
Review Article
Austin J Med Oncol. 2021; 8(3): 1068.
Nanotechnology to Cure Breast Cancer and Obstacles in its Way
Nawaz AF¹*, Riaz A¹, Iqbal A¹, Arshad R¹, Shoukat S² and Zia A²
¹Department of Biochemistry and Biotechnology, University of Gujrat, Gujrat, Pakistan
²National Institute of Genomics and advanced studies in Biotechnology, National Agriculture Research Center, Islamabad, Pakistan
*Corresponding author: Ayesha Fazal Nawaz, Department of Biochemistry and Biotechnology, University of Gujrat, Gujrat, 50700, Pakistan
Received: October 12, 2021; Accepted: November 03, 2021; Published: November 10, 2021
Abstract
Nanomaterials owing to their remarkable pharmaceutical properties and potential to load large quantities of drugs are considered an ideal vector for carrying medicine to the target site. The treatment of breast cancer has been a pressing issue due the use of conventional methods of treatment resulting in many side effects. Nanotechnology offers a more targeted approach in breast cancer treatment. The present review examines the role of Carbon Nanotubes (CNTs) and nanoparticles in the treatment of breast cancer and the obstacles in the way of nanotechnology in becoming one of the best methods in breast cancer treatment and drug delivery. Use of monoclonal antibody trastuzumab against HER-2 biomarkers by attaching it with nanotubes or nanoparticles is considered the best-targeted therapy along with photo thermal ablation. The absence of human tumor models and lack of toxicological assays are the major factors contributing to differences between the preclinical and clinical trial results.
Keywords: Breast cancer; Doxorubicin; Gold nanoparticles; Trastuzumab; Targeted drug delivery
Introduction
Cancer is one of leading causes of death worldwide. It results from the disruption of balance between cell proliferation and cell death leading to development of abnormal cells. This imbalanced condition arises due to gene deletion, mutation, and translocation, causing genetic variation [1]. Breast cancer manifests because of abnormal growth of cells in breast tissues leading to lobular carcinoma; cancer of lobules and ductal carcinoma; cancer of ducts, both can be invasive or non-invasive. Mutations in genes like BRCA1, BRCA2, RB, PIK3, MDM2, HER2 and TPK53 result in the development of breast cancer [2]. Breast cancer is the second most commonly diagnosed cancer (11.6% of the total cases) after lung cancer and is the leading cause of cancer death in women in over 100 countries (Graphical Abstract). Each year 2.1 million women are affected and in 2018, out of 2,048,849 women diagnosed with breast cancer, 626,789 died making 15% of the total cancer deaths in women. According to GLOBOCAN 2018 estimate, the age standardized (world) incidence rate (ASIR) was 43.6 and age standardized (world) mortality rate (ASMR) was 13.0 [3].
Graphical Abstract :
The possible factors associated with development of breast cancer include reproductive, menstrual, and hormonal factors. The family history, obesity, high caloric diet, lack of breastfeeding, use of oral contraceptive pills and ingestion of hormones like estrogen in HRT are all contributing factors to breast cancer [4,5].
Traditional treatment of breast cancer involves approaches like surgery, radiotherapy and chemotherapy, all of which have certain limitations and many side effects. Chemotherapy involves the use of anticancer drugs, which are non-specific and cytotoxic. They target not only cancer cells but also healthy cells in the body and leads to Multiple Drug Resistance (MDR). Radiotherapy can also cause side effects due to damage to tissues surrounding the cancer site. This can also cause impairment of immune system and other side effects like fatigue, anemia, diarrhea and vomiting [1]. The use of radiation therapy is also influenced by the presence of breast cancer gene variants. Individuals having pathogenic variants of TP53 are at higher risk of development of new cancers form radiation therapy. The use of radiotherapy and chemotherapy can also cause increase risk of heart disease [6,7]. Surgery can be used for localized cancer removal but it fails in the treatment of metastasized cancer [1]. Nanotherapy on the other provides a solution to all above problems by providing targeted drug delivery to cancer cells with reduced toxicity, enhanced imaging, monitoring and effective drug delivery [8].
