients heavily pretreated with trastuzumab (ASCO Annual Meeting Abstract [45]). More investigations are needed to further validate the role of retaspimycin in Her-2+ breast cancer. Though the preclinical and clinical studies associated with geldanamycin and its derivatives were promising and validated the proof of concept for targeting HSP90 in Her-2+ breast cancer, these first generation inhibitors presented tremendous toxicities and formulation difficulties.
Second generation HSP90 inhibitors overcame the solubility and safety issues associated with geldanamycin and its derivatives and have shown more efficacy as an anti-tumor agent in solid tumors and malignancies. NVP-AUY922 is a resorcinol core HSP90 inhibitor developed by a structure optimization of a lead compound, CCT018159, which was identified during a high throughput screening for HSP90 inhibitors in yeast. In preclinical studies, NVP-AUY922 has demonstrated promising anti-tumor activity at low nano-molar ranges in various breast cancer cell lines and xenograft models. Its mechanism of inhibition involves the dissociation of HSP90-p23 complex (required for HSP90 activity and client protein stability) followed by proteasomal degradation of Her-2 protein and inhibition of cell growth by apoptosis [46]. A recently concluded clinical study of NVP-AUY922 plus trastuzumab in patients with Her-2+ metastatic breast cancer (who were previously treated with trastuzumab) established the tolerability and activity of NVP-AUY922 [47]. However, further validation and investigation is required in larger cohorts.
Ganetespib, or STA-9090, is a potent synthetic HSP90 inhibitor with a unique structure, increased potency and a favorable safety profile without hepatotoxicity and ocular toxicity [48]. In vitro studies involving ganetespib, alone or in combination with other drugs [49], reported improved efficacy and cytotoxicity in various hematological and solid tumors, including those harboring mutant kinases, such as B-Raf, EGFR and c-Kit [48]. In vivo, ganetespib has shown significant inhibition of tumor growth in xenograft models of EGFR-mutant and wild type Non-Small Cell Lung Cancer (NCSLC) [50], Small Cell Lung Cancer (SCLC) [49], Triple-Negative Breast Cancer (TNBC) [51,52] and Her-2+ breast cancer [53]. The anti-tumor effects of ganetespib are attributed to its ability to inhibit Akt, MAPK/Erk and mTOR signaling pathways and inhibit cell growth by inducing cell cycle arrest and apoptosis [53]. In a Phase II open label study of ganetespib in Metastatic Breast Cancer (MBC) patients, the ORR was not achieved, but clinical activity was evident in trastuzumabrefractory Her-2+ and TNBC patients [54]. The results of ongoing trial of ganetespib in combination with paclitaxel and trastuzumab in Her-2+ MBC patients were reported at the San Antonio Breast Cancer Symposium [55]. According to a Phase I trial, the combination of trastuzumab and paclitaxel with ganetespib was well-tolerated with grade 1/2 toxicities - diarrhea and fatigue. The ORR was 25% and the clinical benefit rate was 50% but PFS and Pharmacokinetic (PK) data was not presented. Other ongoing trials of ganetespib include in combinational treatment with capecitabine and radiation in rectal cancer and with paclitaxel in women with primary peritoneal cancer [56].
Another HSP90 inhibitor, FW-04-0806, has also shown antitumor effects in Her-2+ breast cancer models [57,58] by disrupting HSP90 and Cdc37 chaperone/co-chaperone interaction and degradation of HSP90 client proteins [57]. When used in combination with lapatinib, FW-04-0806 resulted in effective reduction of Her-2 expression and downstream signaling pathways PI3K/Akt and Ras/ MEK/Erk signaling pathways in cell and xenograft models of Her-2+ breast cancer [58].
