
Research Article
Austin J Med Oncol. 2020; 7(2): 1051.
Secondary Substernal Goiters: Surgical Treatment and Challenges
Avgoustou C¹*, Constantinou P¹, Sioula M¹ and Avgoustou E²
¹Department of Surgery, General Hospital of Nea Ionia “Constantopoulion - Patission”, Athens, Greece
²Department of Internal Medicine, General Hospital of Athens “G. Gennimatas”, Athens, Greece
*Corresponding author: Avgoustou C, Department of Surgery, General Hospital of Nea Ionia “Constantopoulion - Patission”, Athens, Greece
Received: September 30, 2020; Accepted: October 17, 2020; Published: October 24, 2020
Abstract
Objective: To investigate clinical presentation, surgical treatment, complications, and malignancy risk of Substernal Goiters (SSGs).
Methods: Among 580 patients who had undergone Total Thyroidectomy (TT) during period 1/2013-12/2019, we encountered 38 (6.55%) with SSG: 32 women/6 men, aged 30-72 years (mean, 57). Five patients were receiving antithyroids and 11 had co-existing thyroiditis. Twelve had severe comorbidities. All presented with palpable cervical mass. Most had compressive symptoms. We tested thyroid function to ensure euthyroidism and Thyroglobulin (TG) to investigate association with malignancy. We performed Computed Tomography (CT) to assess the thyroid swelling. Thirteen (34.21%) had increased TG values (83.7-456.5 ng/mL, mean 195 ng/mL; normal 0-60 ng/mL). CT scan revealed anterior swelling and tracheal/ esophageal compression. All underwent TT via cervicotomy. Among the remaining 542 patients (control group), we encountered 98 (18.08%) with differentiated thyroid malignancy: microcarcinoma (TMC) 70, carcinoma (TC) 20, coexistent TMC +TC 8.
Results: Among SSGs, no death occurred. The overall complication rate was 36.84% (14 patients), including transient hypoparathyroidism (7, 18.42%) and transient monolateral vocal cord palsy (2, 5.26%). Histology revealed papillary malignancy in 9 (3 TCs, 6 TMCs/23.68%), all without extrathyroid invasion, and all but 2 TMCs located substernally. Among patients with high TG, 2 had TC and 3 TMC. Malignancy rate in SSGs was higher compared to that of control group (p=0.007). SSG patients were discharged 2-6 days after surgery (mean, 3.4).
Conclusion: The substernal nature of goiter does not have major impact on postoperative complications. The rate of unanticipated thyroid malignancy is high in SSG patients.
Keywords: Substernal goiter; Tracheal compression; Cervicotomy; Thyroidectomy; Complications; Malignancy
Introduction
A Substernal Goiter (SSG), also called as retrosternal or mediastinal goiter, is an enlarged thyroid gland that has descended through the thoracic inlet into the mediastinum [1-4]. This definition refers to secondary lesions. It is estimated that 85-90% of the SSGs are located in the anterior mediastinum [1]. The prevalence of SSGs ranges from 2% to as high as 20% among all patients with a goiter [1,5]. Most SSGs are identified during the fifth or sixth decade of life, and their incidence is three to four fold greater among women than men [4,6]. These slowly progressive and space-occupying lesions often compress adjacent structures, and may also cause hyperthyroidism and malignant changes [1,4,7,8]. Most of the investigators advocate for the removal of SSGs before dangerous compressive symptoms appear [3-5,8-11]. Surgery is technically demanding, with greater risk of injury of the native structures [12,13].
This study evaluated the clinico-laboratory characteristics, the perioperative difficulties and challenges, and the surgical outcomes of 38 patients treated for SSG at the Dept. of Surgery of the General Hospital of Nea Ionia “Constantopoulion - Patission”, Athens, Greece.