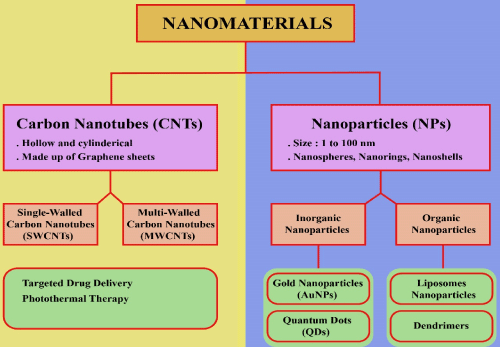
Carbon Nanotubes (CNTs) are hollow cylindrical structures of graphene sheets [9]. CNTs are classified as Single-Walled Carbon Nanotubes (SWCNTs) and Multi-Walled Carbon Nanotubes (MWCNTs) [10]. CNTs, in combination with the drugs like doxorubicin, are used extensively as improving agents in cancer therapy and diagnosis because of their large surface area, conjugation ability, and encapsulation of drugs [11,12]. To make CNTs tumorspecific, they can be conjugated with ligands against biomarkers that are over-expressed in cancerous tissues. For example, SWCNTs can be conjugated with CD44 antibodies to target CD44 receptors on breast cancer stem cells [13]. Although CNTs are hydrophobic in nature, they can be functionalized with hydrophilic substances to improve their biocompatibility and solubility [9]. Because of their unique physiochemical properties, CNTs have numerous applications in diagnosis and treatment of different diseases especially cancer [11].
Chemotherapeutic drugs are encapsulated in nano-scale devices to increase the targeted drug delivery and bioavailability of breast cancer drugs [14]. Nanomaterials are nano-carriers of drugs; having the ability of imaging, tracking, and targeting [9].
Nanoparticle is a nanomedicine for cancer treatment and it has been reported by researchers that the uptake of nanoparticles (organic or inorganic) can improve the limitations of traditional therapeutics [15]. Particles having size between 1 and 100nm are referred as nanoparticle [16]. Basically, nanoparticles (NPs) are involved in targeted drug delivery at the tumor site. Micro-particles cannot pass through tiny capillaries having 5-6 microns diameter, so nanoparticle are more suitable to reach remote areas at cancer site [17].
Drug carriers are of several types such as micelles, polymeric dendrimers, microspheres, Quantum Dots (QDs), nano-emulsions, liposomes, hydrogels, and Gold Nanoparticle (GNPs), which adopt diverse methods of drug attachment such as covalent binding, adsorption, and encapsulation [14]. Gold Nanoparticle are considered as a best vaccine vector because they have appropriate payload and are highly biocompatible [16]. Systematic administration of nanoparticle at the tumor site causes accumulation of nanoparticle in the tumor cells through enhanced permeability and retention (EPR) effect; this is due to poor drainage of lymphatic system [18].
In cancer, therapy nano-medicines face certain obstacles in becoming a new paradigm due to incomplete understanding of nano-bio interactions, complexities, heterogeneity of tumor biology, chemistry and good manufacturing practice (GMP). Difference between the efficacies obtained in preclinical studies and the outcomes from clinical trials, is another well-recognized obstacle due to lack of tumor models that can recapitulate human cancers [19]. Enhanced permeability and retention effect (EPR) is one of the example in which small animal models show positive results but such evidences are lacking in humans during clinical trials [20]. In vivo and in vitro toxicity testing assays show that nanomaterials have detrimental effects to biological system at molecular and cellular level because of varying physiochemical properties as, these can impact numerous biological pathways. Because it is impossible to study toxicological properties of all Nano material variants, it is a challenge to testing toxicity of nanomaterials [21].
Carbon Nanotubes in Breast Cancer Treatment
The promising size, needle-like structure and unique physiochemical properties of carbon nanotubes make them a good tool for fighting breast cancer [9]. CNTs are mostly used for targeted drug delivery and photothermal therapy for breast cancer [11].
One of the major causes of treatment resistance in breast cancer is hypoxia. This is because tumor cells initiate multiple adaptation processes under hypoxic conditions, which results in treatment failure. In 2017, Jia, Weng et al. determined the increase in chemosensitivity and radiosensitivity of MDA MB 231 and ZR 75 1 human breast cancer cell lines by synthesizing a new functionalized single walled carbon nanotube carrying oxygen. They synthesized an oxygen carrying tombarthite modified folic acid conjugated chitosan (R O2 FA CHI) SWCNT nanocarrier and treated cancer cell lines with this nanocarrier along with chemotherapy drugs or radiotherapy. It was observed that the R O2 FA CHI SWCNTs significantly enhanced the chemosensitivity and radiosensitivity of human breast cancer cell lines by downregulating the expression of B cell lymphoma 2 (Bcl 2), survivin, hypoxia inducible factor 1a (HIF 1a), multidrug resistance associated protein 1 (MRP 1), P glycoprotein (P gp), RAD51 and Ku80 [22].