The potential of HSP90 inhibitors in attenuating Her-2 activity and/or associated signaling pathways in preclinical and clinical studies has caught the attention of scientist and more such inhibitors are currently being tested for their activity. Table 1 summarizes various HSP90 inhibitors and their mechanism of action in targeting Her-2+ breast cancer and Table 2 summarizes the relevant clinical trials (completed and ongoing) for HSP90 inhibitors.
HSP90 Inhibitors
Class of inhibitor/Core structure
Molecular target/mechanism
References
17-AAG
First Generation/ansamycin based
Ubiquitinylation and lysosomal degradation of Her-2, cell cycle arrest and induction of apoptosis
IPI-504
First Generation/ansamycin based
Inhibition of Akt and MAPK pathways
NVP-AUY-922
Second generation/ resorcinol based
Dissociation of HSP90-p23 complex, proteasomal degradation of Her-2 and induction of apoptosis
[46]
STA-9090
Second generation/ resorcinol based
Inhibition of Akt, MAPK/Erk and mTOR pathways, cell cycle arrest and apoptosis
FW-04-0806
Second generation/ resorcinol based
Disruption of HSP90-Cdc37 complex, induction of apoptosis and proteasomal degradation of Her-2
Table 1: HSP90 inhibitors and their mechanism of action in targeting Her2+ breast cancer.
Drug Treatment Regime
Phase
Outcomes
References
Tanespimycin (17-AAG) +Trastuzumab
II
ORR: 22%
PFS: 6 months
OS: 17 months
[41]
Ganetespib (STA-9090)
II
CBR: 9%
PFS: 7 weeks
OS: 11.5 months
[54]
Ganetespib + Paclitaxel + Trastuzumab
I
RP2D determined
[55]
Ganetespib (ENCHANT-1 trial)
II
Ongoing
Ganetespib, Paclitaxel, Trastuzumab and Pertuzumab
I
Ongoing
NVP-AUY922
I/II
Ongoing
**ORR: Overall Response Rate; PFS: Progression Free Survival; OS: Overall Survival; CBR: Clinical Benefit Rate; RP2D: Recommended Phase 2 Dose.
Table 2: HSP90 inhibitors targeting Her-2+ breast cancer in clinical development.
Recent advances in HSP90/Her-2 therapies
Considering the array of data obtained from targeting the N-terminal domain of HSP90 and the promising anti-cancer benefits of HSP90 inhibitors in Her-2+ breast cancer, other aspects of HSP90 are being targeted as anti-cancer strategies. New small molecule inhibitors are being investigated that either target the C-terminal domain of HSP90, and/or target the association of HSP90 with cochaperones such as Cdc37, Bag-1 and CHIP [22]. Recently, a new novel HSP90 inhibitor, CPUY201112, with a radicicol scaffold was identified using a fragment-based approach and structure optimization. This inhibitor binds the ATP binding pocket of HSP90 and degrades its client proteins, including Her-2, Akt and c-RAF, via activation of p-53-dependent apoptosis [59]. Similarly, a natural product, celastrol, was studied as an inhibitor of the HSP90-Cdc37 complex and was effective in down regulating Her-2 levels in cell and xenograft models [60].
A great deal of interest has also shifted towards increasing the targeted delivery of HSP90 inhibitors using antibody-conjugated Nanogels (NGs) and Nanoparticles (NPs) in order to reduce systemic toxicity [61]. A study involving the synergistic delivery of doxorubicin and 17-AAG encapsulated in nanogel to target Her-2-driven tumors in vitro and in vivo provided the proof of concept for using nanogel in the targeted delivery systems [62]. This was followed by another study using trastuzumab-conjugated nanogels loaded with the cytotoxic drug doxorubicin (Tras-NG/Dox) in combination with 17-AAG in Her-2-driven breast cancer cell and xenograft models [63]. Both of the studies have shown that the targeted delivery of drugs using nanogels resulted in enhanced anti-tumor activity and potency when compared to either of the drugs alone in Her-2-driven breast cancer xenograft models [62,63].
