
Research Article
Austin J Med Oncol. 2021; 8(3): 1067.
Second Line Chemotherapy in Patients with Advanced Pancreatic Cancer after Failure of First-Line FOLFIRINOX: A Retrospective Analysis
Lee EM*
Department of Internal Medicine, Gospel Hospital, Kosin University College of Medicine, Korea
*Corresponding author: Lee EM, Department of Internal Medicine, Kosin University College of Medicine, 262 Gamcheon-ro, Seo-gu, Busan, Korea
Received: August 26, 2021; Accepted: September 20, 2021; Published: September 27, 2021
Abstract
Background: The first-line combination chemotherapy regimens, FOLFIRINOX and gemcitabine/nab-paclitaxel, improved survival outcomes in patients with advanced pancreatic cancer. However, there is no consensus therapy after failure of first-line chemotherapy. This objective of this study was to analysis of the clinical characteristics and outcomes of subsequent chemotherapy in patients who failed first-line FOLFIRINOX.
Methods: This retrospective study analyzed the clinical data of patients with advanced pancreatic cancer receiving second-line chemotherapy after failure of FOLFIRINOX at Kosin University Gaspel Hospital from January 2013 to July 2020.
Results: Sixty-three patients with advanced pancreatic cancer received first-line FOLFIRINOX, and 33 (51.7%) of those patients received at least one cycle of second-line chemotherapy. At the start of second-line chemotherapy, the median age of patients was 59 years (range, 31-79), and 54.5% (61 patients) was male. The second-line chemotherapy regimens included gemcitabine/ nab-paclitaxel (21, 63.6%), gemcitabine/erlotinib (6, 18.2%), and gemcitabine monotherapy (6, 18.2%). Of twenty-five patients who had measurable disease, only 1 patient (4.0%) achieved a partial response, and the disease control rate was 56% (14 patients). The median Overall Survival (OS) was 8.7 months (95% Confidence Interval [CI], 5.2-12.2), and the median progression-free survival was 3.2 months (95% CI, 1.7-4.8). The median OS from starting FOLFIRINOX was14.7 months (95% CI, 10.4-18.3). There was no significant difference of median OS between second-line regimens.
Conclusion: Gemcitabine-based chemotherapy had modest survival benefits in patients with advanced pancreatic cancer after failure of FOLFIRINOX.
Keywords: Pancreatic adenocarcinoma; FOLFIRINOX; Second-line chemotherapy; Gemcitabine
Introduction
Pancreatic cancer is one of the most lethal malignant tumors worldwide, with a 5-year survival rate of 9% in all stages [1]. Surgical resection is the only potentially curative treatment, but only 15-20% of patients with pancreatic cancer are diagnosed with resectable disease, and most patients relapse after surgery. Therefore, palliative chemotherapy is the main treatment modality for patients with advanced pancreatic cancer. Gemcitabine showed clinical benefits and modest survival advantages over treatment with bolus 5-Fluorouracil (5-FU) in a randomized clinical trial published in 1997. Thus, for more twenty years, gemcitabine became the standard of care for advanced pancreatic cancer [2]. The ACCORD11/ PRODIGE4 trial published by Conroy et al. in 2011 was a milestone in first-line treatment for advanced pancreatic cancer. FOLFIRINOX (a combination of 5-FU, leucovorin, irinotecan, and oxaliplatin) demonstrated dramatic improvements in Overall Survival (OS), as first-line therapy for pancreatic cancer, compared with gemcitabine monotherapy (11.1 vs. 6.8 months, Hazard Ratio [HR] 0.57, 95% Confidence Interval [CI] 0.45-0.73, p <0.01) [3]. Another phase 3 trial, the MPACT trial, compared gemcitabine plus nab-paclitaxel with gemcitabine monotherapy; the addition of nab-paclitaxel significantly improved OS (8.7 vs. 6.6 months, HR 0.72, 95% CI 0.62- 0.83, p <0.001) [4]. These two combination chemotherapy regimens have been the standard first line chemotherapy for patients with good Performance Status (PS). However, optimal subsequent treatment after failure of initial chemotherapy has not been established.