Targeted drug delivery
In order to make carbon naotubes tumor-specific, they can be conjugated with monoclonal antibodies (mAb) or aptamers [10]. In 2015, Al Faraj, Shaik et al. determined the efficiency of SWCNTs to improve the clinical utility of doxorubicin (DOX) in breast cancer murine model. They synthesized Iron-tagged SWCNTs conjugated with Endoglin/CD105 antibody with or without DOX. After characterizing the nanoparticles for biocompatibilty, they were actively and magnetically targeted to tumor sites in murine model. It was observed that DOX-loaded SWCNTs induced significant increases in apoptosis, DNA damage, and oxidative stress. These results showed enhanced therapeutic efficacy of DOX delivered through antibody-conjugated magnetic SWCNTs [23].
Another approach to control the cancer cell proliferation and metasis to other tissues is by blocking certain molecular pathways through small molecules or monoclonal antibody. Trastumzumab an anti-HER monoclonal antibody is considered to be the besttargeted therapy so far. The nanodelivery of trastuzumab can be done by decorating the surfaces of nanocarriers with antibody and allowing targeted delivery of trastuzumab to HER2+ cells. Antibody conjugated with gold sulfide nanoparticles can be used for photoablation therapy and ln-labeled trastuzumab conjugated to modified gold nanoparticles can be used for radiation therapy [24].
Abraxane, also called as nab-paclitaxel or nanoparticle albumin bound paclitexal, is another example of targeted drug delivery to breast cancer cells [24]. Paclitaxel (PTX) has been an important contribution to the treatment of breast cancer. A Single Walled Carbon Nanotube Based Drug Delivery System (SWNT-HAS) was established using human albumin proteins. This combination displayed 80% of intracellular drug delivery in MCF-7 breast cancer cell along with high growth inhibition; suggesting high delivery efficiency and effectiveness [25].
A cisplatin modified poly(d,1-lactide-co-glycolide) (PLGA) has shown significant efficacy in breast cancer patients [14].
Photothermal therapy
Because of the intrinsic hyperthermic property of CNTs induced by near infrared (NIR) irradiation, several attempts have been made to use them for the photothermal ablation of cancer cells [26]. In 2016, Han, Han et al. synthesized SWCNTs conjugated with PEG and thioaptamer and determined their effect in human breast cancer targeted photothermal therapy in-vivo and in-vitro. In vivo testing was performed via injection to the caudal vein to subcutaneous breast cancer mice models while HCC1937 human breast cancer cells were used for in vitro testing. In vitro results showed that SWCNT-PEG-TA significantly decreased the cell viability. Similar results were observed in in vivo study as well [27]. In another study carried out by Oh, Jin et al. in 2017, MDA-MB-231 cell death promoted by SWCNTs-Dox was investigated. Dox-release from SWCNTs was accelerated by NIR irradiation. It was observed that Dox released from SWNTs- Dox effectively localized into the MDA-MB-231 cell nucleus and accumulated inside the cells at high concentration. SWNTs-Dox and PPT together promoted an effective cell death in MDA-MB- 231 cells by mitochondrial disruption and Reactive Oxygen Species (ROS) generation [28].
Nanoparticles - An Emerging Nanomaterial to Treat Breast Cancer
Conventional chemotherapeutics have systemic toxicity and adverse effects on normal cells. Nanotechnology has revolutionized the treatment of breast cancer by targeted drug delivery. Nanoparticles have some important parameters such as size, charge, and shape. These parameters are significant in targeted drug delivery at cancer site. There are many advantages concerned with nanoparticles: (1) prevention of early degradation of drug, (2) absorption rate of drug is increased in selected tumor areas, (3) intracellular penetration of drug is increased by nanosystem, (4) lowers the rate of systemic toxicity, (5) premature interaction of drug and biological system is inhibited, (6) have capacity to absorb or load more than one therapeutic drugs, (7) Nanomedicine are more stable as compared to conventional ones. So Nanomedicine are easily accessible to tumor site and have great potential to treat cancer [29] [30].
Nanoscale dispersions of solid particles are referred as nanoparticles. According to American society for Testing and Materials, nanoparticles are three dimensional small bits of matter, having size from 1-100nm [31]. Nanoparticle are classified into different types; nanospheres, nanorings, nanoshells, nanocapsules, fullerenes, liposomes, dendrimers and quantum dots [32].