Summary
A proposed model of the action of HSP90 and its inhibitors in targeting Her-2 stability and signaling is shown in Figure 1. The binding of ATP molecule to HSP90 complex (consisting of cochaperones) mediates the stability and maturation of Her-2 and other client proteins. The functional Her-2 protein forms homodimers or heterodimers with other family members and activates a plethora of downstream signaling pathways, including PI3K/Akt, mTOR and MAPK pathways, which promote cell growth and survival. HSP90 inhibitors target the stability and maturation of the Her- 2 protein by preventing ATP binding in the NTD of HSP90 or disrupting the chaperone/co-chaperone complex, thus initiating the ubiquitinylation and proteasomal degradation of the Her-2 protein. HSP90 inhibitors can also inhibit the downstream signaling of the Her-2 protein, such as PI3K, MAPK and mTOR pathways in Her- 2-expressing cancer cells, as well as in drug-resistant (trastuzumabor lapatinib-resistant) cancer cells, thereby inhibiting their growth, survival and angiogenesis.
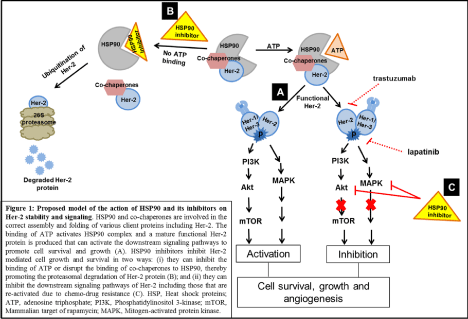
Figure 1: Proposed model of the action of HSP90 and its inhibitors on Her-2 stability and signaling. HSP90 and co-chaperones are involved in the correct assembly
and folding of various client proteins including Her-2. The binding of ATP activates HSP90 complex and a mature functional Her-2 protien is produced that can
activate the downstream signaling pathways to promote cell survival and growth (A). HSP90 inhibitors inhibit Her-2 mediated cell growth and survival in two ways;
(i) they can inhibit the binding of ATP or disrupt the binding of co-chaperons to HSP90, there by promoting the proteasomal degradation of Her-2 protein (B); and
(ii) they can inhibit the downstream signaling pathways of Her-2including these that are re-activated due to chemo-drug resistance (C). HSP, Heat shock proteins;
ATP, adenosine triphosphates; PI3K, Phosphatidylinositol 3-kinase; mTOR, Mammalian target of rapamycin; MAPK, Mitogen-activated protein kinase.
Conclusion
The clinical development of Her-2-targeted therapies is progressing rapidly. The current line of therapies involving humanized monoclonal antibodies, kinase inhibitors and drug conjugates has improved the overall response rate and progression free survival in metastatic Her-2+ breast cancer patients, yet the disease is still not curative and relapse is evident. The new emerging small molecule HSP90 inhibitors have provided a new proof of concept/principle to target Her-2-mediated signaling and appear to be promising therapeutics. Many of these inhibitors are currently in Phase III clinical trials and show effective management of various types of cancer, including those harboring mutations and resistance to first line therapies. Thus, designing a logical and rational treatment combination that targets pivotal players in Her-2-mediated breast cancer signaling and delays regression is required for the patients who will benefit the most. Finally, with the ample data obtained from preclinical and clinical studies along with the ongoing new developments in the field of HSP90 inhibition, there is a need to carefully analyze the available data and utilize it to its utmost potential against the most advance and aggressive tumor types.
Acknowledgement
We thank Dr. Erin Howard for critical reading and editing of the manuscript. Work in the Yang lab is supported, in part, by the NIEHS grant (R21ES025337), a Research Scholar grant from the American Cancer Society (RSG-08-138-01-CNE), a pilot project of a U54 grant from NCI (5U54CA156735), a pilot project of a U54 grant from NIAAA (U54 AA019765), and a UNC Research Opportunities Initiative (ROI) Award.