Methodology
Study design
This retrospective study was approved by the Institutional Review Board of the General Hospital of Nea Ionia “Constantopoulion - Patission”. A SSG was defined as any case where part of the thyroid gland descends beyond the plane of the thoracic inlet and the level of the clavicle, radiologically evidenced by Computed Tomography (CT) scan. Among 580 patients with various thyroidopathies who had undergone Total Thyroidectomy (TT) or completion TT at the Dept. of Surgery between January 2013 and December 2019, 38 (6.55%) were identified as having SSG and were enrolled in the study. Their clinical records were reviewed and data for various parameters were collected for analysis: age, sex, physical findings, symptoms, imaging findings, preoperative and operative methologies, histology, postoperative complications and surgical outcomes. The remaining 542 patients consisted the control group for comparison of the malignancy rates.
Preoperative evaluation and results
Thirty-two women and six men (ratio, 5.33:1) with SSG, aged 30- 72 years (median, 57 years) consisted the study group. They all had known thyroid goiter for 3-22 years (mean, 7.5 years). Preoperative clinical, physical and laboratory findings, and diagnoses for these 38 patients are presented in Table 1. At presentation, 33 patients had non-toxic nodular goiter and 5 were suffering from toxic goiter. Other coexistent severe pathology was encountered in 12 patients. The constant physical finding in all patients was the palpable cervical mass (with positive Pemberton’s sign in 7), and the most common symptom was respiratory distress/short breath during the last few weeks or months (18 patients, 47.36%). Neck/chest radiographs showed deviation of the trachea in 16 (42.1%) patients. Index thyroid ultrasonography (U/S)-associated with Fine Needle Aspiration/ Cytology (FNAC) of the cervical goiter component in 23 patientsrevealed thyroid nodules in 35 patients and swelling in all. Neck/chest CT scans (Figure 1) were performed in all these patients so as to better assess the lesion and revealed: (i) anterior mediastinal thyroid swelling with extension beyond the plane of the thoracic inlet and the level of the clavicle, not exceeding the aortic arch or the tracheal bifurcation, (ii) extension unilaterally in 21 (55.26%) patients, bilaterally in 17, (iii) tracheal deviation in 16 (42.1%) cases, associated with significant stenosis in 12 of them (31.57%), and (iv) esophageal compression with no prestenotic dilation in 8 (21%) cases. For better preoperative assessment, 5 patients -among them 2 with Chronic Obstructive Pulmonary Disease (COPD)-underwent a spirometry; other 2 with sleep apnea syndrome had a sleep apnea study. All patients had routine function tests, that included thyroid antibodies’ titles and levels of serum calcium and Thyroglobulin (TG). Five (13.15%) patients were receiving anti-thyroid medication for hyperthyroidism, leaving to our care to achieve an euthyroid status for at least 6 weeks prior to surgery. Eight patients with significant respiratory symptoms related to tracheal compression, among whom 2 with COPD, or suffering from bronchospasm, were treated with dexamethazone (10 mg/day) and aminophylline (per os, 0.1 g 10 mg bid) during the previous few days. Cardiac arrhythmias in 3 other patients were also timely controlled. In all patients, the vocal cords and their movement were visualized on laryngoscopy the day before surgery.
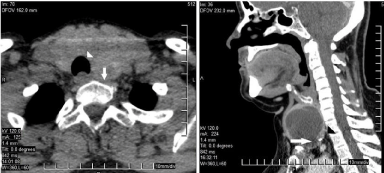
Figure 1: CT scan of upper mediastinum. Arrows point to descending goiter.
Arrowheads indicate compressed trachea (deviation, stenosis).
No of patients
Preoperative diagnosis (U/S, FNAC, function test)
Non-toxic nodular goiter
Nodules only
21
Lymphocytic thyroiditis
11
Hurthle/oxyphylic cell (neoplasm)
1
Thyrotoxicosis
Graves' disease
3
Toxic adenoma
1
Hashitoxicosis*
1
Coexistent other disease
12
Diabetes mellitus
6
Coronary disease (coronary artery bypass in 2)
3
COPD
2
Morbid obesity and sleep apnea (mild COPD)
2
Rheumatoid arthritis
1
Physical findings
Palpable cervical mass
38
Distended cervical veins
8
Positive Pemberton's sign
7
Symptomatology
Compressive symptoms
Respiratory distress/dyspnea
18
Voice changes
10
Dysphagia/swallowing difficulty
8
Thyrotoxicosis (anti-thyroid medication)
5
Neck/chest CT scan findings
SSG/anterior swelling - unilateral (right:11)
21
- bilateral
17
Tracheal compression - deviation
16
- stenosis (deviation)
12
Esophageal compression
8
*Transient hyperthyroidism in the course of Hashimoto's thyroiditis.