Cancer treatment by nano-system
Tumor is optimally treated by using Nanoparticles (NPs), either organic or inorganic in nature. Organic nanoparticles are composed of organic and inorganic materials. Nanoparticles are made from components of particular metals having specific physicochemical properties and these properties are related to that particular metal [15].
Inorganic nanoparticles
Inorganic nanoparticles are made up of two basic parts: shell and core. Core of inorganic NP is made up of metals such as gold, silver, quantum dots, and iron oxides. Shell is composed of either organic polymers or metals. The function of shell is to protect the inner core from chemical interactions and act as a binding substrate for biomolecules [15].
Gold nanoparticle
Gold nanoparticles are highly selective anti-cancerous nanodrugs having unique biological compatibility, and increased payload efficiency. Various biomolecules are coated over gold nanoparticles to make their surface functionalized and more stable in biological environment. Gold nanoparticles are pharmaceutical cargos for targeted drug delivery at tumor site [34].
Characteristics of gold nanoparticles
Gold nanoparticles are found in different sizes, shapes, compositions, and structures. Gold nanospheres (AuNPs) are solid gold balls, which are synthesized by the reduction of chloroauric acid, having diameter from few to more than 100nm. Gold nanoshells (AuNSs) are composed of silica cores and thin gold shells protecting that silica cores, having size between 50-150nm. [31]. Inorganic Nanoparticle such as nanoshells and GNPs are used in combination with MIR and high-resolution superconducting quantum interference devices to enhance the therapeutic effect [35].
Gold nanoparticles conjugated with trastuzumab increases the uptake of AuNPs by breast cancer cells and trastuzumab conjugated AuNPs is potentially strong therapeutic agent to treat breast cancer [31] (Figure 1).
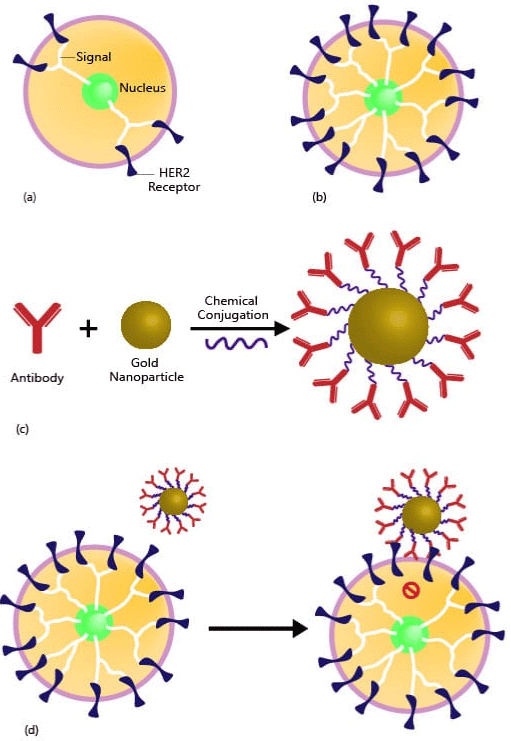
Figure 1: AuNPs (Gold Nanoparticles) to treat/kill breast cancer. (a) Normal
amount of HER2 in normal breast cell. HER2 Receptors sending signals to
breast cell to grow and divide in normal fashion as in mitotic cell division. (b)
Huge amount of HER2+ in cancerous breast cell. Too much HER2 receptors
sending more signals, causing cell to grow and divide quickly in uncontrolled
manner by losing mitotic mechanism. (c) Trastuzumab (Antibody) is
chemically conjugated with AuNP (Gold Nanoparticle) via a protein called
a linker molecule. Trastuzumab is used to treat breast cancer that is HER2
receptor positive, where AuNP has a huge payload efficiency. (d) Gold
Nanoparticle is pharmaceutical cargo and able to transport drug at targeted
tumor site and thus blocking the HER2+ receptors from signaling the cell to
divide and grow in uncontrolled manner.
Quantum dots (QDs)
Nanocrystals, inorganic in nature, 1-10 nm in diameter having properties of semiconductor are called quantum dots (QDs). Group II-VI or groups III-V elements of periodic table are concerned with synthesis of QDs. CdSe nanoparticles are quantum dots. CdSe acts as a core of QDs and shell is made up of another semiconductor, usually ZnS acts as a shell [36].