References
- Harari D, Yarden Y. Molecular mechanisms underlying ErbB2/HER2 action in breast cancer. Oncogene. 2000; 19: 6102-6114.
- Revillion F, Bonneterre J, Peyrat JP. ERBB2 oncogene in human breast cancer and its clinical significance. Eur J Cancer. 1998; 34: 791-808.
- Arteaga CL, Engelman JA. ERBB receptors: from oncogene discovery to basic science to mechanism-based cancer therapeutics. Cancer Cell. 2014; 25: 282-303.
- Moasser MM. The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene. 2007; 26: 6469-6487.
- Moasser MM. Targeting the function of the HER2 oncogene in human cancer therapeutics. Oncogene. 2007; 26: 6577-6592.
- Baselga J, Swain SM. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nature reviews Cancer. 2009; 9: 463-475.
- Rimawi MF, Schiff R, Osborne CK. Targeting HER2 for the treatment of breast cancer. Annu Rev Med. 2015; 66: 111-128.
- Konecny GE, Pegram MD, Venkatesan N, Finn R, Yang G, Rahmeh M, et al. Activity of the dual kinase inhibitor lapatinib (GW572016) against HER-2-overexpressing and trastuzumab-treated breast cancer cells. Cancer res. 2006; 66: 1630-1639.
- Burris HA, 3rd. Dual kinase inhibition in the treatment of breast cancer: initial experience with the EGFR/ErbB-2 inhibitor lapatinib. Oncologist. 2004; 9: 10-15.
- Ryan Q, Ibrahim A, Cohen MH, Johnson J, Ko CW, Sridhara R, et al. FDA drug approval summary: lapatinib in combination with capecitabine for previously treated metastatic breast cancer that overexpresses HER-2. Oncologist. 2008; 13: 1114-1119.
- Jagiello-Gruszfeld A, Tjulandin S, Dobrovolskaya N, Manikhas A, Pienkowski T, DeSilvio M, et al. A single-arm phase II trial of first-line paclitaxel in combination with lapatinib in HER2-overexpressing metastatic breast cancer. Oncology. 2010; 79: 129-135.
- Harbeck N, Beckmann MW, Rody A, Schneeweiss A, Muller V, Fehm T, et al. HER2 Dimerization Inhibitor Pertuzumab - Mode of Action and Clinical Data in Breast Cancer. Breast Care (Basel). 2013; 8: 49-55.
- Baselga J, Cortes J, Kim SB, Im SA, Hegg R, Im YH, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012; 366: 109-119.
- Swain SM, Kim SB, Cortes J, Ro J, Semiglazov V, Campone M, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 studies. Lancet Oncol. 2013; 14: 461-471.
- Lewis Phillips GD, Li G, Dugger DL, Crocker LM, Parsons KL, Mai E, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer research. 2008; 68: 9280-9290.
- Junttila TT, Li G, Parsons K, Phillips GL, Sliwkowski MX. Trastuzumab-DM1 (T-DM1) retains all the mechanisms of action of trastuzumab and efficiently inhibits growth of lapatinib insensitive breast cancer. Breast Cancer Res Treat. 2011; 128: 347-356.
- Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012; 367: 1783-1791.
- Zhu X, Verma S. Targeted therapy in her2-positive metastatic breast cancer: a review of the literature. Curr oncol. 2015; 22: 19-28.
- D'Amato V, Raimondo L, Formisano L, Giuliano M, De Placido S, Rosa R, et al. Mechanisms of lapatinib resistance in HER2-driven breast cancer. Cancer treatment reviews. 2015; 41: 877-883.
- Jolly C, Morimoto RI. Role of the heat shock response and molecular chaperones in oncogenesis and cell death. J Natl Cancer Inst. 2000; 92: 1564-1572.
- Li J, Buchner J. Structure, function and regulation of the hsp90 machinery. Biomed J. 2013; 36: 106-117.