Table 1: Preoperative findings of 38 patients with substernal goiter who underwent total thyroidectomy.
Twelve (31.57%) patients had increased values of anti-TG and anti-TPO antibodies, which correlated well with the FNAC findings of thyroiditis. We also examined whether the preoperative TG levels would have a predictive value of malignancy in SSG cases treated with TT: thirteen (34.21%) patients had increased TG values (83.7-456.5 ng/mL, mean 195 ng/mL; normal values: 0-60 ng/mL).
Among the patients with various cervical thyroidopathies consisting the control group, 533 underwent TT and 9 completion TT. All completion TT cases, except for one, were referred to us from other hospitals for incomplete index surgery (benign nodular goiter recurrence 7, unanticipated papillary thyroid cancer 2). Among the rest of this group, 33 patients had a preoperative FNAC result positive for malignancy, which was postoperatively confirmed in 32. Postoperative histology revealed differentiated malignancy in 98 (18.08%) patients of this group: thyroid carcinoma (TC) in 20 (papillary 18, follicular 2), papillary Thyroid Microcarcinoma (TMC) in 70, and coexistent TCs and TMCs in 8 patients.
Postoperative assessments
Postoperative evaluation included monitoring and recording respiration and voice status, swallowing, wound condition, and the presence of hypocalcaemia symptoms. Serum calcium and parathyroid hormone concentrations were assessed routinely the morning after surgery, and the same day if symptoms of hypocalcaemia had occurred. Permanent hypocalcaemia was defined as serum parathyroid hormone concentration <14 pg/mL with >6 months of medication required for maintaining normocalcaemia. A laryngoscopic evaluation of vocal cord palsy was effectuated if a patient developed dyspnea, hoarseness or difficulty in swallowing. Permanent Recurrent Laryngeal Nerve (RLN) injury was defined as persistent vocal cord palsy for >6 months with no intervention.
Results
Anaesthesia and surgery
Conventional position for standard thyroid surgery through a low cervical incision was adopted. General anaesthesia in difficult cases (i.e. COPD, tracheal deviation/stenosis) was induced with the use of fiberoptic bronchoscope guided tracheal intubation.
Successive ligation of the middle thyroid vein -which commonly lied deeply- and the superior thyrovascular bundle, allowed mobilization of the lateral portion of the cervical goiter and always helped to free the mediastinal component. This last portion was pulled up upwardly following gentle sweeping with the finger along the carotid sheath down into the mediastinum and sharp/blunt dissection along the thyroid capsule to break the mediastinal negative pressure. In some cases with descending pole that was firmly adhered to the superior pleura and mediastinal connective tissues, the use of ultrasonic knife facilitated separation and helped the hemostasis. In one case, needle aspiration was required in order to shrink a large cystic mass and prevent rupture. The SSG was unilateral in 21 (55.26%) cases and bilateral in 17 (Figure 2).
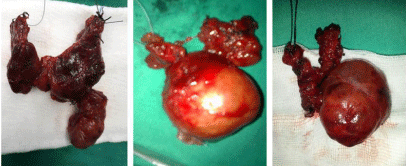
Figure 2: SSG surgical specimens.
When performing an extracapsular TT, we paid special attention to preserve the integrity of the capsule of the posterior thyroid in order to protect both the parathyroid glands with their vascular bundle and the RLNs. We avoided exposing the RLN at the SSG side using routine measures; instead, we performed a dislocation of the RLN clinging it tightly to the outer surface of the preserved thyroid capsule. However, visual identification of the RLNs near the cricothyroid joint at the SSG side(s) was possible unilaterally in 7 (18.42%) cases and bilaterally in 3 (7.89%). After removal of the gland and a perfect hemostasis, we examined the extent and degree of probable tracheal softening, particularly in patients with larger SSGs. Tracheomalacia was identified as a narrowed and soft trachea (~2 rings long) in two patients with bilateral SSG and Graves’ disease; in the one with more severe damage (known also from preoperative CT scan) we performed an intraoperative suspension of the trachea by sewing it to the anterior strap muscles. In all, negative pressure drain was conducted with silicone fine soft tubes that were introduced through separate stab openings out of the surgical incision, and placed deeply in the large residual cavities.