QDs can load large amount of drug, we can say they offer large payload without altering the properties of therapeutic agents [15]. QDs are beneficial and attractive in biological imaging, cancer diagnosis and treatment because they can emit fluorescent when subjected to an energy source. QDs are highly photo stable for longer period because of their amazing surface composition. In breast cancer research, QDs are used for the purpose of targeted drug delivery. Limitation associated with QDs is toxicity and excretion pathway of drug. Elements that are involved in the composition of QDs make them either toxic or non-toxic. Cadmium causes toxicity of QDots. Sometimes high dose of QDs cause alteration in nucleus and mitochondria of the cell thus leading to the cell death and toxicity [9].
Organic nanoparticles
Liposomes nanoparticles: Spherical vesicles of hydrophilic or hydrophobic in nature having bilayer membranous structures are called liposomes. Single lipid bilayer liposomes have watery core for entrapping hydrophilic drugs, but liposomes having multi lipid bilayer, encapsulate lipid soluble drugs. PlasmidDNA and siRNA are efficiently encapsulated in the hydrophobic core of liposomes based Nanoparticle [15]. Liposomes differ in size from 50-100nm and can aggregate in cancer cells. Liposomes are categorized into different types based on their structure, size, wetness of surface, and preparation methods [14].
Drug delivery system comprised of liposomes is significant in biodistribution of drugs by improving the pharmacological structures and properties of traditional chemotherapeutic drugs. Liposome based cancer drugs have overcome the limitations of toxic drugs. Now-a-days, different methods are used to load Nanomedicine on lipid-based system. Liposomes are excellent carriers to load anticancer drugs because of their unique pharmacological structure. Liposomes reduce the toxicity of conventional drugs by changing the biodistribution of encapsulated drug [37].
Paclitaxel (LEP-ETU/Insys) is loaded on liposomes and is used to treat phase II of breast cancer whereas Doxorubicin (Myocettm/ Teva UK) has been approved to treat metastatic breast cancer [29]. Liposomal anthracycline has an active anticancer agent to treat breast cancer [38].
Dendrimers: Polymers having nanoscale branched system are dendrimers. “Dendron” is a Greek word and meaning is tree like. Dendrimers are unique structures synthesized from monomers by step growth polymerization technique having stability and uniformity in branching system. Three main regions of dendrimers are surface, complex branching system, and the core molecule in the central region of the dendrimer. Dendrimers are categorized based on branches, dendrimer having only one branch, or one core molecule is called generation 0 (G0) dendrimer. Addition of one more branch to existing core of dendrimers is called generation 1 (G1) dendrimer. The continuous and successive addition of branches in the dendrimer structure form G2, G3, G4 and so on. Dendrimers have bigger diameter because of complex and enhanced branching system [39,40].
Due to stable structure, they are best nano drug carriers. Anticancer drugs are either encapsulated in the dendrimers or stick to the dendrimers by forming complex structure. Branched structure of dendrimersis involved in drug delivery as well as gene delivery. Dendrimers usually attach to polyamidoamine (PAMAM), poly (ethylene glycol) (PEG), chitin and chitosan [33].
Bio-inspired virus nanoparticles: Natural biomaterials that are biodegradable and bio-suitable are virus nanoparticles (VNPs). VNPs consist of self-assembling and self-proliferative system. Viral nanoparticles reproduce many times in a short time span but maintaining their symmetry throughout proliferating activity. Potato virus X (PVX) having size of 515 × 12 nm is most popular viral nanoparticles. Plant viral nanoparticles (VNPs) have many applications in nanobiotechnology. Most common application of VNPs is targeted drug delivery and has ability to surround cancer cells by penetrating and homing tumor. Herceptin (HER) is delivered via PVX by conjugating with Herceptin (PVX-HER) to kill cell lines involved in two types of breast cancers [41,42].
Obstacles: Carbon nano materials used to treat breast cancer, in their native form are insoluble in biological aqueous environment and these lack the molecular aggregates to provide specific activities, leading to their excessive accumulation in body and hinder their elimination from body [43]. Nano carriers used for target drug delivery purposes still have limited therapeutic benefits because of physiochemical barriers such as tumor penetration, tumor heterogeneity, relative hypoxia, and endosomalescape [44].