- Garg G, Khandelwal A, Blagg BS. Anticancer Inhibitors of Hsp90 Function: Beyond the Usual Suspects. Adv Cancer Res. 2016; 129: 51-88.
- Calderwood SK, Khaleque MA, Sawyer DB, Ciocca DR. Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem Sci. 2006; 31: 164-172.
- Schulz R, Streller F, Scheel AH, Ruschoff J, Reinert MC, Dobbelstein M, et al. HER2/ErbB2 activates HSF1 and thereby controls HSP90 clients including MIF in HER2-overexpressing breast cancer. Cell Death Dis. 2014; 5: 980.
- Calderwood SK, Neckers L. Hsp90 in Cancer: Transcriptional Roles in the Nucleus. Adv Cancer Res. 2016; 129: 89-106.
- Citri A, Kochupurakkal BS, Yarden Y. The achilles heel of ErbB-2/HER2: regulation by the Hsp90 chaperone machine and potential for pharmacological intervention. Cell cycle. 2004; 3: 51-60.
- De Mattos-Arruda L, Cortes J. Breast cancer and HSP90 inhibitors: is there a role beyond the HER2-positive subtype?. Breast. 2012; 21: 604-607.
- Pick E, Kluger Y, Giltnane JM, Moeder C, Camp RL, Rimm DL, et al. High HSP90 expression is associated with decreased survival in breast cancer. Cancer Res. 2007; 67: 2932-2937.
- Zagouri F, Bournakis E, Koutsoukos K, Papadimitriou CA. Heat shock protein 90 (hsp90) expression and breast cancer. Pharmaceuticals (Basel). 2012; 5: 1008-1020.
- Xu W, Mimnaugh E, Rosser MF, Nicchitta C, Marcu M, Yarden Y, et al. Sensitivity of mature Erbb2 to geldanamycin is conferred by its kinase domain and is mediated by the chaperone protein Hsp90. J Biol Chem. 2001: 276: 3702-3708.
- Mimnaugh EG, Chavany C, Neckers L. Polyubiquitination and proteasomal degradation of the p185c-erbB-2 receptor protein-tyrosine kinase induced by geldanamycin. J Biol Chem. 1996; 271: 22796-22801.
- Sidera K, Gaitanou M, Stellas D, Matsas R, Patsavoudi E. A critical role for HSP90 in cancer cell invasion involves interaction with the extracellular domain of HER-2. J Biol Chem. 2008; 283: 2031-2041.
- Citri A, Gan J, Mosesson Y, Vereb G, Szollosi J, Yarden Y. Hsp90 restrains ErbB-2/HER2 signalling by limiting heterodimer formation. EMBO Rep. 2004; 5: 1165-1170.
- Xu W, Yuan X, Beebe K, Xiang Z, Neckers L. Loss of Hsp90 association up-regulates Src-dependent ErbB2 activity. Mol Cell Biol. 2007; 27: 220-228.
- Bertelsen V, Stang E. The Mysterious Ways of ErbB2/HER2 Trafficking. Membranes (Basel). 2014; 4: 424-446.
- Raja SM, Clubb RJ, Bhattacharyya M, Dimri M, Cheng H, Pan W, et al. A combination of Trastuzumab and 17-AAG induces enhanced ubiquitinylation and lysosomal pathway-dependent ErbB2 degradation and cytotoxicity in ErbB2-overexpressing breast cancer cells. Cancer Biol Ther. 2008; 7: 1630-1640.
- Munster PN, Marchion DC, Basso AD, Rosen N. Degradation of HER2 by ansamycins induces growth arrest and apoptosis in cells with HER2 overexpression via a HER3, phosphatidylinositol 3'-kinase-AKT-dependent pathway. Cancer Res. 2002; 62: 3132-3137.