The Anaesthesiologist always assessed the vocal cords upon endotracheal tube removal. The patient with Graves’ disease and “suspended”-sewn trachea remained intubated in the Intensive Care Unit for 24 hours; he was then extubated successfully and transferred to the ward. No thyroid storm or immediate postoperative active bleeding occurred in any patient. Steroid medication (hydrocortisone) was initiated after extubation in the operating room in 5 patients who had a difficult intubation in order to reduce transient hoarseness caused by inflammatory vocal cord edema (3 patients) or to prevent laryngeal edema (2 patients); this medication was administered in small doses for 2-4 days. The suction drains were removed 2-7 days after surgery; nine patients with history of huge goiter and postoperative large residual cavity had their drains removed in outpatient setting. The patients were discharged 2-6 days (mean, 3.4 days) after surgery. Every patient was advised to be examined in outpatient setting during the following 14 days or until collecting histology results, and especially if there were any airway or wound problem.
Complications
The overall complication rate was 36.84% (Table 2). No death occurred.
No of patients (%)
Overall complications
14 (36.84%)
Transient RLN injury, unilateral (tracheomalacia: 1)
2 (5.26%)
Transient hoarseness from inflammatory edema
3 (7.89%)
Transient hypoparathyroidism
7 (18.42%)
Wound seroma (residual cavity)
1 (2.63%)
Wound infection (subcuticular)
1 (2.63%)
Pathology
Benign disease
29 (76.32%)
Thyroid nodules
26
Hashimoto's/ lymphocytic thyroiditis
8
Graves' disease
3
Plummers' disease
1
Toxic adenoma
1
Hürthle cell "benign" neoplasm
1
Malignant (papillary)
9 (23.68%)
TC (+lymphocytic thyroiditis: 2)
3
TMC (+lymphocytic thyroiditis: 2)
6
Table 2: SSG surgical specimens.
Unilateral RLN microtraumatism with transient vocal cord palsy happened in 2 (5.26%) patients with bilateral SSG. The first was a young patient with Graves’ disease and limited tracheomalacia (“nonsuspended” trachea), who had a difficult intubation and experienced acute respiratory distress and hoarseness after extubation; he was immediately re-intubated and underwent an emergent tracheostomy; he had a hemi-paresis of the left vocal cord and laryngeal edema on urgent laryngoscopy, he fully recovered in the Postanaesthetic Care Unit, and was transferred to the ward. Before discharge on day 6, his tracheostomy tube was replaced with a new “speaking” one; he was freed from the tube on day 25. The second patient suffered mild respiratory distress and hoarseness after extubation, he had rightsided vocal cord hemi-paresis on urgent laryngoscopy, and was treated conservatively (low dose of hydrocortisone, O2 on demand, bronchodilators); he was discharged on day 6, after undergoing a new laryngoscopy that showed improved vocal cord movement.
Symptomatic hypocalcaemia due to transient hypoparathyroidism was observed in 7 (18.42%) patients; only one required initial intravenous administration of calcium glyconate (1 gr of a 10% solution few times per day) for 3 days, all received oral Ca++ or oral Ca++/Vit D (50000-100000 Units per day); six patients needed medication for 2-3 weeks, one required oral Ca++/Vit D for 3 months.
Histology and TG
Histology revealed bilateral nodules in 14 bilateral SSG patients and in all unilateral (SSG side) cases; nodules were identified in the cervical (4 patients), the mediastinal (10 patients) and the cervicomediastinal (21 patients) components of SSGs. Twentynine patients (76.32%) had a benign diagnosis (one intraoperative FNAC result included), while differentiated malignant tumours were identified in 9 (23.68%) patients (Table 2). Lymphocytic/Hashimoto’s thyroiditis coexisted in 12 patients (31.57%), among whom 4 with malignancy (2 TCs, 2 TMCs). Malignancies included papillary TMCs (tumour size <1.0 cm) in 6 patients and papillary TCs with tumour size of 1.2-2.2 cm in 3 patients. All malignancies, apart from 2 TMCs, had developed in the substernal component of the gland. Upon pathologic review, all malignancies were limited to the thyroid gland, with no lesion showing extracapsular invasion. Among patients with high TG value, 2 had TC and 3 TMC (all in substernal component); no one was preoperatively diagnosed. The weight of goiters ranged 85-150 g, with mean 110 g.