Three randomized phase 3 clinical trials for second-line chemotherapy of advanced pancreatic cancer has been conducted. In the CONKO-003 trial, second-line chemotherapy with a combination of oxaliplatin and 5-FU/leucovorin showed OS benefits in patients who failed gemcitabine monotherapy [5]. In contrast, the PANCREOX trial did not demonstrate survival benefits for the addition of oxaliplatin to infusional 5-FU/leucovorin (6.1 vs. 9.9 months, p=0.024) after failure of gemcitabine [6]. The NAPOLI-1 trial assessed the effects of nanoliposomal irinotecan, a new formulation of irinotecan, alone or in combination with 5-FU/leucovorin, in patients who previously received gemcitabine-based chemotherapy. In this trial, OS was longer for the combination of nanoliposomal irinotecan with 5-FU/leucovorin compared to 5-FU/leucovorin (6.1 months vs. 4.2 months, p = 0.012). The combination of nanoliposomal irinotecan plus 5-FU/leucovorin was approved for second-line treatment after failure of gemcitabine-based chemotherapy [7].
These prospective clinical trials for second-line chemotherapy regimens were conducted in patients who previously received gemcitabine-based chemotherapy. No randomized trials accessed subsequent treatment after failure of first-line FOLFIRINOX in patients with advanced pancreatic cancer. This retrospective study was designed to assess the clinical characteristics and outcomes of second-line chemotherapy in patients with advanced pancreatic cancer after failure of first-line FOLFIRNOX.
Materials and Methods
Patients
This retrospective study analyzed the clinical data of patients with advanced pancreatic cancer who received palliative chemotherapy in Kosin University Gaspel Hospital from January 2013 to July 2020.
This study included patients who had histologically confirmed pancreatic adenocarcinoma and locally advanced or metastatic disease, and received first-line FOLFIRINOX and at least one cycle of second-line chemotherapy. Histologic findings other than adenocarcinoma were excluded. Clinical feature, treatment information, and outcomes were retrospectively obtained from the medical records. The Institutional Review Board of our hospital approved this study (KUGH 2021-07-017).
Statistical analysis
OS was defined as the time from the date of starting second-line chemotherapy to the date of death. Progression-Free Survival (PFS) was defined as the time from starting second-line chemotherapy to the date of disease progression or death from other causes. Categorical variables were compared using Fisher exact test. The Kaplan-Meier method was used to estimate survival, and differences between groups were analyzed by using the log-rank test. Statistical analyses were performed using SPSS 23.0 (SPSS, Inc., Chicago, IL, USA), and the values of p < 0.05 were defined as statistically significant.
Results
Patients characteristics
Between January 2013 to July 2020, 63 patients with advanced pancreatic cancer received palliative first-line chemotherapy with FOLFIRINOX, and 33 (51.7%) of these patients received at least one cycle of second-line chemotherapy. The patient characteristics are summarized in Table 1. The median age of patients at the time of starting second-line chemotherapy was 59 years (range, 31-79), and 54.5% of patients was male. Twenty-six patients (78.8%) had the good Eastern Cooperative Oncology Group (EGOG) PS (0 or 1), and 72.7% of patients had metastatic disease. The median PFS from starting firstline FOLFIRINOX to disease progression was 6.7 months (95% CI, 4.6-10.2), and the median cycles of FOLRINOX were 8 (range, 1-22).
Characteristics (n=33)
N (%)
Median age (years)*
59 (31-74)
Sex
Male
18 (54.5)
Female
15 (45.5)
Smoking history
Never
24 (72.7)
Current/former
9 (27.3)
DM
Yes
12 (36.4)
No
21 (63.6)
EGOG PS*
0–1
26 (78.8)
=2
7 (21.2)
Primary tumor location
Head
18 (54.5)
Body
4 (12.1)
Tail
11 (33.3)
Disease extent*
Locally advanced
9 (27.3)
Metastatic
24 (72.7)
Metastatic sites*
Liver
15 (45.5)
Peritoneum
11 (33.3)
Lung
6 (18.2)
Bone
1 (3.0)
Anemia*
24 (72.7)
Hypoalbuminemia*
25 (75.8)
Median CA 19-9* (U/mL) (95% CI)
123.00 (42.14-260.00)
Median CEA* (ng/mL) (95% CI)
6.4 (4.4-9.41)
Medina PFS for first-line FOLFIRINOX (months) (95% CI)
6.7 (4.6-10.2)
Median cycles of first-line FOLFIRINOX (range)
8 (1-22)
*At start of second line chemotherapy.