- Zsebik B, Citri A, Isola J, Yarden Y, Szollosi J, Vereb G. Hsp90 inhibitor 17-AAG reduces ErbB2 levels and inhibits proliferation of the trastuzumab resistant breast tumor cell line JIMT-1. Immunol Lett. 2006; 104: 146-155.
- Basso AD, Solit DB, Munster PN, Rosen N. Ansamycin antibiotics inhibit Akt activation and cyclin D expression in breast cancer cells that overexpress HER2. Oncogene. 2002; 21: 1159-1166.
- Chandarlapaty S, Scaltriti M, Angelini P, Ye Q, Guzman M, Hudis CA, et al. Inhibitors of HSP90 block p95-HER2 signaling in Trastuzumab-resistant tumors and suppress their growth. Oncogene. 2010; 29: 325-334.
- Modi S, Stopeck A, Linden H, Solit D, Chandarlapaty S, Rosen N, et al. HSP90 inhibition is effective in breast cancer: a phase II trial of tanespimycin (17-AAG) plus trastuzumab in patients with HER2-positive metastatic breast cancer progressing on trastuzumab. Clin Cancer Res. 2011; 17: 5132-5139.
- Garcia-Carbonero R, Carnero A, Paz-Ares L. Inhibition of HSP90 molecular chaperones: moving into the clinic. Lancet Oncol. 2013; 14: 358-369.
- Leow CC, Chesebrough J, Coffan KT, Fazenbaker CA, Gooya J, Weng D, et al. Antitumor efficacy of IPI-504, a selective heat shock protein 90 inhibitor against human epidermal growth factor receptor 2-positive human xenograft models as a single agent and in combination with trastuzumab or lapatinib. Mol Cancer Ther. 2009; 8: 2131-2141.
- Scaltriti M, Serra V, Normant E, Guzman M, Rodriguez O, Lim AR, et al. Antitumor activity of the Hsp90 inhibitor IPI-504 in HER2-positive trastuzumab-resistant breast cancer. Mol Cancer Ther. 2011; 10: 817-824.
- Modi S SC, Henderson CA, Lin NU, Mahtani RL, Goddard J, Rodenas E, et al. Efficacy and safety of retaspimycin hydrochloride (IPI-504) in combination with trastuzumab in patients (pts) with pretreated, locally advanced or metastatic HER2-positive breast cancer. Journal of Clinical Oncology. 2011; 29.
- Jensen MR, Schoepfer J, Radimerski T, Massey A, Guy CT, Brueggen J, et al. NVP-AUY922: a small molecule HSP90 inhibitor with potent antitumor activity in preclinical breast cancer models. Breast Cancer Res. 2008; 10: 33.
- Kong A, Rea D, Ahmed S, Beck JT, Lopez RL, Biganzoli L, et al. Phase 1B/2 study of the HSP90 inhibitor AUY922 plus trastuzumab in metastatic HER2-positive breast cancer patients who have progressed on trastuzumab-based regimen. Oncotarget. 2016.
- Jhaveri K, Modi S. Ganetespib: research and clinical development. Onco Targets Ther. 2015; 8: 1849-1858.
- Lai CH, Park KS, Lee DH, Alberobello AT, Raffeld M, Pierobon M, et al. HSP-90 inhibitor ganetespib is synergistic with doxorubicin in small cell lung cancer. Oncogene. 2014; 33: 4867-4876.
- Smith DL, Acquaviva J, Sequeira M, Jimenez JP, Zhang C, Sang J, et al. The HSP90 inhibitor ganetespib potentiates the antitumor activity of EGFR tyrosine kinase inhibition in mutant and wild-type non-small cell lung cancer. Targete Oncol. 2015; 10: 235-245.
- Xiang L, Gilkes DM, Chaturvedi P, Luo W, Hu H, Takano N, et al. Ganetespib blocks HIF-1 activity and inhibits tumor growth, vascularization, stem cell maintenance, invasion, and metastasis in orthotopic mouse models of triple-negative breast cancer. J Mol Med (Berl). 2014; 92: 151-164.