Comparison of rates of coexistent malignancy in SSG and surgically treated cervical thyroidopathies.
The results were further compared with regard to malignancy rates. There was a 33.33% rate (32/96) of successful preoperative FNAC result positive for malignancy in the control group, while no one from the SSG group was preoperatively diagnosed. When compared the histology rates of malignancy between the two groups, a preponderance of the SSG group against the control group was documented (23.68% vs 18.08% respectively: p=0.007).
Discussion
Numerous definitions of SSG have been proposed [2-4,8,14]. In the present study we used a less rigid definition with a SSG defined as when any part of the thyroid descends beyond the constant landmarks of the thoracic inlet and the level of the clavicle, radiologically evidenced by CT scan. The SSG is characterized by slow progression and most patients present with palpable neck masses causing remarkable regional deformity and/or compressive symptoms resulting from space-occupying lesions [1,4,9,11,15- 17]. If the adjacent trachea, esophagus, nerves or blood vessels are compressed, the corresponding symptoms would occur: respiratory (dyspnea, wheezing, cough), dysphagia, neurologic (vocal cord paresis, hoarseness, Horner’s syndrome), vascular (superior vena cava syndrome, ischemic attacks), and metabolic (e.g. thyrotoxicosis) [1,4,6,9,10,15,18]. In this study, 38 SSG cases were encountered among 580 consecutive thyroidectomies for various thyroidopathies (6.55%). In our experience, SSGs with unilateral extension were slightly more common than those with bilateral extension (21/17), and right-and left-sided SSGs showed a nearly identical frequency (11/10). All of our patients presented with palpable neck mass and their most common symptom was respiratory distress, which could be exacerbated with raising arms or hyperextending neck (positive Pemberton’s sign [17]).
The CT scan is reported to be the most valuable radiologic investigation for evaluating SSGs with regard to location, extension, anatomical relationships, and the justified choice of the surgical approach [4,9,18-20]. Thyroid function tests help to detect and correct functional abnormalities [1]. FNAC is not recommended because the substernal portion of the goiter is hardly accessible and puncture may lead to hemorrhage or pneumothorax [1,4]. Finally, the vocal cords and their movement can be preoperatively visualized on fiberoptic laryngoscopy.
Most agree that “the presence of a SSG is in itself an indication for surgery”: a long history of having a large SSG precludes neither hyperfunction and malignancy nor complications such as aerodigestive tract compression; 131I to treat large thyrotoxic goiters may precipitate acute reactions that can result in respiratory distress; malignancy occurs in a significant number of these lesions, which may be inaccessible to needle biopsy [1,3,9,10,15,21]. Obviously, conditions such as hyperthyroidism, cardiac arrhythmias and bronchospasm have to be appropriately controlled prior to surgery [1,22]. The present study did not include cases with lesions descending beyond the carina or into the posterior mediastinum, both of which have been proposed as indicators of the need to use a thoracic approach (sternotomy, video-assisted thoracic surgery, lateral thoracotomy) [2,4,7,16,18,23].
TT in SSGs is technically demanding, with greater associated difficulties in blunt dissection of the descending lobe(s) and chances to injury to native structures [8,9,11-14,24-26]. Risks are increased in chronic large SSGs associated with compressive symptoms, and when toxicosis or active thyroiditis coexist [24,25]. For a safe removal of the gland, most investigators propose a primary ligation of the middle thyroid vein and the superior thyrovascular bundle, followed by a meticulous extracapsular dissection of the cervical portions of the lobes, with special attention to preserve the integrity of the posterior (cervical) thyroid, and the mobilization of the descending component(s) by gentle sweeping with the finger along the carotid sheath and the thyroid capsule down into the mediastinum [1]. The ultrasonic knife can deliver fine tissue separation and secure resection and it ensures effective hemostasis within a small surgical space [27].