DM: Diabetes Mellitus; ECOG PS: Eastern Cooperative Oncology Group Performance Status; CI: Confidence Interval; CEA: Carcinogenic Embryonic Antigen.
Table 1: Baseline characteristics.
Treatment with second-line chemotherapy
Table 2 shows treatment pattern of second-line chemotherapy. All regimens used for subsequent chemotherapy after failure of FOLFIRINOX were gemcitabine-based regimens. Gemcitabine/ nab-paclitaxel was administered to 21 patients (63.6%), gemcitabine/ erlotinib was administered six patients (18.2%), and gemcitabine monotherapy was administered to six patients (18.2%). Second-line chemotherapy was stopped in twenty patients (60.6%) due to disease progression, seven patients (21.2%) discontinued treatment due to toxicity of chemotherapy or deterioration of PS. A median three cycles of chemotherapy was performed (range 1-18), and the median treatment duration of second-line chemotherapy was 2.5 months (95% CI, 1.7-3.6).
N (%)
Second-line chemotherapy regimens
Gemcitabine + nab-paclitaxel
21 (63.6)
Gemcitabine + erlotinib
6 (18.2)
Gemcitabine monotherapy
6 (18.2)
Reason for treatment discontinuation
Disease progression
20 (60.6)
Toxicity/deterioration of PS
7 (21.2)
Patient’s withdrawal
6 (18.2)
Cycles of second-line chemotherapy
Median (range)
3 (1–18)
Duration of second-line chemotherapy
Median (months) (95% CI)
2.5 (1.7–3.6)
PS: Performance Status; CI: Confidence Interval.
Table 2: Treatment pattern of second-line chemotherapy.
Tumor response and survival outcomes
Twenty-five of the 33 patients had measurable disease based on the Response Evaluation Criteria in Solid Tumor version 1.1, and tumor responses were assessed in these patients (Table 3). None of the patients attained a complete response (CR). One patient, who was administered gemcitabine/nab-paclitaxel, had a Partial Response (PR). The Disease Control Rate (DCR), including CR, PR and Stable Disease (SD) was 56% (14 patient). Among 8 patients received gemcitabine ± erlotinib, and six patients achieved SD. SD was achieved in 8 of 17 patients who received gemcitabine/nab-paclitaxel. There was no statistically significant difference in the DCR between second-line chemotherapy regimens.
Gemcitabine ± erlotinib
Gemcitabine + nab-paclitaxel
Total
p value
Tumor response (n=25)
n=8
n=17
N (%)
PR
0
1
1 (4)
SD
6
7
13 (52)
PD
1
6
7 (28)
NE
1
3
4 (16)
DCR
6
8
14 (56)
0.234
Median survival (n=33)
n=12
n=21
PFS, month (95% CI)
3.6 (3.0-4.2)
2.3 (1.1-3.5)
3.2 (1.7-4.8)
0.531
OS, month (95% CI)
12.1 (4.2-20.1)
5.9 (4.8-7.0)
8.7 (5.2-12.2)
0.08
PR: Partial Response; SD: Stable Disease; PD: Progressive Disease; NE: Not Evaluable; DCR: Disease Control Rate; PFS: Progression-Free Survival; CI: Confidence Interval; OS: Overall Survival.
Table 3: Clinical outcomes of second-line chemotherapy.
The median follow-up was 12.7 months from starting secondline chemotherapy. The median PFS and OS from starting secondline chemotherapy were 3.2 months (95% CI, 1.7-4.8) and 8.7 months (95% CI, 5.2-12.2), respectively (Table 3). The 6-month and 12-month survival rates were 54.0% and 25.8%, respectively (Figure 1A). The median total OS from starting first-line FOLFIRINOX was 14.7 months (95% CI, 10.4–18.9) (Figure 1B). The median PFS from starting second-line chemotherapy were not statistically different between second-line chemotherapy regimens (3.6 months in gemcitabine ± erlotinib group vs. 2.3 months in gemcitabine/ nab-paclitaxel group, p = 0.531) (Figure 2A). The median OS from starting second-line chemotherapy was longer in patients receiving gemcitabine ± erlotinib than patients who received gemcitabine/nabpaclitaxel; however, the difference was not statistically significant (12.1 vs. 5.9 months, p = 0.080) (Figure 2B).