Doxorubicin (DOX) is a broad-spectrum anticancer drug used to treat breast cancer. Because of low therapeutic index and life, its high dose is required, which cause some unwanted results to normal tissues and induce cumulative cardiac toxicity [12]. By using nanotechnology PEGylated liposomal doxorubicin (Doxil) formulated, and used to treat breast cancer [45]. Pegylated Liposomal Doxorubicin (PLD) improve the efficacy and lower the side effects of Doxorubicin as it has different pharmacokinetics profile. Through a consistent review of phase II clinical trials the side effects of PLD in metastatic breast cancer was studied [46]. Doxil limits the cardiotoxicity but resulted to another side effects because of high concentration accumulation in skin. Skin eruptions, which were encountered, are hand-foot syndrome, diffuse follicular rash, intertrigo like eruption, and new formation of melanotic macules. Thus, to identifying a right nanoparticle is crucial as an effort used to solve one problem can leads to another issue [45].
Raw carbon nanotubes and functionalized multi wall Carbon nanotubes have cytotoxicity potential in human breast cancer cells (MCF-7) but they also show adverse effects to human health [47]. Their small size and high surface area lead to increase their chemical reactivity and induce changes to permeability to biological membrane [48].Due to which they induce health risks because they can reach to every part of tissue, organ and can interact with cellular metabolic pathways to cause adverse effects [47].
Some of the nanoparticles have cytotoxicity effects because of their non-specific activity, can damage to normal replicating cells. Trustuzumab and pertuzumab used to treat breast cancer but on other hand, also heart related problems was showed in women. Tamoxifen also have serious side effects as increased 1.9 times thromboembolic disease and 2.4 times endomaterial cancer chances in a woman of 50 years or older. Therefore, for treatment of breast cancer selective delivery of cytotoxic nanoparticles to tumor cells and to keep the efficacy-toxicity balance is critical [49].
Due to certain problems, there are some nanoparticles, which are restricted to use, like magnetic nanoparticles, Quantum dots, and Carbon nanotubes have potential material toxicity and solid lipid nanoparticles have low capacity to load drugs and these all still have no clinical trials. Polymeric nanocarrier leads to degradation of carrier and go through to only phase 1 clinical trials e.g. paclitaxel [24].
For targeted drug delivery purposes, the complex formulation of nanomaterials is required, which increased the risks of toxicity, immunogenicity, higher production cost, and good manufacturing practice issues. Breast cancer is more fatal and destructive when metastasis occurs, and it spread to other parts of body like brain, bone, lung, liver. So such Nanomedicine need to develop which can percolate to these site without causing any side effects [24].
Another challenge related to nanoparticles is their effects to environment. During formation, these nanoparticles require the special precautions as airborne nanoparticles can spread into environment as aerosol. Which can lead to lungs deposition to cause pulmonary diseases. Similarly, some nanoparticles can penetrate skin barrier to cause dermatological problems [45,50].
Conclusion
Breast cancer is being studied extensively to design new drugs and methods for treatment, and nanotechnology has brought tremendous advancements in treatment methods. Nanomaterials provide numerous functionalization options from conjugation with anti-breast cancer antibodies to surface modifications for enhanced drug delivery. Although several techniques have been developed, there is a room for more research to fully understand the capabilities and potential of the nanomaterial to further advance the treatment methods and minimize the toxicity issues. The absence of human tumor models presents an ongoing problem in the advancement of nanotechnological approaches. Currently there have not been many clinical studies regarding the use of nanomaterials in breast cancer treatment due to toxicity problems but many studies showed that the future seems bright for the nanomedicine in breast cancer treatment.
References
- S Yalcin, U Gunduz. The Magnetic Nanobased Strategies to Overcome Drug Resistance in Breast Cancer Therapy. Handbook of Nanomaterials for Industrial Applications. 2018; 577-586.
- A Amja, I Khan, Z Kausar, F Saeed, L Azhar, PJCMB Anwar, Risk Factors in Breast Cancer Progression and Current Advances in Therapeutic Approaches to Knockdown Breast Cancer. 2018; 4: 2471.
- F Bray, J Ferlay, I Soerjomataram, RL Siegel, LA Torre, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. 2018; 68: 394-424.
- Salahuddin, Arifullah, Najma, Mehreen. Risk Assessment Model of Rural and Urban Breast Cancer Patients of Khyber Pakhtunkhwa Province, Pakistan. 2018; 57: 71-76.
- C La Vecchia, G Carioli. Epidemiology Biostatistics and Public Health. The epidemiology of breast cancer, a summary overview. 2018; 15: 1.