An issue of paramount importance is the preservation of the native structures in the surgical field of TT for SSG. It is expected that the normal route of the RLN may be altered by the descending spaceoccupying mass [4]. However, the surgical exposure of the RLN during mobilization remains controversial [11,12,20]. Many researchers [27,28] have proposed that, when performing an extracapsular removal in SSG surgery, the RLN should not be routinely exposed; instead, it should be dislocated with the preserved capsule of the posterior thyroid. Others have suggested avoiding blind finger manipulation of a non-accessible inferior aspect of a mediastinal lesion without visual nerve identification and, instead, have recommended adopting a complementary thoracic approach [4,16]. The authors believe that, in the cervical approach, the deliberate dissection of the RLN during an extracapsular TT in SSG cases with accessible inferior aspect, that are commonly associated with mild chronic inflammation in the tracheoesophageal groove, altered anatomy and tracheal deviation, is unnecessary. However, postoperative hoarseness may also be due to transient vocal cord edema or vocal cord injury caused by the endotracheal tube used for anaesthesia [18,24]. The reported frequency of permanent postsurgical RLN palsy in SSGs ranges from 0% to 14.3% [4,6,8,9,18,20]. In the present study, two patients with bilateral SSGs suffered a transient unilateral RLN hemi-paresis (5.26%); only one with Graves’ disease and tracheomalacia required a temporary tracheostomy. Three (7.89%) others experienced mild transient hoarseness that was attributed to vocal cord edema and they were successfully treated conservatively.
Injury or blood impairment of the parathyroid glands leads to postoperative hypocalcaemia [8,13,18,20,27-30]. In a retrospective analysis [8] of 1767 cases with SSG surgically treated in a referral centre for endocrine surgery during a twenty-eight-year period, transient and permanent hypoparathyroidism were encountered in 14% and 4.1% respectively. In the present study, there were 7 (18.42%) patients who suffered transient hypoparathyrodism. Similarly to RLN protection against traumatism, the maintenance of the capsule of posterior thyroid, which permits the preservation of the parathyroids’ arterial branch, is the key to provide a viable gland [13]. If a parathyroid is inadvertently removed, autotransplantation into the sternocleidomastoid is performed.
Another concern is the possible tracheal destruction (tracheomalacia) in chronic large SSGs, that could result in respiratory distress and even in suffocation due to collapse of the tracheal wall following an inattentive extubation [1,22,24,27]. If that happens, then intraoperative tracheal suspension with the patient left intubated for 24-48 hours may be necessary [ 1]. Conventional tracheostomy is not recommended except for the cases with destruction of more than 2 rings in CT scan, tracheal narrowing with difficulty in intubation, or trachea collapse after the tumour removal [27].
Another issue of interest is the better control of the residual cavity, which is left after the removal of a large cervicomediastinal mass [27]. In general, empty body cavities might get soon filled up with reactive serum fluid, that may result in increase of the endo-space pressure, and re-compression of native structures. The authors believe that, in SSG surgery, the vital function of breath is safely protected with the use of negative pressure fine tubes for drainage; these tubes should be extracted later than usual, even in an out-patient setting, and when daily draining is less than 20 ml. We think that such drain secures the required time for healing and functions as an equilibrium that allows smoother cavity shrinkage.
A controversial issue remains the type of surgical removal of SSGs with regard to malignancy. In most studies, the incidence of malignancy among SSGs is increased when compared to patients with cervical nodular goiters, and ranges from 3.7 to 22.6% [4,6,8- 10,14,19,21]. Cancer foci of SSGs are most commonly located in the intrathoracic component [1,4,21]. Also, commonly, malignant tumours are limited to the thyroid gland without extrathyroidal invasion, which should require a combined cervical-thoracic approach [4]. In our study, all SSG malignancies (9 cases, 23.68%) were papillary tumours diagnosed postoperatively, with all of them limited to the thyroid gland. Seven of these cases (3 TCs, 4 TMCs) were located in the substernal component and, thus, they would have been inaccessible to preoperative FNAC. During the same period, we encountered other 98 cases (18.08%) with differentiated thyroid malignancy, who had undergone TT or completion TT for various cervical thyroidopathies, among whom 32 had a preoperative FNAC diagnosis of malignancy which was confirmed postoperatively.