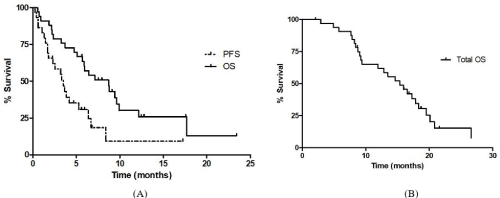
Figure 1: (A) Kaplan-Meir survival curves for PFS and OS from starting second line chemotherapy. (B) Kaplan-Meir survival curve for OS from starting first-line
FOLFIRINOX.
PFS: Progression-Free Survival; OS: Overall Survival
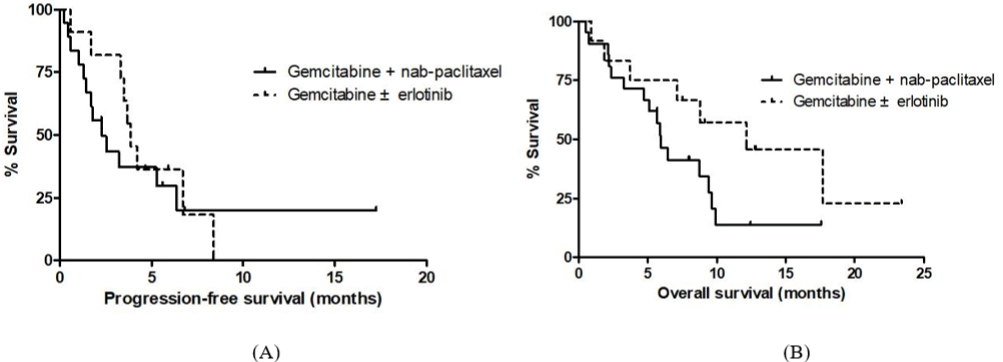
Figure 2: (A) Kaplan-Meir survival curves for PFS according to second line chemotherapy regimens. (B) Kaplan-Meir survival curves for OS according to second
line chemotherapy regimens.
PFS: Progression-Free Survival; OS: Overall Survival
Third-line chemotherapy
Of the twenty patients with disease progression after secondline chemotherapy, nine patients received third-line chemotherapy, including six patients who had received second-line gemcitabine/ nab-paclitaxel, and three patients who had received second-line gemcitabine ± erlotinib. The third-line chemotherapy regimens were nanoliposomal irinotecan plus 5-FU/leucovorin (3 patients), 5-FU/cisplatin (2 patients), S-1 (1 patient), nivolumab (1 patient), gemcitabine/nab-paclitaxel (1 patient), and FOLFIRINOX (1 patient).
Discussion
Although combination chemotherapy regimens, such as FOLFIRINOX or gemcitabine/nab-paclitaxel, improved survival outcomes in patients with advanced pancreatic cancer over gemcitabine monotherapy, no standard of care after failure of initial chemotherapy has been established. Three randomized clinical trials of second-line chemotherapy for advanced pancreatic cancer showed survival benefits in patients after failed gemcitabine-based chemotherapy [5-7]. However, there are no prospective data of subsequent chemotherapy after failure of first-line FOLFIRINOX. This retrospective study was conducted to analyze the characteristics and clinical outcomes of subsequent chemotherapy in patients with advanced pancreatic cancer after failure of first-line FOLFIRINOX in real clinical practice. All patients received gemcitabine-based chemotherapy, and the median OS was 8.7 months (95% CI 5.2-12.2). These outcomes were similar to other retrospective studies of secondline chemotherapy after failure of FOLFIRINOX (3.6-12.4 months) [8-15].