- JC Rehammar, MB Jensen, P McGale, EL Lorenzen, C Taylor, SC Darby, et al. Oncology, Risk of heart disease in relation to radiotherapy and chemotherapy with anthracyclines among 19,464 breast cancer patients in Denmark, 1977-2005. 2017; 123: 299-305.
- AM Falstie-Jensen, A Kjærsgaard, EL Lorenzen, JD Jensen, KV Reinertsen, OM Dekkers, et al. Hypothyroidism and the risk of breast cancer recurrence and all-cause mortality-a Danish population-based study? 2019; 21: 44.
- S Naz, H Shahzad, A Ali, M Zia. nanomedicine, biotechnology, Nanomaterials as nanocarriers: a critical assessment why these are multi-chore vanquisher in breast cancer treatment. 2018; 46: 899-916.
- JJ Lee, LS Yazan, CAC Abdullah. A review on current nanomaterials and their drug conjugate for targeted breast cancer treatment. IJN. 2017; 12: 2373-2384.
- EJ Comparetti, VdA Pedrosa, R Kaneno. Carbon nanotube as a tool for fighting cancer. JBc. 2017; 29: 709-718.
- M Sheikhpour, A Golbabaie, A Kasaeian. Carbon nanotubes: a review of novel strategies for cancer diagnosis and treatment. 2017; 76: 1289-1304.
- A Ünlü, M Meran, B Dinc, N Karatepe, M Bektas, FS Güner. Cytotoxicity of doxrubicin loaded single-walled carbon nanotubes. 2018; 45: 523-531.
- A Al Faraj, AS Shaik, B Al Sayed, R Halwani, I Al Jammaz. Specific targeting and noninvasive imaging of breast cancer stem cells using single-walled carbon nanotubes as novel multimodality Nano probes. 2016; 11: 31-46.
- SK Singh, S Singh, JW Lillard Jr, R Singh. Drug delivery approaches for breast cancer. 2017; 12: 6205-6218.
- ME Grigore. Organic and inorganic nano-systems used in cancer treatment. J Med Res Health Educ. 2017; 1: 1.
- F Leng, F Liu, Y Yang, Y Wu, W Tian. Strategies on nanodiagnostics and nanotherapies of the three common cancers. J. N. 2018; 8: 202.
- R Sebastian. Nanomedicine-the future of cancer treatment: A review. J.J.C.P.C.R. 2017; 8: 00-265.
- O Akakuru, H Louis, O Oyebanji, B Ita, PI Amos. Utility of nanomedicine for cancer treatment. J.N.N. 2018; 9: 1-6.
- J Shi, PW Kantoff, R Wooster, OC Farokhzad. Cancer nanomedicine: progress, challenges and opportunities. J.N.R.C. 2017; 17: 20-37.
- AC Anselmo, S Mitragotri. Nanoparticles in the clinic. J.B.T. Medicine. 2016; 1: 10-29.
- S Halappanavar, U Vogel, H Wallin, CL Yauk. Promise and peril in nanomedicine: the challenges and needs for integrated systems biology approaches to define health risk. J.W.I.R.N. Nanobiotechnology. 2018; 10: e1465.
- Y Jia, Z Weng, C Wang, M Zhu, Y Lu, L Ding, et al. Increased chemosensitivity and radiosensitivity of human breast cancer cell lines treated with novel functionalized single-walled carbon nanotubes. J.O.L 2017; 13: 206-214.
- A Al Faraj, AP Shaik, AS Shaik. Magnetic single-walled carbon nanotubes as efficient drug delivery nanocarriers in breast cancer murine model: noninvasive monitoring using diffusion-weighted magnetic resonance imaging as sensitive imaging biomarker. I.J.N. 2015; 10: 157-168.
- Taherian, N Esfandiari. Nanomedicine, a new therapeutic strategy in breast cancer treatment. J.A.o.B.C. 2019; 2: 67-78.
- W Shao, A Paul, L Rodes, S Prakash. A new carbon nanotube-based breast cancer drug delivery system: preparation and in vitro analysis using paclitaxel. J.C.b. Biophysics. 2015; 71:1405-1414.
- Y Hashida, H Tanaka, S Zhou, S Kawakami, F Yamashita, T Murakami, et al. Photothermal ablation of tumor cells using a single-walled carbon nanotube– peptide composite. J.C. Release. 2014; 173: 59-66.
- Z Han, X Han, Z Wang, S Wu, R Zheng. Thioaptamer conjugated single-wall carbon nanotubes in human breast cancer targeted photothermal therapy invivo and in-vitro. J.I.J.o.C. E. Medicine. 2016; 9: 58-64.