- Proia DA, Zhang C, Sequeira M, Jimenez JP, He S, Spector N, et al. Preclinical activity profile and therapeutic efficacy of the HSP90 inhibitor ganetespib in triple-negative breast cancer. Clin Cancer Res. 2014; 20: 413-424.
- Friedland JC, Smith DL, Sang J, Acquaviva J, He S, Zhang C, et al. Targeted inhibition of Hsp90 by ganetespib is effective across a broad spectrum of breast cancer subtypes. Invest New Drugs. 2014; 32: 14-24.
- Jhaveri K, Chandarlapaty S, Lake D, Gilewski T, Robson M, Goldfarb S, et al. A phase II open-label study of ganetespib, a novel heat shock protein 90 inhibitor for patients with metastatic breast cancer. Clin Breast Cancer. 2014; 14: 154-160.
- Jhaveri K TE, Chandarlapaty S, Solit D, Cadoo K, Speyer J, D'Andrea G, et al. A phase I trial of ganetespib (heat shock protein 90 inhibitor) in combination with paclitaxel and trastuzumab in patients with human epidermal growth factor receptor-2 positive (HER2+) metastatic breast cancer (MBC). Cancer Res. 2016; 76: 2015.
- https://www.syntapharma.com/pipeline-ganetespib-presentations.php
- Huang W, Ye M, Zhang LR, Wu QD, Zhang M, Xu JH, et al. FW-04-806 inhibits proliferation and induces apoptosis in human breast cancer cells by binding to N-terminus of Hsp90 and disrupting Hsp90-Cdc37 complex formation. Mol Cancer. 2014; 13: 150.
- Huang W, Wu QD, Zhang M, Kong YL, Cao PR, Zheng W, et al. Novel Hsp90 inhibitor FW-04-806 displays potent antitumor effects in HER2-positive breast cancer cells as a single agent or in combination with lapatinib. Cancer Lett. 2015. 356: 862-871.
- Xu XL, Bao QC, Jia JM, Liu F, Guo XK, Zhang MY, et al. CPUY201112, a novel synthetic small-molecule compound and inhibitor of heat shock protein Hsp90, induces p53-mediated apoptosis in MCF-7 cells. Sci Rep. 2016; 6: 19004.
- Raja SM, Clubb RJ, Ortega-Cava C, Williams SH, Bailey TA, Duan L, et al. Anticancer activity of Celastrol in combination with ErbB2-targeted therapeutics for treatment of ErbB2-overexpressing breast cancers. Cancer Biol Ther. 2011; 11: 263-276.
- Ngamcherdtrakul W, Castro DJ, Gu S, Morry J, Reda M, Gray JW, et al. Current development of targeted oligonucleotide-based cancer therapies: Perspective on HER2-positive breast cancer treatment. Cancer Treat Rev. 2016; 45: 19-29.
- Desale SS, Raja SM, Kim JO, Mohapatra B, Soni KS, Luan H, et al. Polypeptide-based nanogels co-encapsulating a synergistic combination of doxorubicin with 17-AAG show potent anti-tumor activity in ErbB2-driven breast cancer models. J Control Release. 2015; 208: 59-66.
- Raja SM, Desale SS, Mohapatra B, Luan H, Soni K, Zhang J, et al. Marked enhancement of lysosomal targeting and efficacy of ErbB2-targeted drug delivery by HSP90 inhibition. Oncotarget. 2016; 7: 10522-10535.
- Leow CC, Chesebrough J, Coffman KT, Fazenbaker CA, Gooya J, Weng D, et al. Antitumor efficacy of IPI-504, a selective heat shock protein 90 inhibitor against human epidermal growth factor receptor 2-positive human xenograft models as a single agent and in combination with trastuzumab or lapatinib. Mol Cancer Ther. 2009; 8: 2131-2141.