No head-to-head randomized clinical studies have compared the efficacies of FOLFIRINOX and gemcitabine/nab-paclitaxel as first-line chemotherapy. The median OS was numerically longer in the FOLFIRINOX group in the ACCORD11/PRODIGE4 trial (11.1 months) than the gemcitabine/nab-paclitaxel group in the MPACT trial (8.5 months). However, comparisons between the two phase 3 clinical trials are limited because the ACCORD11/PRODOGE4 trial included patients with better PS and younger age than the MAPCT trial [3,4]. Pusceddu et al. reported a meta-analysis of 16 retrospective studies to compare FOLFIRINOX and gemcitabine/nab-paclitaxel. In this meta-analysis, a median weighted OS difference favored FOLFRIINOX (mean difference: 1.15, 95% CI 0.08-2.22, p = 0.03). Grade 3 and 4 neutropenia, febrile neutropenia, and nausea were lower with gemcitabine/nab-paclitaxel, while grade 3 and 4 neurotoxicity and anemia were lower with FOLFIRINOX [16]. In a large realworld cohort study of 1130 patients, FOLFIRINOX had longer OS, but more febrile neutropenia-related hospitalizations compared to gemcitabine/nab-paclitaxel (weighted HR for OS 0.77, 95% CI, 0.70- 0.85, odd ratio for febrile neutropenia-related hospitalization 2.21, p = 0.001) [17]. Considering these results, FOLFIRINOX preferentially could be preferred in patients with good PS and young age for firstline chemotherapy, and gemcitabine/nab-paclitaxel is an acceptable and potentially less toxic alternative to FOLFIRINOX.
Half of the patients remain in good clinical condition after failure of first-line chemotherapy. Thus, further treatment is feasible. The combination of nanoliposomal irinotecan with 5-FU/leucovorin improved survival outcomes in patients after gemcitabine-based chemotherapy in the phase 3 NAPOLI-1 trial [7]. However, no subsequent treatment after failure of FOLFIRINOX has been established. Although no randomized data demonstrate the optimal second-line chemotherapy in patients who failed firstline FOLFIRINOX, gemcitabine-based regimens are acceptable therapeutic options. In the ACCORD11/PRODIGE4 study, 46.8% of patients in the FOLFIRINOX group had received secondline chemotherapy with gemcitabine monotherapy (82.5%) and gemcitabine-based combination therapy (12.5%) [3]. In the present study, all patients received gemcitabine-based regimens as secondline chemotherapy, gemcitabine/nab-paclitaxel (63.6%), gemcitabine/ erlotinib (18.2%), and gemcitabine monotherapy (18.2%).
Although no prospective data to compared second-line gemcitabine monotherapy with the best supportive care, several small retrospective studies reported the outcomes of gemcitabine monotherapy. The DCR ranged 20% to 44%, the PFS ranged from 1.7 months to 2.5 months, and the median OS ranged from 3.6 to 6.8 months (8-11, 15). Gemcitabine/nab-paclitaxel showed promising outcomes after failure of FOLFIRINOX in several studies. According to a small retrospective study by Nguyen et al., second-line gemcitabine/nab-paclitaxel resulted in a median PFS of 3.8 month and a median OS of 12.4 months [12]. The AGEO prospective trial reported the efficacy of second-line gemcitabine/nab-paclitaxel in 58 patients who failed FOLFRINOX; the DCR was 18%, the median PFS was 5.8 months (95% CI, 3.2-6.2), and the OS was 8.8 months (95% CI, 6.2-9.7) [18]. Similar results were reported by Mita et al. in a small phase 2 trial that evaluated second-line gemcitabine/nabpaclitaxel in 30 patients after failure of FOLFIRINOX; the DCR was 47%, the median PFS and OS were 3.8 months (95% CI, 3.3–4.8) and 7.6 months (95% CI, 5.7-8.6), respectively [19]. However, grade 3 and 4 adverse events occurred in 38% of patients in the AGEO trial, and 70% in the phase 2 trial by Mita et al. [18,19]. Therefore, gemcitabine/ nab-paclitaxel should be recommended in selected patients who have good PS, a relatively favorable comorbidity profile, and patient preference and a support system for aggressive medical therapy [20].