- Y Oh, JO Jin, J Oh. Photothermal-triggered control of sub-cellular drug accumulation using doxorubicin-loaded single-walled carbon nanotubes for the effective killing of human breast cancer cells. J.N. 2017; 28: 125101.
- M Rocha, N Chaves, S Báo. Nanobiotechnology for breast cancer treatment. J.B.C.-F.B.t.M. 2017.
- Y Li, B Humphries, C Yang, Z Wang. Nanoparticle-mediated therapeutic agent delivery for treating metastatic breast cancer-challenges and opportunities. J.N. 2018; 8: 361.
- J Lee, DK Chatterjee, MH Lee, S Krishnan. Gold nanoparticles in breast cancer treatment: promise and potential pitfalls. J.C.L. 2014; 347: 46-53.
- MdP Rodriguez-Torres, LS Acosta-Torres, LA Diaz-Torres. Heparin-based nanoparticles: An overview of their applications. J.J.o.N. 2018; 2018: 489.
- DJ Patel, PA Mistri, J. Prajapati. Treatment of cancer by using nanoparticles as a drug delivery. I.J.D.D.R. 2012; 4: 14-27.
- SK Surapaneni, S Bashir, K Tikoo. Gold nanoparticles-induced cytotoxicity in triple negative breast cancer involves different epigenetic alterations depending upon the surface charge. J.S.r. 2018; 8: 12295.
- D Peer, JM Karp, S Hong, OC Farokhzad, R Margalit. Nanocarriers as an emerging platform for cancer therapy. Nature Nanotechnology. 2007; 2: 751- 760.
- LD True. Quantum dots for molecular pathology: their time has arrived. J Mol Diagn. 2007; 9: 7-11.
- A Sharma, N Jain. Nanocarriers for diagnosis and targeting of breast cancer. BioMed Res Int. 2013; 2013.
- MV Yezhelyev, X Gao, Y Xing, A Al-Hajj, S Nie. Emerging use of nanoparticles in diagnosis and treatment of breast cancer. Lancet Oncol. 2006; 7: 657-667.
- F Diederich. Supramolecular chemistry of dendrimers with functional cores. Proc Natl Acad Sci USA. 2002; 99: 4778-4781.
- JB Christensen. Dendrimers-based nanoparticles for cancer therapy and bioimaging. Nanooncology. 2018: 281-304.
- N Esfandiari. Targeting breast cancer with bio-inspired virus nanoparticles. Arch Breast Cancer. 2018: 90-95.
- AA Aljabali, A Berardi, DJ Evans. Nature’s nanoparticles: using viruses as nanomedicines and for bioimaging. Fundamentals of nanoparticles. 2018: 29-50.
- M Casais-Molina, C Cab, G Canto, J Medina. Carbon nanomaterials for breast cancer treatment. 2018.
- D Rosenblum, N Joshi, W Tao, JM Karp. Progress and challenges towards targeted delivery of cancer therapeutics. Nat Commun. 2018; 9: 1-12.
- N Desai. Challenges in development of nanoparticle-based therapeutics. AAPS J. 2012; 14: 282-295.
- L Ansari, F Shiehzadeh, Z Taherzadeh, S Nikoofal-Sahlabadi, A Momtazi- Borojeni, A Sahebkar, et al. The most prevalent side effects of pegylated liposomal doxorubicin monotherapy in women with metastatic breast cancer: a systematic review of clinical trials. Cancer Gene Ther. 2017; 24: 189-193.
- MA Siddiqui, R Wahab, J Ahmad, NN Farshori, J Musarrat. Evaluation of cytotoxic responses of raw and functionalized multi-walled carbon nanotubes in human breast cancer (MCF-7) cells. Vacuum. 2017; 146: 578-585.
- BM Rotoli, O Bussolati, MG Bianchi, A Barilli, C Balasubramanian, S Bellucci, et al. Non-functionalized multi-walled carbon nanotubes alter the paracellular permeability of human airway epithelial cells. Toxicol Lett. 2008; 178: 95-102.
- R Vivek. Tactics of Breast Cancer Therapeutics and Future Outlooks. J Cancer Biol Res. 2018; 6: 1120.
- K Greish, G Thiagarajan, H Herd, R Price, H Bauer, D Hubbard, et al. Size and surface charge significantly influence the toxicity of silica and dendritic nanoparticles. Nanotoxicology 2012; 6: 713-723.