TG level fails to get certain value as a diagnostic screening tool for malignancy. In this study, elevated TG levels in SSG patients undergoing TT constituted an index of suspicion for malignancy, but not always corresponded to thyroid malignancy; on the contrary, patients with normal TG values were found to have TMCs (3) or TCs (1).
On a multivariate analysis [10], substernal location was found to be the only variable independently associated with an unexpected thyroid cancer on surgical pathology. Thus, surgery is not only the most effective treatment for symptomatic patients with SSG, but also gives the definite answer to possible or unanticipated malignancy [4,10,29].
Conclusion
SSG is frequent, representing a 6.55% of all surgically treated thyroidopathies in this study. The descending goiter associated with present or imminent compressive symptoms, mostly respiratory distress, and the risk of malignancy, constitutes a major indication for thyroid surgery. All secondary SSGs not exceeding the aortic arch on CT scan can be approached transcervically. TT is the treatment of choice. With greater care taken during surgical removal, the substernal nature of the goiter does not have major impact on postoperative complications. In this study, the incidence of transient monolateral RLN palsy (5.26%) and transient hypoparathyroidism (18.42%), and the overall morbidity are comparable to the acceptable respective values after TT for cervical goiters. The rate of postoperatively discovered thyroid malignancy in patients with SSG of this study is 23.68%, and it is increased when compared to patients with cervical thyroidopathies (p=0.007). Finally, the elevated TG levels in SSG patients undergoing TT are not always a confirmation of thyroid cancer. Depending on these findings, we suggest that all SSGs should be surgically treated.
References
- Mack E. Management of patients with substernal goiters. Surg Clin North Am. 1995; 75: 377-394.
- Page C, Strunski V. Cervicothoracic goitre: an anatomical or radiological definition? Report of 223 surgical cases. J Laryngol Otol. 2007; 121: 1083- 1087.
- Nankee L, Chen H, Schneider DF, Sippel RS, Elfenbein DM. Substernal goiter: when is a sternotomy required? J Surg Res. 2015; 199: 121-125.
- Lin YS, Wu HY, Lee CW, Hsu CC, Chao TC, et al. Surgical management of substernal goitres at a tertiary referral centre: A retrospective cohort study of 2,104 patients. Int J Surg. 2016; 27: 46-52.
- Mercante G, Gabrielli E, Pedroni C, Formisano D, Bertolini L, et al. CT crosssectional imaging classification system for substernal goiter based on risk factors for an extracervical surgical approach. Head Neck. 2011; 33: 792-799.
- White ML, Doherty GM, Gauger PG. Evidence-based surgical management of substernal goiter. World J Surg. 2008; 32: 1285-1300.
- Kacprzak G, Karas J, Rzechonek A, Blasiak P. Retrosternal goiter located in the mediastinum: surgical approach and operative difficulties. Interact Cardiovasc Thorac Surg. 2012; 15: 935-937.
- Polistena A, Sanguinetti A, Lucchini R, Galasse S, Monacelli M, et al. Surgical approach to mediastinal goiter: An update based on a retrospective cohort study. Int J Surg 28 Suppl. 2016; 1: S42-46.
- Testini M, Gurrado A, Avenia N, Bellantone R, Biondi A, et al. Does mediastinal extension of the goiter increase morbidity of total thyroidectomy? A multicenter study of 19,662 patients. Ann Surg Oncol. 2011; 18: 2251-2259.
- Campbell MJ, Candell L, Seib CD, Gosnell JE, Duh QY, et al. Unanticipated thyroid cancer in patients with substernal goiters: are we underestimating the risk? Ann Surg Oncol. 2015; 22: 1214-1218.
- Bove A, Di Renzo RM, D’Urbano G, Bellobono M, D’Addetta V, et al. Preoperative risk factors in total thyroidectomy of substernal goiter. Ther Clin Risk Manag. 2016; 12: 1805-1809.