Gemcitabine/nab-paclitaxel, the intensive combination chemotherapy regimen, seems to have improved outcome in patients with advanced pancreatic cancer after failure of FOLFIRINOX, compared to gemcitabine monotherapy. Zhang et al. demonstrated that longer median OS in second-line gemcitabine/nab-paclitaxel than gemcitabine monotherapy (5.7 vs. 3.8 months, hazard ration 2.66, p = 0.03) in a small retrospective study [14]. However, in the present study, gemcitabine/nab-paclitaxel did not improve OS compared with gemcitabine (± erlotinib). Rather, the median OS was lower but not statistically different in patients who received gemcitabine/nabpaclitaxel, compared with gemcitabine monotherapy (5.9 vs. 12.1 months, p = 0.080). Because this study had only a small number of patients and the comparative group was not unified, further clinical trials are needed to confirm the superiority of gemcitabine/nabpaclitaxel over other regimens.
In this retrospective study, the median OS from starting firstline FOLFIRINOX was 14.7 months (95% CI, 10.4-18.9), consistent with other studies of second-line chemotherapy after failure of FOLFIRINOX (11.2-18 months) [9-13,18,19]. The median OS of the first-line FOLFIRINOX group was 11.1 months in the ACCORD11/PRODIGE4 trial; thus, subsequent chemotherapy after FOLFIRINOX seems to improve survival for advanced pancreatic cancer. Therefore, identification of patients who will benefit from subsequent chemotherapy after failure of FOLFIRINOX is important. Several studies reported factors to predict survival for second-line chemotherapy of advanced pancreatic cancer, and PS is one of the most common and important prognostic factors [21-23]. Viaud et al. analyzed 96 patients with advanced pancreatic cancer who received second-line gemcitabine after failure of FOFLIRINOX; poor ECOG PS (>1) and old age at diagnosis were associated with poor OS [11]. Most of patients (78.8%) had good PS in the present study, and prognostic factors analysis to predict survival benefits could not be performed due to the small number of patients.
Our study had several limitations. This was a retrospective study, and all data were collected by reviewing of medical records. Therefore, collecting adverse events for second-line chemotherapy was difficult, and analysis of adverse events according to chemotherapy regimens could not be performed. Because of the small number of patients, identifying prognostic factors to predict survival outcomes was difficult and the results did not demonstrate that a specific regimen had superiority over other regimens.
Conclusion
In conclusion, this study demonstrated that gemcitabine-based chemotherapy in patients with advanced pancreatic cancer had modest survival benefit; the median PFS with 3.2 months (95% CI, 1.7-4.8) and the median OS with 8.7 months (95% CI, 5.2-12.2 months), consistent with other retrospective studies. Prospective randomized clinical studies to confirm the survival benefits of second-line chemotherapy after failure of FOLFIRINOX are needed.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020; 70: 7-30.
- Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997; 15: 2403-2413.
- Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Eng J Med. 2011; 364: 1817-1825.
- Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Eng J Med. 2013; 369: 1691-1703.
- Oettle H, Riess H, Stieler JM, Heil G, Schwaner I, Seraphin J, et al. Secondline oxaliplatin, folinic acid, and fluorouracil versus folinic acid and fluorouracil alone for gemcitabine-refractory pancreatic cancer: outcomes from the CONKO-003 trial. J Clin Onco. 2014; 32: 2423-2429.
- Gill S, Ko YJ, Cripps C, Beaudoin A, Dhesy-Thind S, Zulfiqar M, et al. PANCREOX: A Randomized Phase III Study of Fluorouracil/Leucovorin With or Without Oxaliplatin for Second-Line Advanced Pancreatic Cancer in Patients Who Have Received Gemcitabine-Based Chemotherapy. J Clin Oncol. 2016; 34: 3914-3920.
- Wang-Gillam A, Li CP, Bodoky G, Dean A, Shan YS, Jameson G, et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet. 2016; 387: 545-557.
- da Rocha Lino A, Abrahão CM, Brandão RM, Gomes JR, Ferrian AM, Machado MC, et al. Role of gemcitabine as second-line therapy after progression on FOLFIRINOX in advanced pancreatic cancer: a retrospective analysis. J Gastrointest Oncol. 2015; 6: 511-5.
- Gilabert M, Chanez B, Rho YS, Giovanini M, Turrini O, Batist G, et al. Evaluation of gemcitabine efficacy after the FOLFIRINOX regimen in patients with advanced pancreatic adenocarcinoma. Medicine (Baltimore). 2017; 96: e6544.