- Testini M, Gurrado A, Bellantone R, Brazzarola P, Cortese R, et al. Recurrent laryngeal nerve palsy and substernal goiter. An Italian multicenter study. J Visc Surg. 2014; 151: 183-189.
- Heineman TE, Kadkade P, Kutler DI, Cohen MA, Kuhel WI. Parathyroid Localization and Preservation during Transcervical Resection of Substernal Thyroid Glands. Otolaryngol Head Neck Surg. 2015; 152: 1024-1028.
- Doulaptsi M, Karatzanis A, Prokopakis E, Velegrakis S, Loutsidi A, et al. Substernal goiter: Treatment and challenges. Twenty-two years of experience in diagnosis and management of substernal goiters. Auris Nasus Larynx. 2019; 46: 246-251.
- Greenblatt DY, Sippel R, Leverson G, Frydman J, Schaefer S, et al. Thyroid resection improves perception of swallowing function in patients with thyroid disease. World J Surg. 2009; 33: 255-260.
- Rafaelli M, De Crea C, Ronti S, et al. Substernal goiters: Incidence, Surgical Approach, and Complications in a Tertiary Care Referral Center. Head Neck. 2011; 33: 1420-1425.
- De Filippis EA, Sabet A, Sun MR, Garber JR. Pemberton’s sign: explained nearly 70 years later. J Clin Endocrinol Metab. 2014; 99: 1949-1954.
- Chávez Tostado KV, Velázquez-Fernandez D, Chapa M, Pantoja Millán JP, Salazar MS, et al. Substernal Goiter: Correlation between Grade and Surgical Approach. Am Surg. 2018; 84: 262-266.
- Simó R, Nixon IJ, Vander Poorten V, Quer M, Shaha AR, et al. Surgical management of intrathoracic goitres. Eur Arch Otorhinolaryngol. 2019; 276: 305-314.
- Wong WK, Shetty S, Morton RP, McIvor NP, Zheng T. Management of retrosternal goiter: Retrospective study of 72 patients at two secondary care centers. Auris Nasus Larynx. 2019; 46: 129-134.
- Sahbaz NA, Tutal F, Aksakal N, Acar S, Aksu KI, et al. Cancer Frequency in Retrosternal Goiter. Am Surg. 2017; 83: 1390-1393.
- Mackie TW, Skinner A. Anaesthesia for massive retrosternal thyroidectomy in a tertiary referral centre. Br J Anaesth. 2014; 112: 756.
- Hanson MA, Shaha AR, Wu JX. Surgical approach to the substernal goiter. Best Pract Res Clin Endocrinol Metab. 2019; 33: 101312.
- Shen WT, Kebebew E, Duh QY, Clark OH. Predictors of airway complications after thyroidectomy for substernal goiter. Arch Surg. 2004; 139: 656-659.
- C. Avgoustou, Eirini Avgoustou. Coexistence of Hashimoto’s thyroiditis and papillary thyroid carcinoma. Hellenic Journal of Surgery. 2017; 89: 73-78.
- Tabchouri N, Anil Z, Marques F, Michot N, Dumont P, et al. Morbidity of total thyroidectomy for substernal goiter: A series of 70 patients. J Visc Surg. 2018; 155: 11-15.
- Gao B, Jiang Y, Zhang X, Zhao J, He Y, et al. Surgical treatment of large substernal thyroid goiter: analysis of 12 patients. Int J Clin Exp Med. 2013; 6: 488-496.
- Shaha AR, Jaffe BM. Parathyroid preservation during thyroid surgery. Am J Otolaryngol. 1998; 19: 113-117.
- Di Crescenzo V, Vitale M, Valvano L, Napolitano F, Vatrella A, et al. Surgical management of cervico-mediastinal goiters: Our experience and review of the literature. Int J Surg. 2016; 1: S47-53.
- Benbakh M, Abou-elfadl M, Rouadi S, Abada RL, Roubal M, et al. Substernal goiter: Experience with 50 cases. Eur Ann Otorhinolaryngol Head Neck Dis. 2016; 133: 19-22.