- Sarabi M, Mais L, Oussaid N, Desseigne F, Guibert P, De La Fouchardiere C. Use of gemcitabine as a second-line treatment following chemotherapy with folfirinox for metastatic pancreatic adenocarcinoma. Oncol Lett. 2017; 13: 4917-4924.
- Viaud J, Brac C, Artru P, Le Pabic E, Leconte B, Bodère A, et al. Gemcitabine as second-line chemotherapy after Folfirinox failure in advanced pancreatic adenocarcinoma: A retrospective study. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2017; 49: 692-696.
- Nguyen KT, Kalyan A, Beasley HS, Singhi AD, Sun W, Zeh HJ, et al. Gemcitabine/nab-paclitaxel as second-line therapy following FOLFIRINOX in metastatic/advanced pancreatic cancer-retrospective analysis of response. J Gastrointest Oncol. 2017; 8: 556-565.
- de Jesus VHF, Camandaroba MPG, Donadio MDS, Cabral A, Muniz TP, de Moura Leite L, et al. Retrospective analysis of efficacy and safety of Gemcitabine-based chemotherapy in patients with metastatic pancreatic adenocarcinoma experiencing disease progression on FOLFIRINOX. J Gastrointest Oncol. 2018; 9: 806-819.
- Zhang H, Kellett C, Lambert P, Kim CA. Efficacy and Tolerability of Secondline Nab-paclitaxel and Gemcitabine After Failure of First-line FOLFIRINOX for Advanced Pancreas Cancer: A Single-institution Experience. Clin colorectal cancer. 2018; 17: e451-e456.
- Girardi DM, Faria L, Teixeira MC, Costa FP, Hoff PMG, Fernandes GS. Second-Line Treatment for Advanced Pancreatic Adenocarcinoma: Is There a Role for Gemcitabine? J Gastrointest Cancer. 2019; 50: 860-866.
- Pusceddu S, Ghidini M, Torchio M, Corti F, Tomasello G, Niger M, et al. Comparative Effectiveness of Gemcitabine plus Nab-Paclitaxel and FOLFIRINOX in the First-Line Setting of Metastatic Pancreatic Cancer: A Systematic Review and Meta-Analysis. Cancers (Basel). 2019; 11: 484.
- Chan KKW, Guo H, Cheng S, Beca JM, Redmond-Misner R, Isaranuwatchai W, et al. Real-world outcomes of FOLFIRINOX vs gemcitabine and nab paclitaxel in advanced pancreatic cancer: A population-based propensity score-weighted analysis. Cancer Med. 2020; 9: 160-169.
- Portal A, Pernot S, Tougeron D, Arbaud C, Bidault AT, de la Fouchardière C, et al. Nab-paclitaxel plus gemcitabine for metastatic pancreatic adenocarcinoma after Folfirinox failure: an AGEO prospective multicentre cohort. Br J Cancer. 2015; 113: 989-995.
- Mita N, Iwashita T, Uemura S, Yoshida K, Iwasa Y, Ando N, et al. Second- Line Gemcitabine Plus Nab-Paclitaxel for Patients with Unresectable Advanced Pancreatic Cancer after First-Line FOLFIRINOX Failure. J Clin Med. 2019; 8: 761.
- Sohal DPS, Kennedy EB, Cinar P, Conroy T, Copur MS, Crane CH, et al. Metastatic Pancreatic Cancer: ASCO Guideline Update. J Clin Oncol. 2020: Jco2001364.
- Kim ST, Choi YJ, Park KH, Oh SC, Seo JH, Shin SW, et al. A prognostic model to identify patients with advanced pancreas adenocarcinoma who could benefit from second-line chemotherapy. Clin Oncol (R Coll Radiol). 2012; 24: 105-111.
- Vienot A, Beinse G, Louvet C, de Mestier L, Meurisse A, Fein F, et al. Overall Survival Prediction and Usefulness of Second-Line Chemotherapy in Advanced Pancreatic Adenocarcinoma. J Natl Cancer Inst. 2017; 109.
- Hsu CC, Liu KH, Chang PH, Chen PT, Hung CY, Hsueh SW, et al. Development and validation of a prognostic nomogram to predict survival in patients with advanced pancreatic cancer receiving second-line palliative chemotherapy. J Gastroenterol Hepatol. 2020; 35: 1694-1703.